Abstract
The mechanisms of interleukin-12 (IL-12) toxicity were studied in mice using a schedule (murine rIL-12, 400 ng/mouse, intraperitoneally [IP] once daily for 5 days) that markedly reduced body weight and food intake. On day 5, IL-12–treated mice had elevated serum and spleen IFN-γ and tumor necrosis factor (TNF). Serum sTNFR-P75 and corticosterone (CS) were also elevated. IL-12 toxicity was partially prevented by anti–IFN-γ antibodies or dexamethasone (DEX). A pre-dose of IL-12 (200 ng/mouse on day −14) completely prevented the toxicity of subsequent IL-12. The IL-12 predose also inhibited IL-12–induced IFN-γ levels, but did not modify IL-12–induced CS, TNF or sTNFR-P75. A protective effect was observed with a predose of lipopolysaccharide (LPS) or murine recombinant (r)IL-10. The protective effect of the IL-12 predose was reduced by coadministration of anti–IFN-γ, but a predose of murine rIFN-γ was not protective, suggesting that IFN-γ is necessary but not sufficient for the protective effect of IL-12. The IL-12 predose specifically protected against IL-12 toxicity and did not modify LPS toxicity. These data indicate that IL-12 can induce tolerance to its own toxicity, probably through a downregulation of IL-12–induced IFN-γ but independently of endogenous glucocorticoids. IFN-γ, and possibly IL-10, might be important in this tolerance.
INTERLEUKIN-12 (IL-12) was originally described as a natural killer (NK) cell stimulatory factor or cytotoxic lymphocyte maturation factor.1 IL-12 is produced by macrophages, dendritic cells, polymorphonuclear leukocytes, and keratinocytes; stimulates the production of interferon-γ (IFN-γ) from T cells and NK cells2; and has potential antitumor effects.3-5 It is implicated as a pathogenic mediator in autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE),6 graft-versus-host disease,7 and insulin-dependent diabetes mellitus in NOD mice.4 Anti–IL-12 antibodies are also protective in murine models of septic shock, including lipopolysaccharide (LPS)-induced lethality8 and generalized Shwartzman reaction.9 Mice treated chronically with IL-12 develop macrophage infiltrates in tissues10 and show body weight loss,11 with induction of tumor necrosis factor (TNF) and IFN-γ synthesis.10 11
The role of IFN-γ in IL-12 toxicity is not clear, since while IL-12–induced splenomegaly is reduced in IFN-γ receptor-deficient mice, this was associated with the appearance of pulmonary edema.10 IL-12 can also induce thymic atrophy which, in virally infected mice, is due to an increase in blood glucocorticoid levels.11
Phase I and II clinical trials with IL-12 indicated that pretreatment with a single dose of IL-12 prevented the toxic effects of a multiple-dose cycle started 2 weeks later, and similar results were reported in mice and monkeys, showing that early pretreatment with IL-12 inhibited subsequent IL-12–induced IFN-γ4,12 (J.P. Leonard et al, submitted for publication).
The aim of this study was to characterize the mechanism of this protective effect of IL-12 on its own toxicity with particular attention to the role of IFN-γ, IL-10, and endogenous glucocorticoids. While IFN-γ and TNF could mediate IL-12 toxicity,10,11 glucocorticoids are potent inhibitors of the synthesis of various inflammatory cytokines including TNF and IFN-γ,13,14 and if increased by IL-12 they could have a protective effect. IL-10 is a feedback inhibitor of TNF production and is protective in animal models of endotoxic shock.15 Recent reports indicate that, in human T cells, IL-12 induces secretion of both IFN-γ and IL-1016 and IL-10 is an endogenous inhibitor of IL-12– and TNF-induced IFN-γ production.17
We studied the mechanisms of IL-12 toxicity using a 5-day treatment cycle. We monitored food intake and body weight changes and measured various parameters including IFN-γ, IL-4, IL-6, IL-10, TNF and its soluble receptor P75 (sTNFR-P75), serum corticosterone (CS), and blood glucose.
We also investigated the role of other cytokines in IL-12 toxicity, particularly the role of IFN-γ, using an anti–IFN-γ monoclonal antibody (MoAb) previously reported to protect against LPS-induced pulmonary edema.18 We also studied the effect of a synthetic glucocorticoid, dexamethasone (DEX), a potent inhibitor of the synthesis of various proinflammatory cytokines,13 which is protective in various models of TNF-mediated toxicity including endotoxic shock.14
In particular, we studied the protective effect of IL-12 “predose” on the toxicity of IL-12 and investigated whether this effect was mediated by IFN-γ or IL-10, either by administering anti–IFN-γ or anti–IL-10 antibodies, murine rIFN-γ, or murine rIL-10. In some experiments, we pretreated mice with LPS, which is an inducer of IL-12, among other cytokines.8 19
MATERIALS AND METHODS
Animals and treatments.C57BL/6 mice (males, weighing 18 to 20 g; Charles River Italia, Calco, Italy) were used as this strain has been used in other in vivo studies with IL-12,11 and they are good producers of IFN-g and IL-12.8
Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national and international laws and policies (EEC Council Directive 86/609, OJ L 358, 1, December 12, 1987; Italian Legislative Decree 116/92, Gazzetta Ufficiale della Repubblica Italiana No. 40, February 18, 1992; NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985).
Mice were housed five per cage. IL-12 (murine rIL-12, 4.6 × 106 U/mg, LPS contamination < 0.641 EU/mg, a kind gift from Genetics Institute, Cambridge, MA) was administered at the dose of 400 ng/mouse, intraperitoneally (IP), once daily for 5 days. IL-12 was administered in PBS containing 0.1% bovine serum albumin (BSA); control mice received vehicle alone.
Body weight and food intake were recorded daily at 10:00 AM. All treatments were administered between 9:00 AM and 11:00 AM. Weight change was calculated based on the weight measured at the beginning of treatment. Data are expressed as mean ± SD (five mice per group). Food intake was evaluated per cage with five mice.
When indicated, murine rIL-12, 200 ng/mouse, IP, was administered (predose) 14 days before the experiment. In some experiments LPS (from Escherichia coli O55:B5, Sigma, St Louis, MO), murine rIFN-γ (a kind gift from Dr G. Garotta, Roche, Basel, Switzerland), murine rIL-10,15 20 or DEX (Sigma) were administered at the doses indicated in the text.
Monoclonal antimurine IFN-γ antibodies were obtained as ascites fluid from nude mice, as previously described.21 This preparation had a neutralizing titer of 1/40,000 against 30 U/mL of murine IFN-γ and was administered at the dose of 0.2 mL/mouse, IP, a dose that we previously reported to protect against LPS-induced pulmonary edema.18 The JES5-2A5 MoAb, a rat IgG1 neutralizing mouse IL-10 previously shown to upregulate LPS-induced TNF production in vivo,22 was a kind gift from Tim Mosmann (Dept of Immunology, University of Alberta, Edmonton, Canada). In some experiments, mice were treated with 0.2 mL of a MoAb against human epidermal growth factor receptor as a control.
Blood was taken from the retroorbital plexus at the times indicated, under ether anesthesia, and serum was prepared. When indicated, spleens were removed, homogenized in 4 volumes of ice-cold saline, centrifuged, and the supernatants were used for cytokine assays.
Miscellaneous assays.TNF was measured by cytotoxicity on L929 cells as previously described,23 using mouse rTNFα as a standard (Genzyme, Cambridge, MA). The sensitivity of the assay was 7.5 U/mL. An anti-murine TNF antiserum was used to check the specificity of the TNF bioassay. IL-6 activity was measured as hybridoma growth factor activity on 7TD1 cells (kind gift from Dr J. van Snick, Bruxelles) and expressed as costimulatory U/mL using rIL-6 as a standard.24 The sensitivity of the assay was 50 U/mL. When indicated, the specificity of the assay toward murine IL-6 in our samples was checked using an anti-murine IL-6 MoAb.25
CS was measured by radioimmunoassay, using an antiserum obtained from Sigma (C-8784) and following the manufacturer's directions. [3H]-corticosterone was purchased from Amersham (Amersham, UK).
IFN-γ, IL-4, and IL-10 were measured using commercially available ELISA kits (Benfer-Scheller, Milan, Italy). sTNFR-P75 was measured by ELISA as previously described.26 All cytokine ELISA used were specific for the indicated cytokine and did not cross-react with any other, according to the manufacturer's specification. The limits of sensitivity of the various assays are shown in Results. Serum glucose was measured by a commercially available kit using the glucose oxidase-peroxidase principle (Boehringer Mannheim).
RESULTS
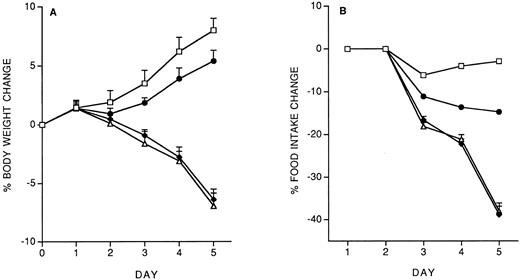
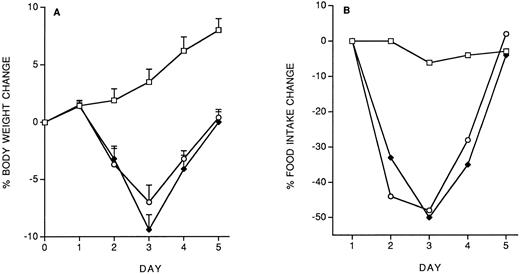
Model of IL-12 toxicity and its modulation.Five days' chronic treatment with murine rIL-12 (400 ng/mouse, IP, once daily) resulted in a reduction in body weight and food intake (Fig 1). Anti–IFN-γ antibodies (injected IP on days 1 and 3) markedly protected mice from the IL-12–induced decrease in body weight and food intake, while an irrelevant antibody used as control (anti-human EGFR) was not protective. Administration of DEX in drinking water throughout IL-12 treatment was also partially protective (on day 5, percentage body weight change was: vehicle, +8.8 ± 1; IL-12 × 5, −6.9 ± 0.9; IL-12 × 5/DEX, +2.5 ± 0.8. Percentage food intake change was: vehicle, −2.9; IL-12 × 5, −37; IL-12 × 5/DEX, −13).
Effect of chronic IL-12 on body weight (A) and food intake (B) and protection by anti–IFN-γ antibodies. IL-12 was administered at the dose of 400 ng/mouse intraperitoneally (IP), once daily; anti–IFN-γ antibodies were administered on days 1 and 3 (0.2 mL/mouse, IP). One group of mice received control antibodies (antihuman EGF receptor). Weight change was calculated based on the weight at the beginning of treatment and data are expressed as mean ± SD (n = 5). Food intake was evaluated per cage with five mice. (□), Vehicle; (♦), IL-12; (•), anti–IFN-γ + IL-12; (▵), control antibody + IL-12.
Effect of chronic IL-12 on body weight (A) and food intake (B) and protection by anti–IFN-γ antibodies. IL-12 was administered at the dose of 400 ng/mouse intraperitoneally (IP), once daily; anti–IFN-γ antibodies were administered on days 1 and 3 (0.2 mL/mouse, IP). One group of mice received control antibodies (antihuman EGF receptor). Weight change was calculated based on the weight at the beginning of treatment and data are expressed as mean ± SD (n = 5). Food intake was evaluated per cage with five mice. (□), Vehicle; (♦), IL-12; (•), anti–IFN-γ + IL-12; (▵), control antibody + IL-12.
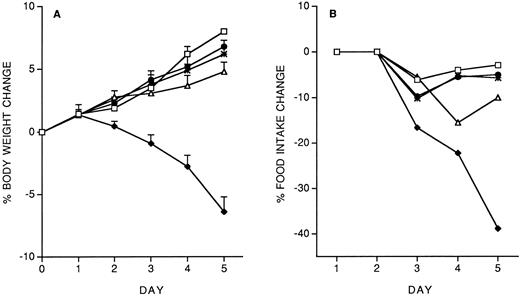
We investigated the effect of a predose of 200 ng/mouse of IL-12, administered 14 days before starting the 5-day chronic treatment with 400 ng/d. The effect on body weight and food intake is shown in Fig 2. The IL-12 pre-dose on day −14 almost completely prevented the toxicity of chronic IL-12. Pretreatment with either IL-10 (1 μg/mouse) or LPS (500 μg/mouse) was also protective (Fig 2, A and B). We also studied the effect of a pretreatment with IFN-γ (1 or 10 μg/mouse). As shown in Fig 2 C and D, IFN-γ provided almost complete protection only at the highest dose tested.
Effect of pretreatment (on day −14) with IL-12 (200 ng/mouse, IP), IL-10 (1 μg/mouse, IP) or LPS (500 μg/mouse, IP) on the decrease in body weight (A) and food intake (B) induced by IL-12 (400 ng/mouse, IP; once daily for 5 days). The effect of a predose of IFN-γ (1 or 10 μg/mouse, IP), as well as of IL-12 (200 ng) as a reference, is shown in panels C (body weight) and D (food intake). Body weight is expressed as mean ± SD (n = 5). Food intake was evaluated per cage with five mice. (A and B) (□), Vehicle; (♦), IL-12; (•), IL-12/IL-12; (▵), LPS/IL-12; (*), IL-10/IL-12. (C and D) (□), Vehicle; (♦), IL-12; (•), IL-12/IL-12; (▵), IFN-γ 10/IL-12; (*), IFN-γ 1/IL-12.
Effect of pretreatment (on day −14) with IL-12 (200 ng/mouse, IP), IL-10 (1 μg/mouse, IP) or LPS (500 μg/mouse, IP) on the decrease in body weight (A) and food intake (B) induced by IL-12 (400 ng/mouse, IP; once daily for 5 days). The effect of a predose of IFN-γ (1 or 10 μg/mouse, IP), as well as of IL-12 (200 ng) as a reference, is shown in panels C (body weight) and D (food intake). Body weight is expressed as mean ± SD (n = 5). Food intake was evaluated per cage with five mice. (A and B) (□), Vehicle; (♦), IL-12; (•), IL-12/IL-12; (▵), LPS/IL-12; (*), IL-10/IL-12. (C and D) (□), Vehicle; (♦), IL-12; (•), IL-12/IL-12; (▵), IFN-γ 10/IL-12; (*), IFN-γ 1/IL-12.
On day 5, mice were killed 2 hours after the last IL-12 injection, various parameters were evaluated, and the results are reported in Table 1. IL-12 raised serum and spleen IFN-γ and serum sTNFR-P75. TNF was also detected in these samples, although it was very low. It should be noted, in fact, that the limit of sensitivity of the assay was 7.5 U/mL. Control mice had a background level of 10.5 ± 6.9 that was not statistically significant, and this background level was only doubled in IL-12–treated mice. No IL-4, IL-6, or IL-10 was detectable within the sensitivity of the assays used. Serum CS was increased and hypoglycemia was observed, although the latter might well be secondary to the decrease in food intake.
Effect of IL-12 Predose on Chronic IL-12–Induced Changes
| Parameter . | Vehicle × 5 . | IL-12 × 5 . | IL-12 (−14) + Vehicle × 5 . | IL-12 (−14) + IL-12 × 5 . |
|---|---|---|---|---|
| Serum TNF (U/mL) | 10.5 ± 6.9 | 26 ± 2.1* | 12.4 ± 4.3 | 32.8 ± 2.8* |
| Spleen TNF (U/g) | 15 ± 6.5 | 31.5 ± 12* | 14 ± 3.8 | 36.8 ± 10.6* |
| Serum sTNFR-P75 (ng/mL) | 20.6 ± 2 | 54.8 ± 3.4† | 23.2 ± 7.1 | 57.3 ± 3.5† |
| Serum IL-4 (pg/mL) | <5 | <5 | <5 | <5 |
| Serum IL-6 (U/mL) | <50 | <50 | <50 | <50 |
| Serum IL-10 (pg/mL) | <7.5 | <7.5 | <7.5 | <7.5 |
| Serum IFN-γ (pg/mL) | <1 | 367 ± 62.5† | <1 | 126 ± 37† |
| Spleen IFN-γ (pg/g) | <1 | 248 ± 36† | <1 | 29.6 ± 8† |
| Serum CS (ng/mL) | 42.1 ± 10 | 135.4 ± 19† | 51.2 ± 16 | 126 ± 18† |
| Glycemia (mg/100 mL) | 169 ± 2.9 | 138.7 ± 3.8* | 166 ± 4.2 | 154.2 ± 3.8 |
| Parameter . | Vehicle × 5 . | IL-12 × 5 . | IL-12 (−14) + Vehicle × 5 . | IL-12 (−14) + IL-12 × 5 . |
|---|---|---|---|---|
| Serum TNF (U/mL) | 10.5 ± 6.9 | 26 ± 2.1* | 12.4 ± 4.3 | 32.8 ± 2.8* |
| Spleen TNF (U/g) | 15 ± 6.5 | 31.5 ± 12* | 14 ± 3.8 | 36.8 ± 10.6* |
| Serum sTNFR-P75 (ng/mL) | 20.6 ± 2 | 54.8 ± 3.4† | 23.2 ± 7.1 | 57.3 ± 3.5† |
| Serum IL-4 (pg/mL) | <5 | <5 | <5 | <5 |
| Serum IL-6 (U/mL) | <50 | <50 | <50 | <50 |
| Serum IL-10 (pg/mL) | <7.5 | <7.5 | <7.5 | <7.5 |
| Serum IFN-γ (pg/mL) | <1 | 367 ± 62.5† | <1 | 126 ± 37† |
| Spleen IFN-γ (pg/g) | <1 | 248 ± 36† | <1 | 29.6 ± 8† |
| Serum CS (ng/mL) | 42.1 ± 10 | 135.4 ± 19† | 51.2 ± 16 | 126 ± 18† |
| Glycemia (mg/100 mL) | 169 ± 2.9 | 138.7 ± 3.8* | 166 ± 4.2 | 154.2 ± 3.8 |
Murine rIL-12, 400 ng/mouse, IP, was administered daily for 5 days, with or without an IL-12 predose (200 ng/mouse on day −14). Measurements were made 2 hours after the last dose. Data are means ± SD of five mice per group. The sensitivity of the TNF assay was 7.5 U/mL.
P < .05, † P < .01 v vehicle by Tukey's test.
Table 1 also shows the effect of the IL-12 predose on these parameters. The only significant change was a decrease (threefold in serum and eightfold in the spleen) in IL-12 (5 days)–induced IFN-γ; TNF and sTNFR-P75 were not affected. CS was also unaffected by the IL-12 predose. Hypoglycemia was partially reversed, although it is not clear whether this was due to restored food intake, as mentioned above. We also studied the effect of DEX (administered for the entire 5-day treatment period in drinking water as described above) on the production of TNF and IFN-γ. No TNF was detectable (<7.5 U/mL) in sera or spleens from DEX-pretreated mice receiving IL-12, while only a partial, but significant, inhibition of IFN-γ levels was observed in serum (pg/mL serum IFN-γ: IL-12, 333 ± 31; DEX/IL-12, 242 ± 17; P < .01) and in spleen (pg/g spleen IFN-γ: IL-12, 206 ± 11; DEX/IL-12, 168 ± 20; P < .05).
The effect of anti–IFN-γ antibodies on the parameters investigated in Table 1 was also studied. Only induction of serum CS and hypoglycemia were inhibited by anti–IFN-γ antibodies (data not shown).
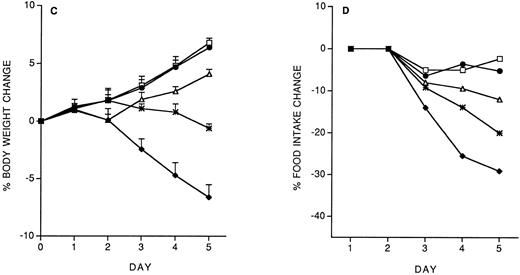
Role of IFN-γ and IL-10 in IL-12 protection against subsequent IL-12.We studied the role of IFN-γ and IL-10 in the protective effect of the IL-12 predose. Mice were administered 200 ng IL-12 (with or without anti–IFN-γ or anti–IL-10 antibodies, administered IP 2 hours before IL-12) on day −14, and then followed the chronic IL-12 schedule. As shown in Fig 3, anti–IFN-γ antibodies, and to a lesser extent, anti–IL-10 antibodies, reduced the protective effect of the IL-12 predose. Anti–IFN-γ antibodies also reverted the inhibitory effect of the predose on IFN-γ induction by chronic IL-12 (serum IFN-γ levels were: control, <1 pg/mL; IL-12 × 5, 418 ± 71 pg/mL; IL-12(−14)/IL-12 × 5, 163 ± 36; IL-12 + anti–IFN-γ (−14)/IL-12 × 5, 359 ± 83). DEX (10 mg/kg, IP, 30 minutes before the IL-12 predose on day −14) did not reverse the protective effect of the IL-12 predose (on day 5, percentage body weight change was: vehicle, +8.8 ± 1; IL-12 × 5, −6.9 ± 0.9; IL-12(−14)/IL-12 × 5, +7.1 ± 0.4; IL-12 + DEX(−14)/IL-12 × 5, +6.8 ± 0.7. Percentage food intake change was: vehicle, −2.9; IL-12 × 5, −37; IL-12(−14)/IL-12 × 5, −5; IL-12 + DEX(−14)/IL-12 × 5, −7).
Effect of IL-12 (200 ng/mouse), IL-12 + anti–IFN-γ (0.2 mL/mouse, IP; 2 hours before IL-12) or IL-12 + anti–IL-10 (0.2 mL/mouse, IP; 2 hours before IL-12), administered on day −14, on the decrease in body weight (A) and food intake (B) induced by chronic IL-12. Body weight is expressed as mean ± SD (n = 5). Food intake was evaluated per cage with five mice. (□), Vehicle; (♦), IL-12; (•), IL-12/IL-12; (▵), anti–IFN-γ + IL-12/IL-12; (*), anti–IL-10 + IL-12/IL-12.
Effect of IL-12 (200 ng/mouse), IL-12 + anti–IFN-γ (0.2 mL/mouse, IP; 2 hours before IL-12) or IL-12 + anti–IL-10 (0.2 mL/mouse, IP; 2 hours before IL-12), administered on day −14, on the decrease in body weight (A) and food intake (B) induced by chronic IL-12. Body weight is expressed as mean ± SD (n = 5). Food intake was evaluated per cage with five mice. (□), Vehicle; (♦), IL-12; (•), IL-12/IL-12; (▵), anti–IFN-γ + IL-12/IL-12; (*), anti–IL-10 + IL-12/IL-12.
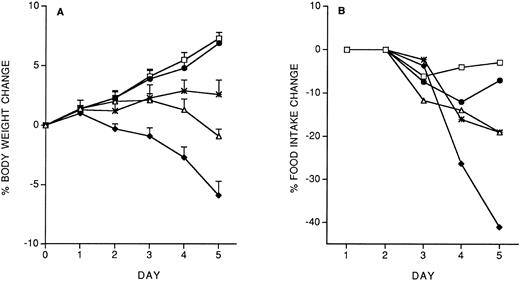
Specificity of the protective effect of IL-12: Interactions between IL-12 toxicity and LPS toxicity.To check whether the protective effect of the IL-12 predose on IL-12 toxicity was specific for IL-12, we studied the effect of the predose on LPS toxicity. Control or IL-12–pretreated mice received, 14 days later, five daily doses of LPS (10 μg/mouse, IP). As shown in Fig 4, IL-12 pretreatment did not protect against the LPS-induced decrease in body weight and food intake. It should be noted that with LPS tolerance developed by day 4.
Effect of IL-12 pretreatment (200 ng/mouse, IP; at −14 days) on LPS (10 μg/mouse, daily, IP)-induced decrease in body weight (A) and food intake (B). Body weight is expressed as mean ± SD (n = 5). Food intake was evaluated per cage with five mice. (□), Vehicle; (♦), vehicle/LPS; (○), IL-12/LPS.
Effect of IL-12 pretreatment (200 ng/mouse, IP; at −14 days) on LPS (10 μg/mouse, daily, IP)-induced decrease in body weight (A) and food intake (B). Body weight is expressed as mean ± SD (n = 5). Food intake was evaluated per cage with five mice. (□), Vehicle; (♦), vehicle/LPS; (○), IL-12/LPS.
IL-12 did not protect against the effect of a single high dose of LPS (1.5 mg/mouse, IP) which induces 100% mortality by 72 hours (data not shown).
DISCUSSION
The data reported indicate that IL-12 induces toxicity, manifested as reduced body weight and food intake, when administered chronically for 5 days. This was associated with high IFN-γ levels in the serum and spleen, whereas TNF reached only twice the background levels, in agreement with a previous report.27 This increase in TNF production was paralleled by an increase of serum levels of sTNFR-P75, which would act as a TNF inhibitor.28
The protective effect of anti–IFN-γ antibodies suggests a role for IFN-γ in IL-12 toxicity. These results are similar to those of Ozmen et al9 who reported that IL-12 can be a priming agent in the generalized Shwartzman reaction and that this activity was inhibited by anti–IFN-γ antibodies, indicating that IL-12, in that experimental model, acted through IFN-γ. IFN-γ is also a mediator of LPS toxicity, as indicated by the protective effect of anti–IFN-γ antibodies in the Shwartzman reaction21 or endotoxic shock29 and by the increased resistance of IFN-γ receptor-deficient mice to endotoxic shock.30
As IL-12 is a potent inducer of IFN-γ production, it was suggested that the protective effect of anti–IL-12 antibodies in endotoxic shock and generalized Shwartzman reaction was actually due to inhibition of IL-12–mediated IFN-γ production.8,9 Our data suggest that the same occurs in mice treated chronically with IL-12. The protective effect of DEX against IL-12 toxicity could well be explained by its ability to inhibit the production of several cytokines, as well as to protect against their action,14 31 and indicates that pharmaco-modulation of IL-12 toxicity is possible.
The protective effect of the anti–IFN-γ MoAbs reported here is in apparent contrast with a previous report that IFN-γ receptor-deficient mice have less splenomegaly after chronic IL-12 treatment but develop sickness and pulmonary edema after a 4-day schedule, whereas this was not observed in normal mice.10 Various factors might explain this difference, including the strain of mice and the dose of IL-12 used, or secondary changes induced by deficiency of the IFN-γ receptor. However, it cannot be excluded that low levels of IFN-γ are protective, and that in IFN-γ receptor-deficient mice inhibition of IFN-γ effects is more effective than neutralization of endogenous IFN-γ with antibody treatment. In fact, it was already noted that IL-12–induced IFN-γ in vivo is not completely inhibited by anti–IFN-γ antibody treatment.32 Other investigators reported protective effects of low levels of IFN-γ in autoimmune and inflammatory diseases.33 34
The other observation from this study is that treatment with IL-12 2 weeks before the toxicity study had a protective effect, in agreement with previous reports4 12 (J.P. Leonard et al, submitted for publication). This protection was associated with a marked decrease in IFN-γ induction, while the levels of TNF or sTNFR-P75 were unaffected, thus confirming the importance of IFN-γ as a key mediator of IL-12 toxicity. The protective effect of the IL-12 predose was inhibited by coadministration of anti–IFN-γ antibodies. In fact, murine rIFN-γ at the higher dose of 10 μg/mouse reproduced the protective effect of the IL-12 predose, although not completely indicating that it might be an important mediator in IL-12's effect but probably not sufficient to explain it.
The protective effect of a single predose of IL-10 suggests that induction of this cytokine, and of IFN-γ, might contribute to the protective effect. This data is strengthened by the observation that anti–IL-10 antibodies also partially inhibit the protective effect of the IL-12 predose. In this respect it is likely that our failure to measure any detectable IL-10 in IL-12–treated mice was due to either low sensitivity of the assay used or to the fact that IL-10 is known to be particularly labile, compared with other cytokines.
The protective effect of LPS pretreatment on day −14 can be explained by the ability of LPS to induce IL-12 and/or IL-10 production, as previously reported.8,19 35
We also evaluated the possibility that the protective effect of the IL-12 predose might be due to increased CS levels. Increases in the levels of serum CS, due to activation of the hypothalamus-pituitary-adrenal axis, is a well-known feedback mechanism that limits the production of proinflammatory cytokines,14,31 and IL-12 was reported to induce serum CS.11 However, in the present study an IL-12 predose did not potentiate the elevation of serum CS induced by chronic IL-12. Although this does not completely rule out the possible involvement of glucocorticoids in the observed phenomenon, our data indicate that protection is not merely due to increased blood CS levels.
Cross-protection experiments with LPS suggest that the protective effect of the IL-12 predose is not aspecific. In fact, mice administered an IL-12 predose were not protected in two different experimental models of LPS-induced lethality and LPS-induced decrease in body weight and food intake. This suggests that the toxicity of IL-12 is mediated by mechanisms different from those of LPS. We did, in fact, observe that the patterns of changes induced by LPS or IL-12 were different. For instance, LPS induced much higher serum IL-6 and TNF levels compared with IL-12 (data not shown).
Furthermore, the protective effect of the IL-12 predose cannot be explained by a complete desensitization to IL-12 due, for instance, to downregulation of its receptor(s). In fact, in mice administered the predose, IL-12 still induced some effects, such as high TNF and sTNFR-P75 levels.
In conclusion, IFN-γ has a key role in IL-12 toxicity, which can be relieved with anti–IFN-γ antibodies or DEX, to inhibit proinflammatory cytokine production. A predose of IL-12 administered as early as 14 days before also gives marked protection, not mediated by the hypothalamus-pituitary-adrenal axis and associated with down-regulated IFN-γ production. Other theories on the mechanism of this protective effect include increased production of protective cytokines, such as IL-4, IL-10, and IL-1317 32 or a decreased response of some cells to IFN-γ or IL-12 itself, and further studies will be necessary to test these possibilities. In the experiments reported here, we could not find any upregulation of circulating IL-10 production by IL-12 pretreatment, at least within the sensitivity of our assay (7.5 pg/mL). Furthermore, neutralization of endogenous IL-10 did not modify the 5-day toxicity of IL-12 in our experimental model (data not shown). However, future studies should look at local production of IL-4, IL-10, or IL-13 in key organs or at the single-cell level.
Address reprint requests to Pietro Ghezzi, MD, “Mario Negri” Institute for Pharmacological Research, via Eritrea 62, 20157 Milan, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal