Abstract
Patients with recurrent or refractory low-grade non-Hodgkin's lymphoma (NHL) are increasingly treated with myeloablative therapy and autologous stem cell transplantation. However, allogeneic bone marrow transplantation (BMT) is only sporadically performed in such patients. Therefore, we wish to compare treatment results of patients with recurrent or refractory low-grade NHL who underwent allogeneic BMT with those who underwent autologous BMT in our center. Twenty-eight patients were studied. The patients had received 2 to 5 lines of conventional chemotherapy before the BMT procedure. Eighteen patients, all with chemotherapy-sensitive disease at the time of transplantation, underwent autologous BMT and 10 patients, of whom 7 with chemotherapy-resistant disease at the time of transplantation, underwent allogeneic BMT. Furthermore, all allogeneic BMT patients had overt lymphoma infiltration of the BM at the time of transplantation. The conditioning regimen consisted of cyclophosphamide plus total body irradiation in all 28 patients. All allogeneic BMT patients achieved complete remission, 3 patients had a treatment-related death, and 7 patients are alive and disease-free with a median follow-up of 41 months. In contrast, none of the autologous BMT patients died of transplant-related complications. However, despite the fact that all autologous BMT patients had chemotherapy-sensitive disease and partial remission was converted to complete remission by the BMT procedure in 67% of them, only 3 of 18 patients are alive and disease-free. The probability of relapse or disease-progression among allogeneic BMT patients was 0% compared with 83% for autologous BMT patients (P = .002). Progression-free survival rates 2 years after BMT were 68% for allogeneic BMT patients and 22% for autologous BMT patients (P = .049). Although the numbers of patients are small, this study suggests that allogeneic BMT offers a better chance for cure than autologous BMT for patients with poor-prognosis low-grade lymphoma, and the difference in relapse or disease progression is strongly suggestive for the existence of a graft–versus–low-grade lymphoma effect.
ALTHOUGH DISSEMINATED low-grade non-Hodgkin's lymphoma (NHL) is characterized by a relatively indolent clinical course, the disease is incurable with conventional chemotherapy and almost always fatal.1 Initial responses to chemotherapy can last for several years, yet all patients eventually relapse. The use of chemotherapy combinations in a more aggressive approach has given a higher response rate, but survival has not been improved.2-4
Previous studies have shown poor survival for patients who fail to respond to initial or subsequent chemotherapy or whose response duration is short-lived.1,5,6 Such poor-risk patients are increasingly subjected to high-dose chemotherapy and autologous bone marrow transplantation (BMT) or peripheral blood stem cell transplantation (PBSCT).4,7 However, allogeneic BMT has only sporadically been performed in these patients.7 8 Therefore, we wish to report the outcome of 10 patients with poor-risk low-grade lymphoma who were treated with myeloablative chemotherapy followed by allogeneic BMT and to compare these results with those of a group of 18 patients with poor-risk low-grade lymphoma treated similarly except for receiving autologous BMT.
PATIENTS AND METHODS
We retrospectively analyzed the results of all patients with low-grade NHL who underwent BMT between July 1988 and November 1996 at our BMT unit. Twenty-eight patients with low-grade lymphoma received BMT. Ten patients received allogeneic BMT and 18 patients received autologous BMT. Characteristics of the patients at the time of BMT are shown in Table 1. Eligibility criteria included disseminated low-grade NHL defined by the Working Formulation as follicular small cleaved cell (FSC), follicular mixed small cleaved and large cell (FM), and small lymphocytic (SL) consistent with chronic lymphocytic leukemia (CLL)9; failure to respond to primary or subsequent chemotherapy; or a very short duration of response (<1 year) after chemotherapy. Patients less than 61 years of age were subsequently subjected to an alternative chemotherapy regimen, generally containing doxorubicin, and were considered as candidates for autologous BMT, when at least a partial response was obtained together with the presence of an uninvolved autologous marrow graft. However, when patients had persistent marrow involvement and/or chemotherapy-resistant disease and were therefore not eligible for autologous BMT, they were offered allogeneic BMT when they had an HLA-identical sibling and were less than 56 years of age. All candidates for BMT must have normal organ functions and a World Health Organization performance status that was ≤2. Patients with active central nervous system involvement and those with a positive human immunodeficiency virus serology were not eligible.
Patient Characteristics at BMT
| Variable . | Allogeneic BMT . | Autologous BMT . |
|---|---|---|
| No. | 10 | 18 |
| Age (yr), median (range) | 43 (31-55) | 47 (28-60) |
| Male/female | 6/4 | 7/11 |
| Stage of disease | ||
| III | 0 | 2 |
| IV | 10 | 16 |
| Tumor histology* | ||
| FSC | 0 | 2 |
| FM | 6 | 16 |
| SL | 4 | 0 |
| No. of prior chemotherapy regimens, median (range) | 2 (2-4) | 3 (2-5) |
| BM involvement† | 10 | 2 |
| Status of disease‡ | ||
| Sensitive | 3 | 18 |
| Resistant | 7 | 0 |
| Variable . | Allogeneic BMT . | Autologous BMT . |
|---|---|---|
| No. | 10 | 18 |
| Age (yr), median (range) | 43 (31-55) | 47 (28-60) |
| Male/female | 6/4 | 7/11 |
| Stage of disease | ||
| III | 0 | 2 |
| IV | 10 | 16 |
| Tumor histology* | ||
| FSC | 0 | 2 |
| FM | 6 | 16 |
| SL | 4 | 0 |
| No. of prior chemotherapy regimens, median (range) | 2 (2-4) | 3 (2-5) |
| BM involvement† | 10 | 2 |
| Status of disease‡ | ||
| Sensitive | 3 | 18 |
| Resistant | 7 | 0 |
According to the classification of the Working Formulation: FSC, follicular small cleaved cell; FM, follicular mixed small cleaved and large cell; SL, small lymphocytic.
Analyzed by histopathologic examination of two-sided iliac crest biopsies.
Response to the final chemotherapy before BMT.
Involvement of the BM was analyzed by bilateral biopsies and aspirations from the iliac crest, including immunophenotyping by immunoperoxidase. Patients were considered to be candidates for autologous BM collection if histopathologic examination of both biopsies was normal. When additional immunologic analysis showed that ≥5% of all nucleated marrow cells in the graft were monoclonal B cells, patients were considered ineligible for autologous BMT. No patient received PBSCT. Informed consent was obtained from all patients.
Chemotherapy protocols for initial or subsequent therapy included (1) chlorambucil and prednisone; (2) CVP (cyclophosphamide, vincristine, prednisone); (3) fludarabine; (4) CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone); and (5) ProMACE-MOPP (prednisone, methotrexate, doxorubicin, cyclophosphamide, etoposide, mechlorethamine, vincristine, procarbazine, prednisone).
Two autologous BMT patients had received involved field radiotherapy for localized disease before they were treated with chemotherapy for disseminated disease. Furthermore, 3 autologous BMT patients and 1 allogeneic BMT patient had experienced histologic transformation to intermediate-grade NHL at some time of their disease, but all had completely responded to chemotherapy. At the time of BMT, all patients with active NHL showed low-grade NHL without any evidence (tissue biopsies, clinical features, and serum lactate dehydrogenase level) of tumor transformation.
Treatment protocol. Pretransplant conditioning therapy consisted of total body irradiation (TBI) of 900 cGy in one fraction for autologous BMT patients and 1,200 cGy in two fractions for allogeneic BMT patients and 120 mg/kg cyclophosphamide administered over 2 days. Because of the increased risk for interstitial pneumonitis in allogeneic BMT recipients receiving TBI in one fraction, these patients were treated with fractionated TBI, but at a higher total dose, so that the single fraction and the two fractions, radiobiologically, were equivalent. Allogeneic BMT patients received partially T-cell–depleted marrow grafts, containing 1 × 105 T cells/kg, and received a 3-month course of cyclosporine posttransplantation, as described previously.10 Autologous marrow grafts were collected, cryopreserved, and thawed as described before,11 and marrow purging was not performed. Posttransplantation care was identical for autologous and allogeneic BMT patients, except that allogeneic BMT patients received prophylaxis with cotrimoxazole and acyclovir for 6 months to 1 year after BMT and received parenteral alimentation in the early posttransplantation period.10 The diagnosis and grading of acute and chronic graft-versus-host disease (GVHD) was established according to the Seattle criteria.12
Response criteria. Complete remission (CR) was defined as the disappearance of all disease and symptoms for more than 1 month. Partial remission (PR) was defined as more than 50% reduction in the sum of products of the two greatest perpendicular diameters of measurable lesions, including at least PR of BM involvement. Partial remission of BM involvement was defined as more than 50% reduction in marrow involvement, measured histopathologically and immunologically. All other outcomes were considered treatment failures. Progression-free survival (PFS) was measured in months and was defined as the time from BMT until disease relapse, progression, or death from any cause. Overall survival (OS) was measured in months from BMT until death from any cause.
Statistical analysis. Survival analysis was performed using the Kaplan-Meier method. The log-rank statistic was used to compare relapse rate, PFS, and OS probabilities between allogeneic and autologous patient groups.
RESULTS
Patient characteristics. Patient characteristics at the time of BMT are shown in Table 1. All patients had disseminated disease; stage IV was present in all allogeneic patients and in 16 of 18 autologous patients. The conventional chemotherapeutic regimen that preceded the BMT procedure produced either PR (n = 12) or CR (n = 6) in autologous patients, whereas PR was achieved in only 3 of 10 allogeneic patients. Seven allogeneic patients had chemotherapy-resistant disease. Furthermore, BM involvement was histopathologically present at the time of BMT in only 2 autologous patients, whereas it occurred in all allogeneic patients. BM collection, which was performed in the majority of patients early (at a median of 2 weeks) before the BMT procedure, showed a low percentage (<5%) of monoclonal B cells in 3 patients, whereas histopathology at the time of collection was normal in all patients. The median time from diagnosis to transplant was 43 months (range, 11 to 98 months) for autologous BMT and was 20 months (range, 12 to 61 months) for allogeneic BMT recipients. The more indolent course and responsiveness to chemotherapy in the autologous BMT group is responsible for this difference in time interval between autologous and allogeneic BMT recipients.
Response. Six of 18 patients were in CR at the time of autologous BMT. Relapse occurred in 4 of them (13, 15, 16, and 51 months after BMT), and 2 patients remained in continuous CR 39 and 45 months after BMT. Twelve of 18 autologous patients were in PR at the time of BMT, 8 achieved CR and 4 remained in PR with the BMT procedure. Seven patients relapsed from 6 weeks to 16 months after BMT and only 1 patient is still in CR, 77 months after BMT. All 4 patients not achieving CR with the BMT procedure had an early disease progression 1 to 4 months after BMT.
Three allogeneic patients were in PR at the time of BMT, although with BM involvement, and 7 allogeneic patients had resistant disease. Three patients died from toxicity 2, 5, and 7 months after BMT. At autopsy, all patients were in CR. All allogeneic patients achieved CR during follow-up, although marrow involvement disappeared slowly in 2 patients (up to 2 years in 1 patient).
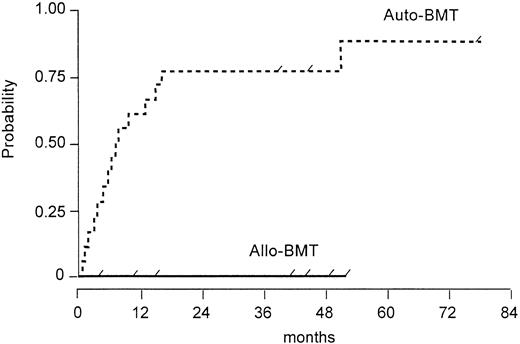
The probability of relapse or disease progression for allogeneic and autologous patients is shown in Fig 1 and the difference between both groups is significantly (P = .002) in favor of the allogeneic group.
Probability of relapse or disease progression after autologous versus allogeneic BMT (log rank test, P = .002). Tick marks depict the duration of survival of patients who did not relapse.
Probability of relapse or disease progression after autologous versus allogeneic BMT (log rank test, P = .002). Tick marks depict the duration of survival of patients who did not relapse.
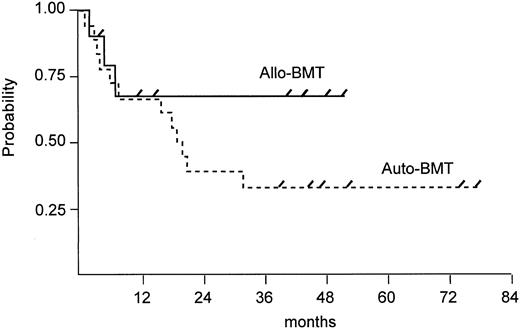
Survival. Seven of 10 allogeneic patients are alive and disease-free with a median follow-up of 41 months (range, 4 to 52 months). In contrast, 3 of 18 autologous patients are alive and disease-free with a follow-up duration of 39, 45, and 77 months. PFS rates 2 years after BMT were 68% for allogeneic BMT patients and 22% for autologous BMT patients (P = .049) and are shown in Fig 2. Of 15 autologous patients who had a relapse or disease progression, 3 patients are still alive with active disease and 12 patients have died of lymphoma. At this time, there is no significant difference (P = .276) in overall survival between both groups, although there is a trend in favor of allogeneic BMT (Fig 3).
Estimated PFS after autologous versus allogeneic BMT (log rank test, P = .049). Tick marks depict patients alive.
Estimated PFS after autologous versus allogeneic BMT (log rank test, P = .049). Tick marks depict patients alive.
Estimated OS after autologous versus allogeneic BMT (log rank test, P = .276).
Estimated OS after autologous versus allogeneic BMT (log rank test, P = .276).
Four of the 28 patients had experienced histologic transformation during the course of disease, though none had transformed disease at BMT. Three underwent autologous BMT and 1 received an allogeneic BMT. One autologous BMT recipient has become a long-term disease-free survivor (>45 months), as did the allogeneic BMT recipient (>41 months). Two autologous BMT patients had relapsed, 1 again as low-grade lymphoma and lived for another 2 years after relapse and the other patient died early after BMT of disease progression.
Toxicity. There were no treatment-related deaths among autologous patients, whereas 3 allogeneic patients died from toxicity. One patient died 2 months after BMT of cerebral infarction, 1 patient died 5 months after BMT of interstitial pneumonitis, and 1 patient died 7 months after BMT of a posttransplant lymphoproliferative disorder.
Acute GVHD developed in 8 of 10 allogeneic patients; it was grade I in 4 patients and grade II in another 4 patients. Chronic GVHD developed in 3 patients (extensive disease in 1) and was absent in 6 patients (1 patient was not evaluable for chronic GVHD because of early death).
DISCUSSION
This report indicates that patients with refractory or recurrent low-grade lymphoma have a better chance of cure when they are treated with allogeneic BMT than with autologous BMT. The difference in outcome between both treatment modalities is even more remarkable in light of the fact that most allogeneic patients had chemotherapy-resistant disease, whereas all autologous patients had chemotherapy-sensitive disease. Although the numbers of patients are small and the results are coming from a nonrandomized comparison, the data are helpful to make treatment options for younger patients with poor-risk low-grade lymphoma.
So far, treatment results of allogeneic BMT for patients with low-grade lymphoma are very scarce. There is only one study, from the Houston group, reporting the results of 10 allografted patients.8 Eight of them achieved CR and none showed disease progression at the time of report. Our results with allogeneic BMT for low-grade are fully compatible with their data. A recent report of the International Bone Marrow Transplant Registry (IBMTR), assembling the patients worldwide, is supportive for the curative potential of allogeneic BMT for low-grade lymphoma.13 It shows that patients surviving disease-free beyond 2 years after BMT are likely to be cured of the disease. On the other hand, numerous patients with intermediate-grade and high-grade lymphoma have been allografted,14-18 and one can conclude from these reports that a substantial number of these patients are cured by allogeneic BMT and that the putative graft-versus-aggressive lymphoma effect appears to exist.15,16 18
There have been numerous reports describing excellent early results with autologous BMT or PBSCT for patients with low-grade lymphoma in an early or later phase of their disease.19-23 However, a continued pattern of relapse is generally seen with prolonged follow-up, consistent with our results of autologous BMT for chemotherapy-sensitive low-grade lymphoma. It might be possible for our patients as well as those reported in the literature that the higher relapse rate occurring after autologous BMT/PBSCT than after allogeneic BMT is due in part to the presence of malignant cells in the autologous transplants.
It is clear that treatment-related mortality differs between autologous BMT/PBSCT and allogeneic BMT and one can assume that T-cell–depleted allogeneic BMT has a 10% to 15% higher mortality rate10,24-26 than autologous BMT/PBSCT.21-23 27 Nevertheless, the much higher rate of relapse or disease progression occurring after autologous BMT eventually will turn the scale in favor of allogeneic BMT for patients with low-grade lymphoma. However, it is important to note that allogeneic BMT is applicable only to a minority of patients with low-grade lymphoma because of the fact that most of the patients are diagnosed when they are more than 60 years of age and the shortage of HLA-compatible donors for many of the younger patients.
We conclude from this study that allogeneic BMT is preferable to autologous BMT in younger patients with poor-risk low-grade lymphoma. Furthermore, the large difference in relapse or disease progression between autologous and allogeneic BMT indicates the existence of a graft-versus-indolent lymphoma effect.
Address reprint requests to Leo F. Verdonck, MD, PhD, University Hospital Utrecht, Department of Haematology (G03.647), PO Box 85.500, 3508 GA Utrecht, The Netherlands.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal