Abstract
We have recently defined the window for marrow stem cell homing into nonablated hosts as the first 24 hours posttransplant. Within this homing window, donor cells rapidly cleared from the peripheral blood and lungs and plateaued in the marrow. We have now assessed the cell-cycle status of the engrafting cells capable of contributing to long-term hematopoiesis using administration of hydroxyurea (HU), a chemotherapy agent with S-phase cell-cycle specificity. HU was given at very short periods following a male bone marrow transplant (0, 3, 6, 12, and 15 hours) into female nonablated hosts, and donor cell engraftment was analyzed after 6 weeks. The data show that quickly after transplant (12 hours), greater than half of the engrafting cells capable of contributing long-term to all levels of the hematopoietic hierarchy are in S-phase. Analysis after 6 weeks included whole bone marrow, peripheral blood, primitive cells with high proliferative potential, and mature lineage-restricted marrow cells. These donor cells appear to be naturally synchronized. When HU was administered at any of the other time points, there was little evidence of cell death 6 weeks postengraftment.
FOLLOWING A BONE MARROW transplant, stem cells in the peripheral blood are thought to selectively migrate to stem cell “niches” within the bone marrow in a process referred to as homing. Once “homed” to the marrow, these cells then proliferate and differentiate. However, little is known about the mechanisms of hematopoietic cell homing, including timing and microenvironmental influences.
Hydroxyurea (HU) is a chemotherapy agent with cell-cycle stage specificity. HU preferentially kills cells in the DNA synthesis phase,1,2 and blocks the entry of G1 cells into this drug-sensitive S phase.1 The plasma half-life of HU after intraperitoneal injection in the BALB/c mouse is approximately 13 to 48 minutes,3,4 and the concentration of HU that affects spleen colony-forming units (CFUs) decreases to ineffective levels by 3 to 4 hours.4 As such, the cell kill observed after a single injection of HU represents the proportion of cells in S-phase at the time of drug exposure.
HU administration in vivo has previously been successfully used for a number of applications, such as to determine the number of different cell populations in cycle at a given time and hence to estimate cell-cycle times of particular populations.4-8 HU has also been used as a method for synchronizing cells capable of giving rise to colonies in the spleen when injected into lethally irradiated mice.4 More recently, HU administration has been used as a method to detect biological activity capable of suppressing the normal proliferative response of cells to HU.9
In the present study, we used HU administration as an in vivo assay for analyzing when engrafting cells capable of contributing to long-term hematopoiesis first enter the cell cycle posttransplant. Specifically, we administered HU at short periods after a bone marrow transplant and determined the number of donor cells long-term. The data show that quickly after transplant (approximately 12 hours), greater than half of the engrafting cells capable of contributing long-term to all levels of the hematopoietic hierarchy are cycling. These donor cells are naturally synchronized, with little evidence of cell death following administration of HU either 0, 3, 6, or 15 hours posttransplant.
MATERIALS AND METHODS
Mice
Six- to 8-week-old BALB/c H-2D mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in a conventional clean facility for at least 1 week before experimental use. All mice received mouse chow and acidified water ad libitum. This strain of mice was specifically chosen for this series of experiments because of their very low to negligible degree of immunoreactivity to the H-Y (Y chromosome–associated histocompatibility) antigen.10
Cell Suspensions
Mice were killed by cervical dislocation. Bone marrow was collected from the femurs and tibiae. This was routinely performed by flushing the femurs and tibiae with cold phosphate-buffered saline (PBS) supplemented with 5% heat-inactivated (HI) fetal calf serum ([FCS] Hyclone, Logan, UT). The cell suspension was filtered through a 40-μm filter (Becton Dickinson, Franklin Lakes, NJ). The cells were washed and resuspended for injection in 0.5 mL PBS per recipient.
Transplants and HU Administration
Male whole bone marrow cell populations were transplanted into nonablated female recipients by tail vein injection. Each transplant contained between 100 × 106 and 120 × 106 cells. Results were mathematically standardized so that each transplant was equal to 100 × 106 whole marrow cells. This correction assumes a linear relationship between the number of cells transplanted and the level of donor cells detected. A linear relationship has previously been shown for whole marrow transplants over the cell doses used.11 At various short intervals posttransplant (0, 3, 6, 12, or 15 hours), a single dose (900 mg/kg) of HU (Sigma Diagnostics, St Louis, MO) dissolved in PBS was administered to each recipient by tail vein injection. Cells were allowed to engraft for 6 weeks, after which the hosts were killed and the presence of donor cells was quantified using fluorescence in situ hybridization (FISH).
Engraftment Quantification
FISH. Peripheral blood and bone marrow cell populations were analyzed for donor cells using FISH. Red blood cells were lysed from peripheral blood samples using 0.83% ammonium chloride at 37°C for 5 minutes. Residual cells were washed twice, first in PBS and then in PBS 5% HI FCS. Cell cytospins were fixed in a 50% Carnoy's (75% methanol/25% acetic acid) and 50% PBS solution for 10 minutes before baking at 72°C for 1 hour. The slides were then further fixed in 100% Carnoy's solution for 5 minutes before permeabilization using proteinase K (0.2 μg/mL; Sigma) enzymatic digestion in 20 mmol Tris buffer at 37°C for 1.5 minutes. The cytospins were dehydrated through graded ethanol and then denatured in 70% formamide (GIBCO-BRL, Gaithersburg, MD) in 2× SSC (0.3 mol/L NaCl and 0.03 mol/L sodium citrate, pH 6.4) at 70°C for 3 minutes. The slides were dehydrated once more, and then hybridized with a digoxigenin-labeled Y chromosome probe12 at 45°C overnight. Unbound probe was removed by stringent washing in three changes of 50% formamide in 2× SSC and two changes of 2× SSC at 45°C. Following a wash in 4× SSC (0.6 mol/L NaCl and 0.06 mol/L sodium citrate, pH 6.4) at room temperature, the slides were blocked using a blocking buffer consisting of 5% FCS, 5% nonfat milk (Shaw's, Bridgewater, MA), and 0.05% Triton X-100 (Sigma) in 4× SSC for 15 minutes. Detection of digoxigenin was made using anti–digoxigenin-rhodamine Fab fragments (Boehringer, Mannheim, Germany) at a concentration of 6.5 μg/mL in PBS containing 2% BSA fraction V (Sigma) for 30 minutes in the dark. Nonbound antibody was removed through three extensive, light-protected washings: first in 4× SSC for 10 minutes, then in 4× SSC containing 0.05% Triton X-100 for 10 minutes, and finally in 4× SSC for 10 minutes. Cytospins were counterstained in 0.4 μmol DAPI (4,6-diamidino-2-phenylindole; Sigma) and mounted in the antifade media Vectashield (Vector Laboratories, Burlingame, CA). Specific positive label was confirmed under a microscope by a visual check at an excitation and emission wavelength other than that of rhodamine. Individual values represent analysis of an average of 185 cells from at least four different fields of focus. Each group contained at least three individual mice. Samples were analyzed by multiple readers for verification of results. In addition, the results were confirmed by Southern blot analysis of whole femoral DNA.
For analysis of donor cells with long-term high–proliferative potential colony-forming ability (HPP-CFC), the FISH protocol was modified as follows to prevent the agar and hence the colonies from being destroyed. The agar containing HPP-CFCs was dried onto a 0.01% poly-L-lysine (Mr 150,000 to 300,000; Sigma) subbed slide. Colonies were fixed and baked as already described. The permeabilization step was extended to 2 minutes, before the same dehydration steps. Denaturation was performed in 0.07 mol/L NaOH in 70% ethanol for 10 minutes at room temperature. The slides were dehydrated once more and then hybridized with the digoxigenin-labeled Y chromosome probe at 37°C overnight. Unbound probe was removed using the same stringent washings as before, but at 37°C. The rest of the labeling procedure was as already described.
Hematopoietic Progenitor Cell Assay
From one of the experiments, HPP-CFCs were grown using a double-layer nutrient agar culture system as previously described,13 except for the use of seven growth factors (interleukin-1 α [IL-1] 250 U/mL, IL-3 100 ng/mL, colony-stimulating factor-1 [CSF-1] 5,000 U/mL, granulocyte-macrophage CSF, 2.5 ng/mL, granulocyte CSF 5 ng/mL, stem cell factor, 100 ng/mL, and basic fibroblast growth factor 5 ng/mL) instead of three. HPP-CFCs typically generated colonies greater than 0.5 mm in diameter consisting of tightly packed cells. Functionally, these are defined as primitive cells by a relative resistance to 5-fluorouracil, synergistic growth factor requirements, and copurification with long-term reconstituting cells in vivo.14 15
Multilineage Analysis
From a separate experiment, bone marrow cells were isolated from the different hematopoietic lineages and analyzed by FISH for the proportion of donor cells 6 weeks posttransplant. Individual bone marrow cell aliquots from mice that received either a whole bone marrow transplant plus HU 12 hours posttransplant or only a whole bone marrow transplant were labeled with lineage-specific rat anti-mouse primary antibodies: B220 (B cells),16 Gr-1 (neutrophils),17 or Lyt-2/L3T4 (CD4/CD8) (T cells)18 (Becton Dickinson, San Jose, CA). Each batch of antibody was evaluated by flow cytometric analysis for the concentration that resulted in the greatest shift in mean channel fluorescence and/or the percentage of positive cells detected. The optimal dilution for each antibody was in the range of 1:10 to 1:100 (final). Following a 15-minute incubation on ice, the labeled cells were washed in PBS 5% HI FCS and resuspended in the same initial volume. The cells were incubated with mouse serum-absorbed fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG (Southern Biotechnology Associates, Birmingham, AL) at 4°C for 20 minutes in the dark. The cells were washed in PBS 5% HI FCS and kept on ice until sorted.
Labeled cell subpopulations were analyzed and sorted on a Moflo cell sorter (Cytomation, Fort Collins, CO) equipped with a 5-W argon ion laser (Coherent Innova 90, Palo Alto, CA) at 100 mW power. Light-scatter signals were collected through a 488-nm band-pass filter and a 1-decade logarithmic neutral-density filter in the forward light-scatter path. FITC fluorescence was collected through a 530/40-nm band-pass filter. Background fluorescence (cell surface binding of FITC-conjugated goat anti-rat second antibody) was less than 5%. Positive cells were collected into individual tubes and analyzed for donor cells using the FISH method already described.
Statistics
An unpaired two-sample t-test was used to test for significant differences in the proportion of donor cells in the peripheral blood, bone marrow, and HPP-CFC populations from HU-treated and untreated groups of mice. A Wilcoxon rank-sum test was used to test for significant differences in the proportion of donor cells from the different mature hematopoietic lineages from HU-treated and untreated groups of mice.
RESULTS
No differences in bone marrow or peripheral blood cellularity or marrow HPP-CFC content were detected between any of the mice groups 6 weeks posttransplant regardless of whether they received HU (data not shown). Therefore, the data are presented as the proportion of donor cells detected for each parameter.
Cycling Status of Cells Engrafting to the Bone Marrow With the Ability to Contribute to Hematopoiesis Long-Term
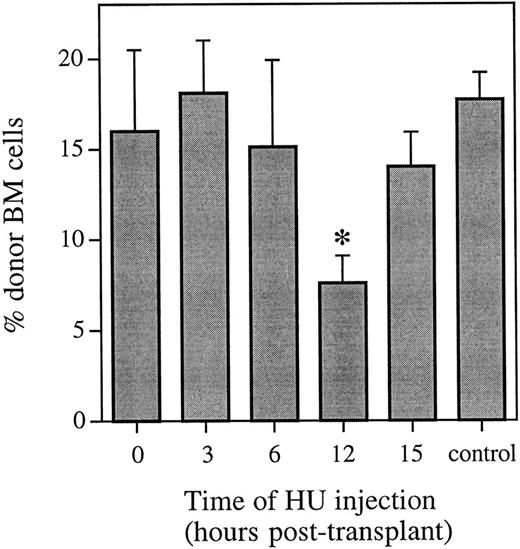
HU was administered either 0, 3, 6, 12, or 15 hours after a transplant of whole bone marrow, and the proportion of donor cells with the ability to contribute to hematopoiesis long-term was analyzed by FISH 6 weeks posttransplant (Fig 1). At this time, there was no difference in the proportion of donor cells in the marrow of mice that received either no HU or HU at 0, 3, or 6 hours posttransplant. However, there was a significant decrease (P < .001) in the proportion of donor cells in the bone marrow of mice that received HU 12 hours posttransplant. This decrease equals a cell kill of greater than 50%, and represents more than half of the cells capable of contributing to long-term hematopoiesis being in cycle. By 15 hours posttransplant, administration of HU again resulted in no difference in the proportion of donor cells in the marrow of mice 6 weeks posttransplant compared with mice that received the same transplant and no HU.
Proportion of donor marrow cells in the bone marrow 6 weeks posttransplant as determined using FISH. Control animals received an equivalent marrow transplant and no HU. Values are the mean ± SEM for 3 to 9 individual animals (6 at 12 hours and 9 in the control group) from two independent experiments. *Significantly different v control (P < .001).
Proportion of donor marrow cells in the bone marrow 6 weeks posttransplant as determined using FISH. Control animals received an equivalent marrow transplant and no HU. Values are the mean ± SEM for 3 to 9 individual animals (6 at 12 hours and 9 in the control group) from two independent experiments. *Significantly different v control (P < .001).
These FISH data were confirmed using Southern blot analysis. The difference in the percentage of male bone marrow cells from mice that received HU 12 hours posttransplant compared with mice that received the same transplant and no HU represented a cell kill of 47% and 44% in two independent experiments (n = 4 or 5 animals per group). The observation of no difference in the proportion of donor cells in the marrow of mice that received no HU compared with those receiving HU at 0, 3, 6, or 15 hours posttransplant was also confirmed (data not shown).
Cycling Status of Engrafting Marrow Cells With the Long-Term Primitive Characteristic of High Proliferative Potential
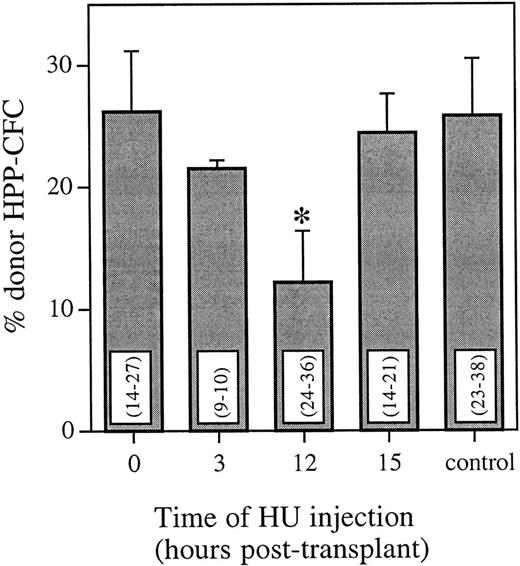
To analyze the cycling status of engrafting marrow cells with long-term primitive characteristics, recipient marrow cells were assayed for HPP-CFCs 6 weeks posttransplant. Using FISH, the proportion of donor colonies from mice that received HU at either 0, 3, 12, or 15 hours posttransplant was compared with mice that received no HU posttransplant (Fig 2). The pattern of HU killing of donor cells was very similar for HPP-CFCs and whole bone marrow. Compared with a marrow transplant and no HU, there was no significant difference in the proportion of donor HPP-CFCs 6 weeks posttransplant when HU was administered either 0, 3, or 15 hours posttransplant. However, when HU was administered 12 hours posttransplant, there was a significant decrease (P < .025) in the proportion of donor HPP-CFCs 6 weeks posttransplant. This equals a cell kill greater than 50% and represents more than half of the cells with long-term HPP colony-forming ability being in cycle.
Proportion of donor HPP-CFCs in the bone marrow 6 weeks posttransplant as determined using FISH. Control animals received an equivalent marrow transplant and no HU. Values are the mean ± SEM for three individual animals. Values in parentheses are the range of individual HPP-CFCs analyzed per animal. *Significantly different v control (P < .025).
Proportion of donor HPP-CFCs in the bone marrow 6 weeks posttransplant as determined using FISH. Control animals received an equivalent marrow transplant and no HU. Values are the mean ± SEM for three individual animals. Values in parentheses are the range of individual HPP-CFCs analyzed per animal. *Significantly different v control (P < .025).
Analysis of Peripheral Blood
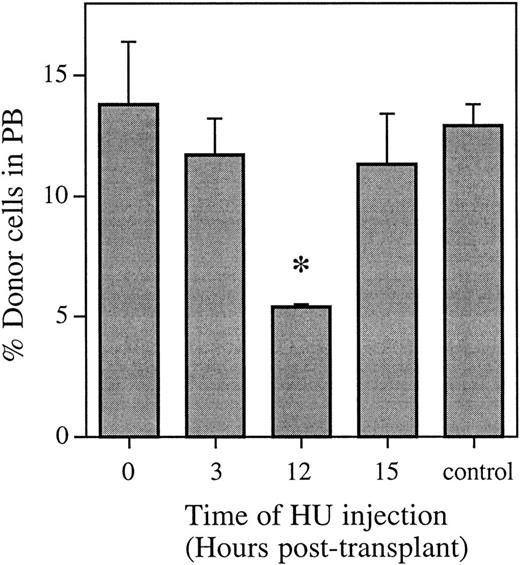
Nucleated peripheral blood cells were also analyzed by FISH to ensure that the effect of HU administration 12 hours posttransplant was the same on peripherally circulating hematopoietic cells as that evident in the bone marrow (Fig 3). The pattern of HU killing of donor cells mirrored the pattern in the whole bone marrow. There was no significant difference in the proportion of donor peripheral white blood cells (WBCs) 6 weeks posttransplant for HU administered either 0, 3, or 15 hours posttransplant compared with a marrow transplant and no HU. However, when HU was administered 12 hours posttransplant, there was a significant decrease (P < .01) in the proportion of donor WBCs 6 weeks posttransplant. This equals a cell kill greater than 55% and represents more than half of the cells with the capability of contributing to hematopoiesis long-term being in cycle.
Proportion of donor bone marrow cells in the peripheral blood 6 weeks posttransplant as determined using FISH. Control animals received an equivalent marrow transplant and no HU. Values are the mean ± SEM for three to four individual animals. *Significantly different v control (P < .01).
Proportion of donor bone marrow cells in the peripheral blood 6 weeks posttransplant as determined using FISH. Control animals received an equivalent marrow transplant and no HU. Values are the mean ± SEM for three to four individual animals. *Significantly different v control (P < .01).
Analysis of Lineage-Restricted Progenitors
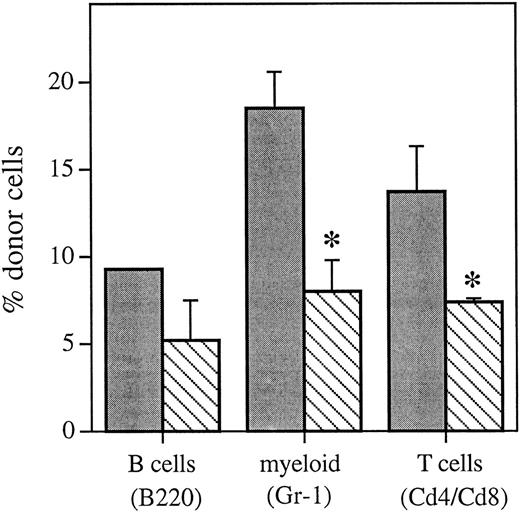
Bone marrow cells with lineage-restricted potential were separated by flow cytometry (Table 1) and analyzed by FISH to determine if administration of HU 12 hours posttransplant resulted in an equivalent decrease in the proportion of donor cells in individual hematopoietic lineages (Fig 4) versus that seen in the whole bone marrow (Fig 1) and peripheral blood (Fig 3) 6 weeks posttransplant. Compared with mice that received no HU posttransplant, mice that received HU 12 hours posttransplant had a decrease in the proportion of donor cells in all of the hematopoietic lineages analyzed 6 weeks posttransplant. This included a significant decrease in myeloid cells and T cells (P < .1) and equals a cell kill of between 26% and 53% across all lineages analyzed.
Restricted Bone Marrow Progenitor Separation
| Lineage . | Antibody . | Antigen Expression . | |
|---|---|---|---|
| . | . | Control . | HU 12 h Posttransplant . |
| Myeloid | Gr-1 | 22.8 ± 5.0 | 18.9 ± 0.8 |
| B cell | B220 | 11.2 ± 1.2 | 10.2 ± 0.7 |
| T cell | CD4/CD8 | 1.9 ± 1.7 | 3.9 ± 1.8 |
| Lineage . | Antibody . | Antigen Expression . | |
|---|---|---|---|
| . | . | Control . | HU 12 h Posttransplant . |
| Myeloid | Gr-1 | 22.8 ± 5.0 | 18.9 ± 0.8 |
| B cell | B220 | 11.2 ± 1.2 | 10.2 ± 0.7 |
| T cell | CD4/CD8 | 1.9 ± 1.7 | 3.9 ± 1.8 |
Values are the mean ± SEM for 3 individual mice.
Proportion of the different hematopoietic lineages of donor marrow origin as determined using FISH 6 weeks posttransplant from control animals that received an equivalent marrow transplant and no HU () or mice that received HU 12 hours posttransplant (▧). Values are the mean ± SEM for two to three individual animals (except for the B220 control group, which only had 1 sample). *Significantly different v control (P < .1).
Proportion of the different hematopoietic lineages of donor marrow origin as determined using FISH 6 weeks posttransplant from control animals that received an equivalent marrow transplant and no HU () or mice that received HU 12 hours posttransplant (▧). Values are the mean ± SEM for two to three individual animals (except for the B220 control group, which only had 1 sample). *Significantly different v control (P < .1).
DISCUSSION
We have demonstrated with in vivo administration of HU that transplanted marrow cells capable of contributing to all levels of the hematopoietic hierarchy long-term are initially quiescent but quickly go into cycle (approximately 12 hours posttransplant). The data indicate that this transplanted population is relatively synchronized in that greater than 50% cycle 12 hours posttransplant but virtually none are cycling either 6 or 15 hours posttransplant. It is important to note that this occurred in a nonablated transplant model, in which the preparative irradiation treatment is not stimulating the engrafting cells to cycle.
Six-weeks posttransplant was chosen as the end point to represent long-term engrafting cells, since previous studies from this laboratory have shown that donor cell engraftment levels detected 6 weeks posttransplant in a nonablated mouse model remain constant at 6 months and through 2 years, the normal lifespan of a mouse.19 20 This held true in both primitive hematopoietic stem cell and whole bone marrow compartments.
It has been demonstrated that HU kills only about 80% of S-phase CFU-spleen, with sensitivity to HU gradually decreasing as the cells approach the middle part of S phase, and increasing again as the cells enter the late portions of S phase.21 This may lead to an underestimation of the S-phase fraction. As a consequence, even more than the estimated number of transplanted cells capable of contributing to hematopoiesis long-term may actually be cycling 12 hours posttransplant. These data, taken with our recent observation that primitive hematopoietic cells home specifically to the bone marrow within 1 to 19 hours (Nilsson et al, manuscript submitted), suggest that immediately posttransplant there is a rapid clearance of dormant G0 /G1 cells from the peripheral blood into the bone marrow, where they very quickly enter into cell cycle. This agrees with the study by Hendrikx et al,22 who suggested that a CFU-spleen–enriched population also specifically homed to the bone marrow of both nonablated and irradiated hosts. Using PKH-26 labeling, many of these cells were shown to proliferate by 24 hours, and all of them by 36 hours.
However, transplanted stem cells (Rhodamine/Hoechstdull cells) maintain high proliferative potential and a subset of cells with Rhodamine/Hoechstdull characteristics long-term.20 In the present study, transplanted primitive cells cannot continue to cycle at the same rate, but probably quiesce upon taking up residence within a stem cell niche. Presumably, these cells will cycle very slowly throughout life, as has been shown to be normal for primitive hematopoietic stem cells in unperturbed bone marrow. Bradford et al23 recently showed that the most primitive hematopoietic stem cells, characterized by the Rhodamine/Hoechstdull phenotype and the capacity to reconstitute a lethally irradiated mouse long-term, are not dormant but continuously cycle slowly in normal steady-state bone marrow.
The data have interesting gene-therapy implications, potentially allowing in vivo incorporation of either cytoplasmically contained or systemically infused virus by the engrafting cells naturally cycling very quickly posttransplant. This would have many advantages over conventional methods, which require cytokine stimuli to force cell proliferation in vivo and have resulted in only limited long-term engraftment success postinduction.
ACKNOWLEDGMENT
The authors acknowledge Joanne Wuu and Chung Hsieh for advice on statistical analysis of the data. We also acknowledge Ruud Hulspas for invaluable help and advice with the flow cytometric analysis and isolation of individual lineage cell populations.
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants No. 49650-02 and 50222-01, and in part by a C.J. Martin Fellowship from the National Health and Medical Research Council, Australia, and the Our Danny Cancer Fund, University of Massachusetts (both to S.K.N.).
Address reprint requests to Susan K. Nilsson, PhD, Trescowthick Research Laboratories, Peter MacCallum Cancer Institute, Locked Bag #1, A'Beckett St, Melbourne, Australia, 3000.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal