Abstract
Expression of B7 family costimulatory molecules on B cells defines their capacity to function as antigen presenting cells (APCs). B cells that do not express B7 costimulatory molecules induce T-cell tolerance. Therefore, the expression of B7 costimulatory molecules on malignant B cells might be critical for their recognition by anti–tumor-specific T cells. Here we show that virtually all germinal center (GC)-derived B-cell lymphomas including follicular lymphoma (FL) and diffuse large cell lymphoma, but not mantle cell lymphoma or small lymphocytic lymphomas (SLL/CLL), express B7-1 (CD80) and B7-2 (CD86) on their cell surface in situ, although at extremely low levels. Despite their expression of low levels of B7-1 and B7-2, FL cells could not induce significant allogeneic T-cell proliferation. However, B7 costimulatory molecules on FL appeared to be functional because they were capable of increasing T-cell proliferation of preactivated T cells in a secondary allogeneic mixed lymphocyte response. Moreover, low B7 expression was sufficient to prevent the induction of alloantigen-specific anergy in vitro. Therefore, we postulate that whereas low-level expression of B7 is not sufficient to initiate a productive antilymphoma T-cell response, it might be sufficient to prevent T-cell tolerance in vivo.
EXPRESSION OF B7 costimulatory molecules on malignant B cells is pivotal for their recognition by antilymphoma specific T cells.1 B cells must express higher levels of costimulatory molecules to induce activation of naive T cells compared with levels necessary to stimulate T cells that have been previously exposed to antigen.2 Moreover, if the level of expression of B7 costimulatory molecules is insufficient, malignant B cells might tolerize rather than activate T cells.3-5 Therefore, the quantity of B7 expression by malignant B cells appears to be essential to direct the outcome of T-cell activation.
Follicular lymphoma (FL) cells have been shown to be either inefficient or ineffective antigen presenting cells (APC) for both autologous and allogeneic T cells in vitro.6-9 Several lines of evidence suggest that this poor APC function is due neither to the absence of antigen nor antigen presentation in the context of major histocompatibility complex (MHC) molecules, but rather to the lack of expression of B7 family costimulatory and/or adhesion molecules.6,7 However, there has been considerable controversy regarding the quantitative expression of B7-1 and B7-2 on non-Hodgkin's lymphoma (NHL).6-12 Low-level B7-1 expression has been detected in some cases of NHL in situ by immunohistochemistry10 and B7-2 has been reported to be frequently expressed.11 Several possibilities could account for the observed discrepancy between the reported in situ expression of B7 family members and their lack of detection by flow cytometric analysis. First, B7-1+ and B7-2+ NHL cells might be depleted during isolation and purification. Second, B7-1 and B7-2 might only be expressed intracellularly and, therefore, be undetectable by standard flow cytometric analysis. Third, in situ B7-1 and B7-2 expression might be too low to be detected by flow cytometry.
Here we show by in situ immunohistochemistry that B7-1 and B7-2 are expressed at extremely low levels on virtually all germinal center (GC) B-cell lymphomas including FL and diffuse large cell lymphoma, but not on mantle cell lymphoma (MCL) or small lymphocytic lymphomas (SLL/CLL). As exemplified by FL, the level of expression of B7 costimulatory molecules is insufficient to induce productive activation of allogeneic T cells. However, the level of B7 expression is sufficient to prevent T-cell anergy, suggesting that it might be capable of preventing anti-FL specific tolerance in vivo.
MATERIALS AND METHODS
Histology and immunohistochemistry. Cases of reactive lymphoid tissue and B-cell lymphoproliferative lesions were retrieved from the surgical pathology files of the Brigham and Women's Hospital and included 67 cases of B-cell NHL and 7 cases of reactive lymphoid hyperplasia. Studies were performed on discarded specimens and institutional approval obtained for these studies. Diagnoses were based on light microscopic appearance and routine immunoperoxidase studies, using standard diagnostic criteria.13 Specimens were fixed in 10% buffered formalin or B5 fixative before conventional tissue processing, paraffin embedding, and preparation of histologic sections for light microscopic examination. Tissue was also snap-frozen in dry ice-isopentane and used for the preparation of frozen sections for routine, diagnostic immunoperoxidase analysis using peroxidase-conjugated reagents. For highly sensitive detection of immunohistochemical staining Vectastain Elite ABC reagents (Vector Laboratories, Burlingame, CA), 5 to 10 times more sensitive then regular Vectastain ABC reagents (Vector Laboratories), were used.14-16 For both methods primary antibodies were used at optimal concentrations for detection (see Table 1). Briefly, for the study of costimulatory molecules, cryostat sections of lymphoid tissue were fixed in acetone for 10 minutes, washed with phosphate-buffered saline (PBS), incubated with primary mouse monoclonal antibody (MoAb) (antibodies used are listed in Table 1) for 1 hour, washed with PBS, incubated with biotinylated horse-antimouse antibody (Vector Laboratories) for 30 minutes, washed with PBS, then incubated with avidin:biotinylated-peroxidase complex (Vector Laboratories) for 40 minutes, followed by reaction with diaminobenzidine/hydrogen peroxide. Sections were subsequently stained with 2% methyl green.
Antibodies Used for Immunohistochemical Staining
| Antibody . | Clone . | Isotype . | Dilution . | Source . |
|---|---|---|---|---|
| Anti–B7-1 (CD80) | EW3.4B2.C4 | IgG2a | 1:250 | Repligen |
| A5.F5 | IgG2a | 1:500 | Repligen | |
| 104 | IgG1 | 1:500 | Coulter | |
| Anti–B7-2 (CD86) | FUN-1 | IgG1 | 1:2,000 | Pharmingen |
| HA5.2B7 | IgG2b | 1:2,000 | Repligen | |
| Anti-CD20 | B1 | IgG2a | 1:4,000 | Coulter |
| Anti-CD3 | UCHT1 | IgG1 | 1:2,000 | Dako |
| Anti-κ | A8B5 | IgG1 | 1:50 | Dako |
| Anti-λ | N10/2 | IgG1 | 1:50 | Dako |
| Antibody . | Clone . | Isotype . | Dilution . | Source . |
|---|---|---|---|---|
| Anti–B7-1 (CD80) | EW3.4B2.C4 | IgG2a | 1:250 | Repligen |
| A5.F5 | IgG2a | 1:500 | Repligen | |
| 104 | IgG1 | 1:500 | Coulter | |
| Anti–B7-2 (CD86) | FUN-1 | IgG1 | 1:2,000 | Pharmingen |
| HA5.2B7 | IgG2b | 1:2,000 | Repligen | |
| Anti-CD20 | B1 | IgG2a | 1:4,000 | Coulter |
| Anti-CD3 | UCHT1 | IgG1 | 1:2,000 | Dako |
| Anti-κ | A8B5 | IgG1 | 1:50 | Dako |
| Anti-λ | N10/2 | IgG1 | 1:50 | Dako |
Dako location: Carpinteria, CA.
Purification of malignant B cells. Lymph node specimens for in vitro studies were obtained under sterile conditions from biopsies of patients with follicular small cleaved NHL (follicular lymphoma; Working Formulation type B) as described previously.6,7 Malignant B cells were isolated as described previously.6 7 For control studies, human normal B cells were purified from human buffy coats in accordance with institutional guidelines.
Induction of B7 molecules on malignant B cells. As described previously, malignant B cells were activated by CD40 stimulation using CD40L transfectants in the presence of IL-4.6 7
Immunocytology. Cytospins were prepared (1) from single cell suspensions of cells derived from lymph nodes after mechanical homogenization and Ficoll density centrifugation, or (2) from malignant B cells cultured for 12, 36, 60, or 96 hours on t-CD40L cells in the presence of interleukin-4 (IL-4). All cell preparations were extensively washed before cytospins were prepared. Cells were then fixed in acetone for 10 minutes. Staining for B7-1 and B7-2 on these preparations was performed as described for the most sensitive immunohistochemistry method applying the Vectastain ABC Elite reagents. For optimal detection of B7-1 the MoAb clone EW3.4B2.C4 was used at a 1:250 dilution, the clone A5.F5 at 1:500. B7-2 MoAb clone HA5.2B7 was used at 1:2,000. To detect an increase in expression of B7-1 and B7-2 during in vitro activation of FL cells, B7-1 and B7-2 MoAbs were used at lower concentrations (EW3.4B2.C4 at a 1:2,000 dilution, A5.F5 at 1:60,000 dilution, HA5.2B7 at 1:12,000 dilution).
Immunofluorescence studies. For direct immunofluorescence MoAbs conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used to detect expression of B7-1 and B7-2 surface molecules.6 7 Anti–B7-1 MoAbs (clones A5.F5 and EW3.4B2.C4) were obtained from Repligen (Cambridge, MA) and conjugated with FITC following standard procedures. Anti-B7.2 (B70-PE, clone IT2.2) was purchased from Pharmingen (San Diego, CA). For indirect staining, cells were incubated at 4°C with unlabeled primary antibodies (clones A5.F5 and EW3.4B2.C4 for B7-1 and clone HA5.2B7 for B7-2 detection) for 30 minutes, washed twice with PBS, followed by incubation with goat-antimouse IgG-PE–labeled antibodies (Southern Biotechnology, Inc, Birmingham, AL) at 4°C for 30 minutes. Immunofluorescence was analyzed on a Coulter EPICS XL (Coulter, Miami, FL).
Mixed lymphocyte reactions (MLR). CD3+ CD4+ T cells were purified from peripheral blood mononuclear cells (PBMC) of healthy donors as described previously.6 Briefly, PBMC were obtained from PB obtained by leukopheresis followed by Ficoll-density centrifugation. T cells were further isolated from PBMC by depleting natural killer (NK) cells, monocytes, and B cells by two rounds of magnetic bead depletion using MoAbs (Coulter, Miami, FL) against CD19 (clone B4, IgG1), CD16 (clone 3G8, IgG1), CD56 (clone N901, IgG1), CD14 (clone Mo2, IgM), and CD11b (Mo1, IgM). Purity was assessed by flow cytometry and T-cell preparations were always greater than 97% CD3+ CD4+ T cells. For primary MLR, T cells were plated at 1 × 105 cells/well with 5 × 104 cells/well irradiated (32 Gy) malignant B cells as stimulators in 96-well round-bottom plates (Nunc, Roskilde, Denmark) in a final volume of 200 μL. A dose-response curve for irradiation was performed for both unstimulated malignant B cells and CD40-activated malignant B cells. Unstimulated malignant B cells either nonirradiated or irradiated with up to 32 Gy induced similarly low but comparable T-cell proliferation of purified allogeneic T cells. APC function was reduced when 64 or 96 Gy were applied. Nonirradiated or irradiated (up to 96 Gy) CD40-activated malignant B cells were similarly efficient APCs (data not shown). Cultures were incubated for 6 days in RPMI-5 (supplemented with 5% human serum and 2 mmol/L glutamine) at 37°C in a 5% CO2 atmosphere. Cells were pulsed with 3H-thymidine (Tdr) (1 μCi/well; Du Pont, Boston, MA) for the last 18 hours of the 6-day culture period. Cells were then harvested onto filters and the radioactivity measured in a β-plate liquid scintillation counter (Pharmacia, Piscataway, NJ). All samples were done in triplicate. For secondary MLR, T cells were incubated with CD40 preactivated FL cells (CD40-FL) in 24-well plates at a ratio of 4:1 (T:CD40-FL) at a concentration of 2.5 × 106 cells/mL/well in RPMI-5. Cells were obtained after 5 days, washed extensively and rested for 2 days in RPMI-5. T cells were then restimulated with unstimulated FL cells (5 × 104/well) in 96-well round-bottom plates either in the presence of CTLA4-Ig or control-fusion protein (10 μg/mL). Cells were pulsed with 3H-Tdr for the last 18 hours of the 3- to 6-day culture period. For assays of anergy, T cells were first stimulated with unstimulated FL cells in the presence or absence of CTLA4-Ig (10 μg/mL) in 24-well plates at ratios of 2:1 or 4:1 (T:FL) at a concentration of 2.5 × 106 cells/mL/well in RPMI-5. Cells were obtained after 5 days, washed extensively, and rested for 2 days in RPMI-5. Rested T cells were restimulated in 96-well round-bottom plates with CD40-FL cells (5 × 104/well) or FL cells plus CD28 MoAbs (clone 3D10, 1 μg/mL). Cells were pulsed with 3H-Tdr for the last 18 hours of the 3- to 6-day culture period.
RESULTS
Low-level in situ expression of B7-1 and B7-2 on GC-derived B-cell lymphomas. Sixty-seven NHL samples were analyzed to determine the in situ expression of B7-1 and B7-2 using the Vectastain ABC Elite biotin-streptavidin immunohistochemistry (IH) method, which is approximately 5- to 10-fold more sensitive than the conventional Vectastain ABC immunoperoxidase technique.14-16 Using this technique, virtually all follicular lymphomas, all diffuse large cell lymphomas, and small noncleaved lymphoma expressed both B7-1 and B7-2 whereas all small lymphocytic leukemia and mantle cell NHL were negative (Table 2). Figure 1 depicts representative expression of B7-1 and B7-2 by IH for 1 of the 32 FL tested. Virtually all malignant B cells expressed both B7-1 and B7-2 on their cell surface. Staining of serial sections with T-cell (CD3), B-cell (CD20) MoAbs, as well as κ or λ light chain MoAbs showed that B7-1 and B7-2 were restricted to the malignant B cells of the GCs. Double staining using CD20 MoAbs and B7-1 or B7-2 MoAbs confirmed that B7 family member expression was indeed on the malignant B-cell population (data not shown). Specificity of staining was confirmed using several isotype-matched control antibodies that were uniformly negative on all lymphoma samples examined (data not shown). Neoplastic cells exhibited uniform staining intensity for B7-1 and B7-2 in histologic sections of moderate to strong intensity, comparable to that of benign, reactive, GC B cells (data not shown).
Immunoperoxidase Staining for Costimulatory Molecules B7-1 (CD80) and B7-2 (CD86) by NHLs Using the Highly Sensitive Technique
| Lymphoma Type . | Pan-B . | MHC . | B7-1 . | B7-2 . |
|---|---|---|---|---|
| SLL/CLL* | 9/9† | 9/9 | 0/9 | 0/9 |
| Mantle cell | 9/9 | 9/9 | 0/9 | 0/9 |
| Follicular | ||||
| Small cleaved | 18/18 | 18/18 | 18/18 | 18/18 |
| Mixed | 7/7 | 7/7 | 7/7 | 7/7 |
| Large cell | 7/7 | 7/7 | 7/7 | 7/7 |
| Diffuse | ||||
| Large cell | 7/7 | 7/7 | 7/7 | 7/7 |
| Immunoblastic | 8/8 | 8/8 | 8/8 | 8/8 |
| Small noncleaved | 2/2 | 2/2 | 2/2 | 2/2 |
| Lymphoma Type . | Pan-B . | MHC . | B7-1 . | B7-2 . |
|---|---|---|---|---|
| SLL/CLL* | 9/9† | 9/9 | 0/9 | 0/9 |
| Mantle cell | 9/9 | 9/9 | 0/9 | 0/9 |
| Follicular | ||||
| Small cleaved | 18/18 | 18/18 | 18/18 | 18/18 |
| Mixed | 7/7 | 7/7 | 7/7 | 7/7 |
| Large cell | 7/7 | 7/7 | 7/7 | 7/7 |
| Diffuse | ||||
| Large cell | 7/7 | 7/7 | 7/7 | 7/7 |
| Immunoblastic | 8/8 | 8/8 | 8/8 | 8/8 |
| Small noncleaved | 2/2 | 2/2 | 2/2 | 2/2 |
Abbreviations: SLL, small lymphocytic lymphoma; CLL, chronic lymphocytic leukemia.
Includes two cases of SLL-plasmacytoid.
No. of immunoreactive cases/total cases studied.
Follicular lymphomas express B7-1 and B7-2 on the cell surface in situ. Immunoperoxidase staining of a representative small cleaved follicular center cell lymphoma using the Vectastain ABC Elite reagents. For B7-1, MoAb clone EW3.4B2.C4 was used and for B7-2 detection clone HA5.2B7. Similar staining for B7-1 and B7-2 were seen using other B7-1 or B7-2 MoAbs or CTLA4-Ig, respectively.
Follicular lymphomas express B7-1 and B7-2 on the cell surface in situ. Immunoperoxidase staining of a representative small cleaved follicular center cell lymphoma using the Vectastain ABC Elite reagents. For B7-1, MoAb clone EW3.4B2.C4 was used and for B7-2 detection clone HA5.2B7. Similar staining for B7-1 and B7-2 were seen using other B7-1 or B7-2 MoAbs or CTLA4-Ig, respectively.
In light of these findings, we sought to establish whether the reported discrepancy between anti–B7-1 and anti–B7-2 staining obtained by flow cytometry6 and IH were simply due to differences in sensitivity of detection. Therefore, we analyzed the expression of B7-1 and B7-2 on follicular lymphoma comparing: (1) the highly sensitive biotin-streptavidin immunohistochemistry (Vectastain ABC Elite), (2) standard biotin-streptavidin immunohistochemistry (Vectastain ABC), (3) highly sensitive biotin-streptavidin immunocytology (Vectastain ABC Elite), (4) direct immunofluorescence by flow cytometry, and (5) indirect immunofluorescence by flow cytometry. In agreement with our previous results, B7-1 could not be detected by either direct or indirect immunofluorescence by flow cytometry in 20 of 20 patient samples (Fig 2A and B, and data not shown) and B7-2 was only detected on a small fraction of malignant B cells in 6 of 20 lymphomas examined. In contrast, expression of both B7-1 and B7-2 could be clearly detected by immunocytochemistry (IC) using the highly sensitive biotin-streptavidin system (Fig 2C). The pattern of staining suggested that cell surface B7-1 and B7-2 was present. These results indicate that the inability to detect B7-1 and B7-2 by flow cytometry on most FL samples is not due to the isolation and purification procedure but rather due to a lower sensitivity of direct or indirect immunofluorescence staining by flow cytometric analysis compared to the highly sensitive IC method. This possibility was confirmed by comparison of the standard IH technique (Fig 2D) with the highly sensitive IH method (Fig 2E). Again, B7-1 and B7-2 could be detected on most GC cells with the highly sensitive IH method, whereas, by standard IH, both molecules were either undetectable or showed very weak staining on few cells in the GC. Taken together, these results support the conclusion that the expression of B7 family costimulatory molecules on FL is extremely low.
Comparison of B7-1 and B7-2 detection on FL cells directly after isolation from a representative small cleaved follicular center cell lymphoma using fluorescence-activated cell sorter (FACS), immunocytology, or immunohistochemistry. (A) FACS analysis using directly fluorescein-labeled MoAbs for B7-1 and B7-2. Unshaded area indicates fluorescence of isotype-matched antibody. (B) Indirect FACS analysis using unlabeled MoAbs to B7-1 and B7-2 followed by goat-antimouse antibodies conjugated with PE. (C) Highly sensitive immunocytology using Vectastain ABC Elite reagents on cytospin preparations of freshly isolated FL cells. (D) Standard IH method (Vectastain ABC), and (E) highly sensitive IH (Vectastain ABC Elite) from the same FL sample in cryostat sections. Antibodies were used at optimal concentrations (see Table 1).
Comparison of B7-1 and B7-2 detection on FL cells directly after isolation from a representative small cleaved follicular center cell lymphoma using fluorescence-activated cell sorter (FACS), immunocytology, or immunohistochemistry. (A) FACS analysis using directly fluorescein-labeled MoAbs for B7-1 and B7-2. Unshaded area indicates fluorescence of isotype-matched antibody. (B) Indirect FACS analysis using unlabeled MoAbs to B7-1 and B7-2 followed by goat-antimouse antibodies conjugated with PE. (C) Highly sensitive immunocytology using Vectastain ABC Elite reagents on cytospin preparations of freshly isolated FL cells. (D) Standard IH method (Vectastain ABC), and (E) highly sensitive IH (Vectastain ABC Elite) from the same FL sample in cryostat sections. Antibodies were used at optimal concentrations (see Table 1).
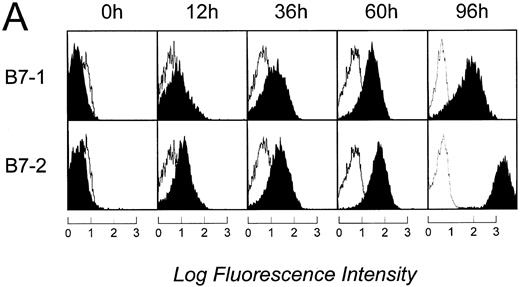
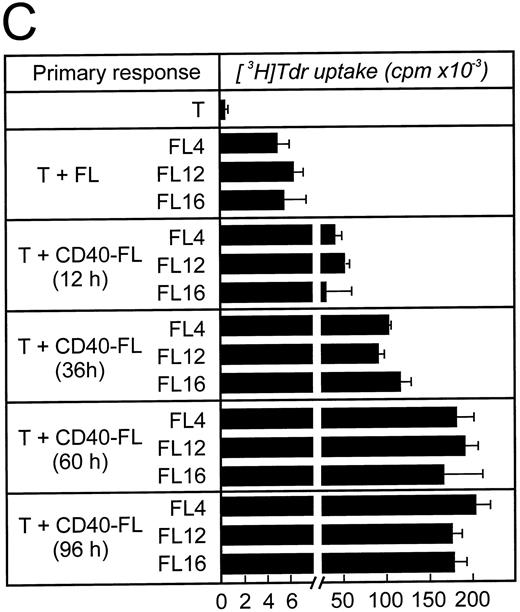
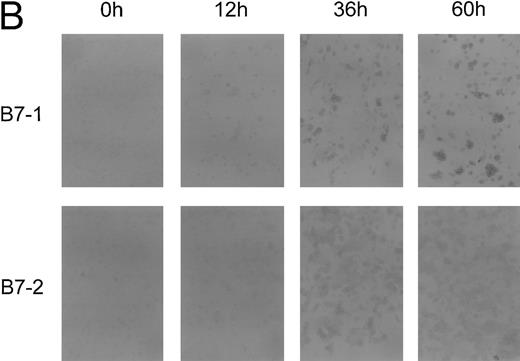
Level of B7 family expression on lymphoma cells correlates with their capacity to induce allogeneic T-cell proliferation. We next sought to determine whether B7 expression closely correlates with APC function of FL cells or whether there is a threshold of B7 expression necessary to induce significant T-cell proliferation. FL cells from patient FL4 and FL6 were activated via CD40 (CD40-FL) for 12, 36, 60, or 96 hours and then analyzed for their expression of B7 family members by flow cytometry and IC and their expression of B7 family members was correlated with their APC capacity. Results from patients FL4 and FL6 were comparable and only flow cytometry and IH from patient FL4 are shown in Fig 3. Using flow cytometry, B7-1 and B7-2 expression could only be detected after 12 hours and further increased until 96 hours (Fig 3A). To detect increase of expression by the highly sensitive IC method, primary antibodies had to be used at significantly lower concentrations than shown in Fig 2 (see Materials and Methods). Under these conditions, B7-1 and B7-2 were still detectable before activation, although very faintly. Moreover, the increase of expression after activation was also shown by IC (Fig 3B). Low levels of B7-1 and B7-2 expressed on FL4, FL12, and FL16 cells induced very low levels of T-cell proliferation (Fig 3C, upper panel). Under these conditions, T-cell proliferation was consistently above background resulting in a stimulation index between 4 and 8. Increased expression of B7 molecules on FL cells after CD40 activation correlated with their capacity to present antigen to allogeneic T cells (Fig 3C). After only 12 hours of CD40 preactivation, CD40-FL cells were then capable of inducing more significant allogeneic T-cell proliferation. Increased B7 family expression correlated with the level of T cell proliferation, which peaked after 60 hours of CD40 activation.
Time course of APC capacity and cell-surface expression of costimulatory molecules B7-1 and B7-2 on follicular lymphoma cells stimulated in vitro with CD40L and IL-4. Induction/upregulation of B7-1 and B7-2 was analyzed by FACS using directly conjugated MoAbs or by IC on cytospin preparations of the same cells. Cells were obtained from culture and extensively washed before analysis at time points indicated. (A) FACS analysis. Unshaded area indicates fluorescence of isotype-matched antibody. (B) IC of cytospin preparations. To detect differences in intensity by IC, primary antibodies were used at lower concentrations than described for Fig 2. B7-1 MoAb (EW3.4B2.C4) was used at a 1:2,000 dilution, B7-2 MoAb (HA5.2B7) at a 1:12,000 dilution. (C) Allogeneic T-cell proliferation induced by unstimulated FL cells and CD40-FL cells. FL cells were activated as described in Material and Methods, washed, irradiated, and used at 5 × 104 stimulator cells/well. Highly purified allogeneic T cells (1 × 105 cells/well) were used as responders. T-cell proliferation was measured at day 3 (data not shown) and day 6 by thymidine incorporation for the last 16 hours of the culture period. Approriate controls (T cells, FL cells, CD40-FL cells) were less than 1,800 cpm. One representative experiment (FL4, FL12, FL16) of three experiments is shown.
Time course of APC capacity and cell-surface expression of costimulatory molecules B7-1 and B7-2 on follicular lymphoma cells stimulated in vitro with CD40L and IL-4. Induction/upregulation of B7-1 and B7-2 was analyzed by FACS using directly conjugated MoAbs or by IC on cytospin preparations of the same cells. Cells were obtained from culture and extensively washed before analysis at time points indicated. (A) FACS analysis. Unshaded area indicates fluorescence of isotype-matched antibody. (B) IC of cytospin preparations. To detect differences in intensity by IC, primary antibodies were used at lower concentrations than described for Fig 2. B7-1 MoAb (EW3.4B2.C4) was used at a 1:2,000 dilution, B7-2 MoAb (HA5.2B7) at a 1:12,000 dilution. (C) Allogeneic T-cell proliferation induced by unstimulated FL cells and CD40-FL cells. FL cells were activated as described in Material and Methods, washed, irradiated, and used at 5 × 104 stimulator cells/well. Highly purified allogeneic T cells (1 × 105 cells/well) were used as responders. T-cell proliferation was measured at day 3 (data not shown) and day 6 by thymidine incorporation for the last 16 hours of the culture period. Approriate controls (T cells, FL cells, CD40-FL cells) were less than 1,800 cpm. One representative experiment (FL4, FL12, FL16) of three experiments is shown.
In vivo B7 expression by FL cells induces significant T-cell proliferation by preactivated T cells. The inability of unstimulated FL cells to induce significant T-cell proliferation in vitro despite detectable expression of B7 could be due to: (1) low levels of B7 expression insufficient to costimulate, or, potentially, (2) malignant B cells might express a nonfunctional form of this molecule. Although the level of T-cell proliferation induced by FL cells was extremely low (note scale change, 4 − 8 × 103 cpm), this result was significant because it could be consistently (P < .05) abrogated by the addition of CTLA4-Ig, which blocks both B7-1 and B7-2 on the FL cells (Fig 4). These data suggest that B7 expressed on FL cells is functional, although this level of expression is insufficient to induce more significant T-cell proliferation. It cannot be excluded that the allogeneic T cells provided signals to the FL cells leading to upregulation of costimulatory signals. To rule out this possibility, we performed two different experiments. First, FL cells were paraformaldehyde fixed instead of irradiated. Paraformaldehyde-treated cells induced the identical pattern of results as irradiated cells (data not shown). Second, we analyzed B7 expression by flow cytometry during coculture of allogeneic T cells and irradiated FL cells and could not detect expression of B7 on the FL cells or the T cells (data not shown).
CTLA4-Ig blocks allogeneic T-cell proliferation stimulated by FL cells. Cocultures of allogeneic T cells and FL cells were performed as described in the Fig 4 legend. CTLA4-Ig (10 μg/mL) was added to the FL cells 30 minutes before coculture and was present throughout the culture. As control an irrelevant fusion protein (control-Ig, 10 μg/mL) was used. Approriate controls (T cells, FL cells) were less than 1,800 cpm. Three experiments with three different T-cell donors were performed (shown here FL4, FL12, and FL16).
CTLA4-Ig blocks allogeneic T-cell proliferation stimulated by FL cells. Cocultures of allogeneic T cells and FL cells were performed as described in the Fig 4 legend. CTLA4-Ig (10 μg/mL) was added to the FL cells 30 minutes before coculture and was present throughout the culture. As control an irrelevant fusion protein (control-Ig, 10 μg/mL) was used. Approriate controls (T cells, FL cells) were less than 1,800 cpm. Three experiments with three different T-cell donors were performed (shown here FL4, FL12, and FL16).
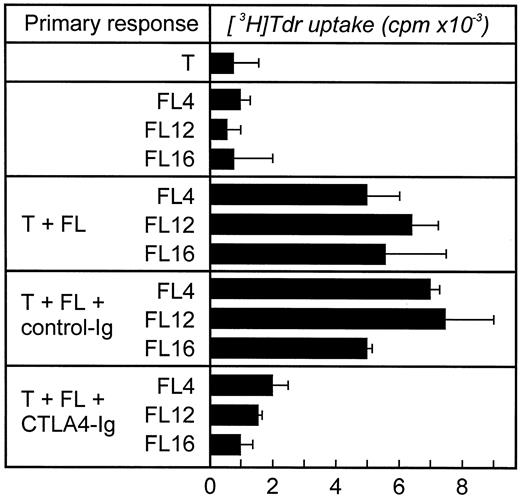
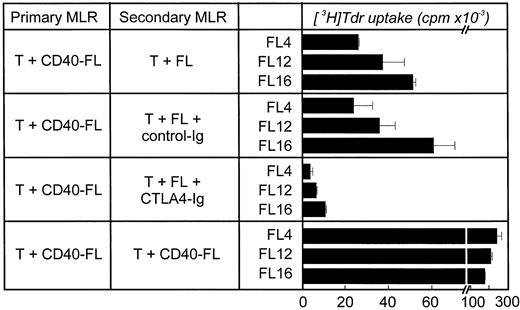
Results obtained in secondary MLRs further support that B7 on unstimulated FL cells is functional. Allogeneic T cells were primarily stimulated with CD40-FL cells and then restimulated with unstimulated FL cells in a secondary MLR either in the presence or absence of CTLA4-Ig (Fig 5). Whereas allogeneic T cells proliferated in response to FL cells in a secondary MLR, the addition of CTLA4-Ig greatly reduced this response. Taken together, these data suggest that the expression of B7 on FL cells is insufficient to significantly costimulate proliferation of resting T cells; however, this low level B7 expression is sufficient to induce significant T-cell proliferation once T cells are previously activated.
Role of B7 on FL cells during restimulation of productively stimulated allogeneic T cells in a secondary MLR. Allogeneic T cells were stimulated primarily with CD40-FL cells for 5 to 6 days. Cells were then harvested, enriched for viable cells by density-centrifugation, and rested for 48 hours before restimulation. Cells were then restimulated with FL cells, FL cells plus control-Ig (10 μg/mL), FL cells plus CTLA4-Ig (10 μg/mL), or CD40-FL cells. T-cell proliferation was assessed after 3 days (shown here) or 6 days (data not shown) by thymidine incorporation for the last 16 hours of coculture. Approriate controls (T cells, FL cells, CD40-FL cells) were less than 3,000 cpm. Control-Ig or CTLA4-Ig were added to FL cells 30 minutes before coculture and were present throughout the culture period.
Role of B7 on FL cells during restimulation of productively stimulated allogeneic T cells in a secondary MLR. Allogeneic T cells were stimulated primarily with CD40-FL cells for 5 to 6 days. Cells were then harvested, enriched for viable cells by density-centrifugation, and rested for 48 hours before restimulation. Cells were then restimulated with FL cells, FL cells plus control-Ig (10 μg/mL), FL cells plus CTLA4-Ig (10 μg/mL), or CD40-FL cells. T-cell proliferation was assessed after 3 days (shown here) or 6 days (data not shown) by thymidine incorporation for the last 16 hours of coculture. Approriate controls (T cells, FL cells, CD40-FL cells) were less than 3,000 cpm. Control-Ig or CTLA4-Ig were added to FL cells 30 minutes before coculture and were present throughout the culture period.
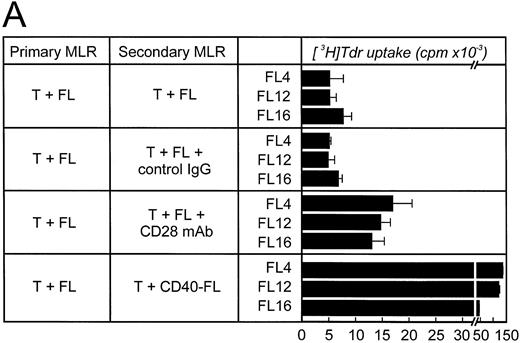
Alloantigen-specific T-cell anergy can be prevented by B7 family members expressed on FLs. To determine whether FL cells could induce alloantigen-specific T-cell anergy, allogeneic T cells were primarily cultured with unstimulated FL cells for 5 days, rested, and then restimulated with either FL cells, FL cells plus anti-CD28 MoAb, or CD40-FL cells. When either FL cells in the presence of CD28 MoAb or CD40-FL cells were used for restimulation, allogeneic T cells proliferated (Fig 6A). These data suggest that unstimulated FL cells did not anergize allogeneic T cells in vitro.
FL cells induce anergy only if CTLA4-Ig is present during primary stimulation. Allogeneic T cells were stimulated in a primary MLR with unstimulated FL cells (A) in the absence or (B) in the presence of CTLA4-Ig. Primary coculture was performed for 5 to 6 days. Cells were then obtained, enriched for viable cells by density-centrifugation, and rested for 48 hours before restimulation. Cells were then restimulated with FL cells, FL cells plus control IgG (2 μg/mL), FL cells plus CD28 MoAb (1 μg/mL), or CD40-FL cells. T-cell proliferation was assessed after 3 (shown here) or 6 days (data not shown) by thymidine incorporation for the last 16 hours of coculture. Approriate controls (T cells, FL cells, CD40-FL cells) were less than 2,000 cpm. Control IgG or CD28 MoAb were added to the T cells 30 minutes before coculture and were present throughout the culture period.
FL cells induce anergy only if CTLA4-Ig is present during primary stimulation. Allogeneic T cells were stimulated in a primary MLR with unstimulated FL cells (A) in the absence or (B) in the presence of CTLA4-Ig. Primary coculture was performed for 5 to 6 days. Cells were then obtained, enriched for viable cells by density-centrifugation, and rested for 48 hours before restimulation. Cells were then restimulated with FL cells, FL cells plus control IgG (2 μg/mL), FL cells plus CD28 MoAb (1 μg/mL), or CD40-FL cells. T-cell proliferation was assessed after 3 (shown here) or 6 days (data not shown) by thymidine incorporation for the last 16 hours of coculture. Approriate controls (T cells, FL cells, CD40-FL cells) were less than 2,000 cpm. Control IgG or CD28 MoAb were added to the T cells 30 minutes before coculture and were present throughout the culture period.
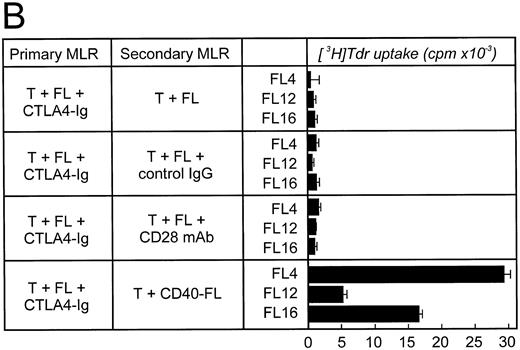
To determine whether low-level expression of B7 on FL cells was responsible for preventing anergy, allogeneic T cells were stimulated by FL cells in the presence of CTLA4-Ig in a primary MLR and then rechallenged (Fig 6B). When the low levels of B7 were blocked during the primary stimulation, no T-cell proliferation could be detected on rechallenge, indicating that the T cells were anergized and were not able to respond to rechallenge with FL and anti-CD28 MoAb. Surprisingly, restimulation with CD40-FL cells resulted in T-cell proliferation, although at significantly reduced levels (5 − 30 × 103 cpm versus 50 − 150 × 103 cpm without CTLA4-Ig during primary culture). This suggests that costimulation provided by CD40-FL cells was capable, at least in part, of reversing anergy.
DISCUSSION
There has been considerable discrepancy regarding the level of expression and potential function of B7 costimulatory molecules on human B cell NHL.6-12 Here we show that both B7-1 and B7-2 costimulatory molecules are expressed on virtually all follicular lymphoma, diffuse large cell lymphoma, and small noncleaved lymphoma, but absent on mantle cell lymphoma and SLL/CLL. The level of expression is extremely low because it is only detectable by the most sensitive IH or IC method. Functional analysis using FL cells showed that the level of expression is insufficient to initiate significant allogeneic T-cell proliferation but augments proliferation of preactivated T cells. Furthermore, low-level B7 expression on FL cells could prevent the induction of T-cell anergy in vitro. Blockade of B7 family–mediated costimulation on FL cells by CTLA4-Ig was necessary to induce T-cell anergy. Taken together, these results are consistent with the hypothesis that low levels of B7 family expression on lymphoma cells might prevent the induction of antilymphoma-specific T-cell tolerance in patients with GC-derived NHLs.
The comparison of different methods for the detection of B7 molecules on lymphoma cells showed that only GC-derived lymphomas expressed B7 family costimulatory molecules. Neither standard immunohistochemistry or flow cytometry were sufficient to detect the expression of B7 on these malignant B cells, leading to the conclusion that expression was indeed very low. These data indicate that the expression of B7 molecules on both normal and neoplastic B cells may be similarly regulated.17,18 Specifically, no technique has detected either B7-1 or B7-2 on normal mantle zone B cells whereas B7-1 and B7-2 expression could be detected in the GC.17,18 Therefore, within the GC, most normal and neoplastic B cells express B7 family molecules, although the level of expression varies widely. CD40 activation in vitro and in vivo is probably the most important pathway to induce B7-1 and B7-2 on B cells.18-22 We (D.M.D. and J.L.S., unpublished results, 1997) and others23 find CD40L-expressing T cells located in normal and malignant follicles, indicating that the expression of CD40L on T cells in B-cell lymphoma is intact. This raises the question why the lymphoma cells do not express sufficient B7 costimulator molecules in vivo, although they can be induced in vitro. Preliminary data in our laboratory (J.L.S., unpublished results, 1997) suggest that induction of B7 costimulatory molecules on B cells by CD40 activation is further regulated by cytokines. Similar to monocytes24,25 and dendritic cells,26 27 IL-10 reduces the expression of B7 molecules induced by CD40 activation. Because lymphomas have been shown to express huge amounts of IL-10, it is tempting to speculate that other mechanisms including the expression of IL-10 might be responsible for the low expression of B7 costimulatory molecules, although the CD40L/CD40 pathway might be intact.
Our data further support the conclusion that different levels of expression of B7 costimulatory molecules are one mechanism to modulate the level of T-cell activation.19,22,28-31 As has been shown by ourselves and others,2,6,22 high expression of costimulatory molecules is either necessary or at least favorable for the initiation of a T-cell response. However, low expression of costimulatory molecules might further support reactivation of primed T cells. Clearly, in vivo this has to be seen in the context of other factors modulating T-cell activation. If the T cells receive inhibitory signals, eg, via downregulatory cytokines, at the same time, low-level expression of B7 molecules on lymphoma cells might not be able to initiate any T-cell activation.4,32-36 However, if stimulatory cytokines such as inflammatory cytokines, in particular IL-12, are present,37-41 the sum of stimulatory signals might be sufficient to initiate an antilymphoma immune response. A dynamic expression of inhibitory and stimulatory factors could, at least in part, account for the natural history of waxing and waning lymph nodes in low-grade NHL. Further in situ analysis of lymph nodes from lymphoma patients at times of regression as well as progression will be necessary to attempt to answer these important questions.
Recent data in a murine model system suggested that low-level expression of B7 on APCs is capable of inducing T-cell tolerance in vivo rather than T-cell activation. The mechanism is postulated to be that low levels of expression of B7 will result preferentially in signaling through CTLA-4 rather than CD28.42 This is clearly not the case in human B-cell NHL. One possible explanation is the lack of CTLA-4 expression on T cells in human B-cell lymphoma (D.M.D. and J.L.S., unpublished results, 1997). Alternatively, the low-level expression of B7 on human B-cell NHL is not comparable to low-level B7 expression described in the murine model system. If FL cells express sufficient B7 to prevent antigen-specific T-cell tolerance in vivo, the general inability of infiltrating T cells to proliferate in vitro43,44 and their multiple signaling defects43-45 have to be explained by other mechanisms. In fact, preliminary data suggest that cytokines such as IL-10 and transforming growth factor-β1, shown to be expressed in lymphoma,46-51 might be at least in part responsible for the unresponsiveness of infiltrating T cells in lymphoma.52
Based on our findings it is tempting to speculate that the impressive clinical success of vaccination strategies in lymphoma using the clonal idiotype as a specific tumor-antigen53-62 are in part due to the expression of B7 costimulatory molecules on the target tumor cells. Clearly, either idiotype in adjuvant,57,59,62 with cytokines63,64 or idiotype-pulsed dendritic cells65-68 might primarily initiate an antilymphoma-specific T-cell response. However, in comparison to other tumor cells, lymphoma cells might be attacked more easily by the idiotype-specific T cells, because the lymphoma cells not only express MHC class I and II molecules, but also costimulatory and some adhesion molecules.69-71 In addition, the idiotype-primed T cells might further be able to upregulate costimulatory and adhesion molecules on lymphoma cells in T-cell B-cell cross-talk,19 further enhancing lymphoma recognition. Therefore, human B-cell lymphoma might be an ideal model to study if B7 expression on the tumor cell itself in addition to MHC class I and II is not only favorable, but mainly responsible for the outcome of immunotherapy in cancer that is based on the induction of antigen-specific T cells either ex vivo or in vivo. In this model the expression of costimulatory molecules at low levels would be capable of enhancing the effector function of T cells that have been previously activated by vaccination strategies using professional APCs. This may then in part explain the impressive results that have been seen to date in preliminary clinical trials.
ACKNOWLEDGMENT
We thank Arnold Freedman, Jim Griffin, Glenn Dranoff, and David Sherr for their helpful discussion and critical reading of this manuscript.
D.M.D. and J.L.S. contributed equally to this manuscript.
Supported in part by National Institute of Health Grants (CA66996) to L.M.N. J.L.S. is a Fellow of the Lymphoma Research Foundation of America. Supported in part by the Deutsche Forschungsgemeinschaft (Schu-950/1-1). S.M. was supported by the Carl Duisberg Gesellschaft.
Address reprint requests to Joachim L. Schultze, MD, Department of Adult Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal