Abstract
CD20 is a B-lineage–specific gene expressed at the pre–B-cell stage of B-cell development that disappears on differentiation to plasma cells. As such, it serves as an excellent paradigm for the study of lineage and developmental stage-specific gene expression. Using in vivo footprinting we identified two sites in the promoter at −45 and −160 that were occupied only in CD20+ B cells. The −45 site is an E box that binds basic helix-loop-helix-zipper proteins whereas the −160 site is a composite PU.1 and Pip binding site. Transfection studies with reporter constructs and various expression vectors verified the importance of these sites. The composite PU.1 and Pip site likely accounts for both lineage and stage-specific expression of CD20 whereas the CD20 E box binding proteins enhance overall promoter activity and may link the promoter to a distant enhancer.
B-LYMPHOCYTE DEVELOPMENT proceeds through discrete stages characterized by ordered DNA rearrangements of the Ig loci that lead to transcription of Ig genes and expression of B-cell antigen receptors.1 In addition, the different stages of this developmental process can be distinguished by the expression of specific cell surface markers. Studies of the promoter and enhancer regions of Ig genes as well as the analyses of the promoter regions of several B-lineage genes have offered some insights into the general mechanisms of lineage specific gene expression.2,3 Such gene expression often depends on a complex interplay between cis-regulatory elements and a large number of transcription factors, some that are ubiquitously expressed and others that are expressed in a lineage-restricted manner.4,5 Often there are direct physical interactions between the different transcription factors resulting in a cooperative regulation of a specific gene.6
Although gene targeting experiments in mice have determined that several transcription factors including BSAP,7 EBF,8 E2A,9 PU.1,10 and Ikaros11 are essential for normal B-cell development, this approach has been less useful in defining the roles of these transcription factors in stage- and lineage-specific gene expression because B-cell development in these mice arrests at a very early stage. For example, the B-cell marker CD20 does not normally appear until the pre–B-cell stage, hence all these mutant mice lack CD20-positive B cells and the potential roles of these transcription factors in CD20 gene expression cannot be assessed. In fact, the molecular mechanisms governing the stage and lineage-specific expression of CD20 remain largely unknown. After its appearance on pre-B cells, CD20 persists until B cells terminally differentiate into plasma cells.12,13 Although the exact function of CD20 remains unknown it is likely important in B-cell activation and it may function as a calcium channel.13
The CD20 promoter has been cloned and CD20 promoter constructs behave in a B-cell–specific manner.14,15 Deletion analysis with subcloned promoter fragments of various sizes identified a positive cis-acting element located between base pairs −186 and −290. Analysis of this interval of the promoter with mobility shift assays showed the presence of a diverged octamer binding site that interacted with the B-cell–specific transcription factor Oct-2 as well as the ubiquitous transcription factor Oct-1.16 However, it is unlikely that this cis-element is sufficient to account for the lineage- and stage-specific activity of the CD20 promoter. Mutational analysis of the diverged octamer site showed that this element is responsible for only about 40% of the promoter activity in B cells. Furthermore, targeted deletion of Oct-2 in the mouse showed that Oct-2 is dispensable for early B-cell development and does not affect expression of the CD20 antigen.17
We have used in vivo footprinting, electrophoretic mobility shift assays (EMSA), and transient transfection experiments to identify two important cis-regulatory elements in the proximal part of the CD20 promoter. One element homologous to the λB element of the λ light chain enhancers is a composite binding site for a B-cell–specific complex composed of the transcription factor PU.1 and its interaction partner Pip. In addition, a μE3 element close to the CD20 transcriptional start sites interacts with members of the basic helix-loop-helix-zipper (bHLHZ) family of transcription factors.
MATERIALS AND METHODS
Cell lines and culture. BJA-B (Epstein Barr virus negative B-cell lymphoma) cell line was kindly provided by Dr E. Oates (University of Miami, Miami, FL). The pre–B-cell lines PB697 and NALM-6 were kindly provided by Dr T. Tedder (Duke University, Durham, NC). The mouse fibroblast cell line NIH3T3, the human multiple myeloma cell lines RPMI 8226 and U266, the human plasmacytoma cell line HS-Sultan, the human cervical carcinoma cell line HeLa, and the Jurkat T-cell line were all obtained from American Type Culture Collection (Rockville, MD). All cells were maintained in RPMI 1640 medium supplemented with 10% to 15% fetal calf serum (FCS), with the exception of the HeLa and NIH3T3 cell lines, which were grown in Dulbecco's modified Eagle's medium supplemented with FCS.
In vivo and in vitro genomic footprinting. For in vivo dimethyl sulfate (DMS) treatment and DNA isolation, 1 × 108 cells were washed once in phosphate-buffered saline (PBS) and resuspended in 2 mL medium with 10% fetal bovine serum. DMS was added to the cells at a final concentration of 0.5%. After incubation for 4 minutes at room temperature the cells were washed twice in ice-cold PBS. The cells were then lysed in sodium dodecyl sulfate (SDS)/Proteinase K solution and incubated at 55°C for 4 hours. DNA was purified by two phenol extractions and two phenol/chloroform extractions followed by one chloroform extraction and ethanol precipitation. For in vitro DMS modification genomic DNA was first extracted in an identical fashion after overnight lysis in SDS/Proteinase K solution. Then 200 μg of genomic DNA were incubated in 200 μL of TE (10 mmol/L Tris Cl and 1 mmol/L EDTA, pH 8.0) with DMS at a final concentration of 0.5% for 90 seconds at room temperature. After addition of 50 μL DMS stop solution the DNA was precipitated with ethanol. Piperidine treatment of in vivo or in vitro methylated DNA was carried out by incubating 200 μg of methylated DNA in 200 μL of 1 mol/L piperidine (Sigma, St Louis, MO) at 90°C for 30 minutes. The piperidine was removed by three rounds of evaporation and by ethanol precipitation of the DNA pellet three times. Ligation-mediated polymerase chain reaction (LMPCR) was performed as described.18 For each region of the CD20 promoter analyzed a set of nested primers with increasing melting temperature (designated primer 1 to 3) were used. Briefly, for first strand synthesis a mixture containing genomic DNA, Vent polymerase (New England Biolabs, Beverly, MA) and primer 1 was incubated at 95°C for 5 minutes, 60°C for 30 minutes, and 76°C for 10 minutes. The linker ligation was performed at 16°C for 16 hours followed by an ethanol precipitation. Subsequently, the first amplification step was carried out using primer 2 and a linker specific primer using the following PCR conditions: denaturation at 95°C for 1 minute, annealing at 65°C for 2 minutes, and extension at 76°C for 3 minutes, with an increase of the extension time of 5 seconds after each cycle (17 cycles). The final amplification was performed with primer 3, which had been labeled with γ-32P adenosine triphosphate (ATP) using polynucleotide kinase (Boehringer-Mannheim, Indianapolis, IN). Two cycles using the following conditions were performed: denaturation at 95°C for 1 minute, annealing at 69°C for 2 minutes, and extension at 76°C for 10 minutes. In both amplification steps Vent polymerase was used. After phenol/chloroform extraction and ethanol precipitation one tenth of the reaction volume was loaded on a 6% acrylamide sequencing gel. The gels were dried under vacuum and exposed for autoradiography for 12 to 24 hours at −70°C. The nucleotide sequences of the primers used were as follows: Primer set 1 visualizes site 1 by amplifying the lower strand of the CD19 promoter; primer 1, 5′-GGTAGCATGAGCATGCCAGGG-3′; primer 2, 5′-GGGTCTTTTTCAAGAAGTGAAACCTGGTAAGGCAG-3′; primer 3, 5′-GGTCTTTTTCAAGAAGTGAAACCTGGTAAGGCAGAAACTTTTTTTGCA CCTCCTT CAGCTATGGTAAGTGT-3′. Primer set 2 visualizes site 1 by amplifying the upper strand; primer 1, 5′-CAAAACCATTCTATACCTTATCCATCACCTCC-3′; primer 2, 5′-GATTCCTTACCTGAGTCTCCAAGGCCTC-3′; primer 3, 5′-GATTCCTTACCTGAGTCTCCAAGGCCTCAAATCTCAAGGGCTG-3′. Primer set 3 visualizes site 2 by amplifying the lower strand of the CD19 promoter; primer 1, 5′-TCCAGGCCTGAAGATGAAATCGCTG-3′; primer 2, 5′-CATCAGGTGACAGGAAATCAGTAGCTTCTGCTAC-3′; primer 3, -5 ;pr - T G A C A G G A A A T C A G T A G C T T C T G C T A C C C T G G G C T T-CGCTCCAATT-3′.
Preparation of nuclear extracts and EMSA. Nuclear extracts were prepared as described.19 Probes for EMSA were synthesized as complementary oligonucleotides with a 1 base pair (bp) overhang. One hundred micrograms of each complementary strand were ethanol precipitated together, and annealed in a PCR machine for 10 minutes at each of the following temperatures: 68°C, 55°C, 37°C, and 24°C. The annealed oligonucleotides were gel purified on a 15% polyacrylamide 1× TBE (Tris-Borate-EDTA) gel. The 5′ ends of the gel shift probes were radiolabeled with γ 32P-ATP using polynucleotide kinase (Boehringer-Mannheim). After labeling at 37°C for 30 minutes the probes were purified over a G50 spin column (5′-3′ Inc, Boulder, CO). For EMSA 0.5 ng of the probe was incubated with 4 to 8 μg of nuclear extract or 1.5 μL of in vitro translated protein in a binding reaction that contained 20 mmol/L HEPES (pH 7.9), 50 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L MgCL2 , 0.1% tween 20, 5% glycerol, and 1 μg of poly(dI-dC) (Pharmacia, Piscataway, NJ) in a final volume of 20 μL. One hundred nanograms of unlabeled probe was used as competitor DNAs. Binding reactions were incubated at room temperature for 20 minutes. For antibody supershift experiments, an anti-TFE3 antiserum (a generous gift from Dr Kathryn Calame, Columbia University, NY), anti-USF1, anti-USF2 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), anti-Pip (a generous gift from Dr Harinder Singh, University of Chicago, Chicago, IL) or preimmune serum was incubated with nuclear extract in the binding buffer for 20 minutes on ice. After addition of the gel shift probe the samples were incubated at room temperature for an additional 20 minutes. For the PU.1 depletion experiment 10 μL of anti-PU.1 (Santa Cruz Biotechnology) or a control antiserum were incubated at 4°C with 16 μL of binding buffer and 2 μL of nuclear extract in the presence of goat antirabbit Ig coupled to magnetic beads (Dynal, Oslo, Norway). One hour later the magnetic beads were removed, and labeled probe and 1 μg of poly(dI-dC) were added to the PU.1-depleted extract. All gel shift samples were electrophoresed on 5% polyacrylamide gels using high ionic strength buffer (50 mmol/L Tris pH 7.5, 380 mmol/L glycine, and 2 mmol/L EDTA) at 25 mA for 2 hours. The gels were dried under vacuum and exposed for autoradiography for 1 to 3 hours. The following EMSA probes were used (only the nucleotide sequence of the upper strand of each probe is shown): CD20 #1, TCCTGTCACCTGATGTCTATC; CD201Mu1, TCCTGTATCCGCATGTCTATC; μE3, GATTGCGTCATGTGGTCTCT; μE5, GATTGCTGCAGGTGTTCTCT; CD20#2, GTCTTTTTTCAAGAAGTGAACCT; CD20#2 Mu, GTCTTTTTTCAAGAAGTCGTACCT; λB, AAATAAAAGGAAGTGAAACCAAG.
Plasmids and in vitro translation. A fragment containing bp −425/+52 of the CD20 promoter was amplified by PCR from an 828-bp promoter fragment that was previously subcloned into the polylinker of the pGEM 4-CAT plasmid.14 The PCR fragment was ligated into the SmaI site of the polylinker of the luciferase expression vector pGL3Basic (Promega, Madison, WI) by T-A overhang cloning. The orientation and the nucleotide sequence of the resulting plasmid, termed pGL3-CD20-425, were verified by DNA sequencing. A plasmid containing PU.1 cDNA and the PU.1 expression vector PU.1-pECE were a gift from Dr R. Maki (LaJolla, CA). The vector pBS-ATG/TFE3 and the TFE3 expression vector pSV2-TFE3 were kindly provided by Dr K. Calame (New York, NY). The vectors for in vitro translation and expression of Pip were a generous gift from Dr H. Singh (Chicago, IL). The USF expression vector CMV-USF was provided by Dr R. Roeder (Rockefeller University, New York, NY) and the Oct-2 expression vector CMV-Oct-2 was obtained from L. Staudt (National Cancer Institute, Bethesda, MD). For in vitro translations 1 μg of the respective plasmid was in vitro transcribed and translated using the appropriate RNA polymerase and a TnT lysate coupled Transcription/Translation kit (Promega). The resulting [35S]-methionine labeled proteins were analyzed by SDS-polyacrylamide electrophoresis before use in mobility shift experiments.
Site-directed mutagenesis. Mutations in the pGL3-CD20-425 construct were generated using 35-bp oligonucleotides carrying 4-bp mutations and a mutagenesis kit from Pharmacia. The following mutations were introduced: the E-Box at bp −45 was changed from CACCTG to AGCCGA and the PU.1/Pip binding site was altered from CAAGAAGTGAAACCT to CACTCCGTGAAACCT. The sequence of the mutated constructs was confirmed by DNA sequencing.
Transfection and luciferase assay. For transfections all plasmids were prepared by alkaline-SDS method followed by purification over Qiagen columns (Qiagen, Chatsworth, CA). HS-Sultan, U266, and RPMI 8226 and Jurkat cells were harvested and resuspended in RPMI 1640 medium containing 10% FCS and 10 mmol/L HEPES buffer, pH 7.5. Five micrograms of reporter gene pGL3-CD20-425 and 1 μg of the β-Gal control plasmid were added to 5 × 106 cells in a volume of 200 μL medium and placed on ice. Electroporation was performed by using a Bio-Rad (Hercules, CA) Gene Pulser (0.4 cm cuvettes) at 250V/960 μFD. Transfected cells were incubated on ice for 5 to 10 minutes, placed in complete medium, and cultured for 16 hours before performing luciferase assays. NIH3T3 cells were transfected using the calcium phosphate method. Six micrograms of reporter plasmid, 2.5 μg of each expression vector, and 1 μg of β-Gal control vector were mixed with CaCl2 and 2× HEPES buffered saline and added to the NIH3T3 cells on a 50% to 60% confluent plate. The total amount of transfected DNA was kept constant by adding empty expression vector. The cells were cultured overnight, washed twice in medium, and harvested after 16 hours. For luciferase assay the cells were pelleted and resuspended in 50 μL of lysis buffer (Promega). After one freeze/thaw cycle the lysates were assayed for luciferase activity using luciferase substrate (Promega) and a luminometer (Analytical Luminescence Laboratory, San Diego, CA). β-Gal activity was measured from the same lysates using galactan chemiluminescent substrate (Tropix, Bedford, MA) in the same luminometer.
Western blot analysis. Whole cell lysates from the different cell lines were prepared in RIPA buffer (150 mmol/L NaCl, 1% NP-40, 0.5% deoxycholic acid (DOC), 0.1% SDS, 50 mmol/L Tris, pH 8) and protein concentrations measured by the Bradford assay (Bio-Rad). One hundred micrograms of lysate were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane, and blocked with Tween-Tris–buffered saline (TTBS) and 10% milk for one hour. The immunoblots were then incubated with rabbit anti-PU.1 antiserum (1:500; Santa Cruz Biotechnologies) in TTBS and 5% milk. This was followed by incubation with biotinylated antirabbit monoclonal antibody (1:5,000), and horse radish peroxidase (HRP)-conjugated streptavidin (1:10,000) before detection. All immunoblots were detected by enhanced chemiluminescence (Amersham, Buckinghamshire, UK).
RESULTS
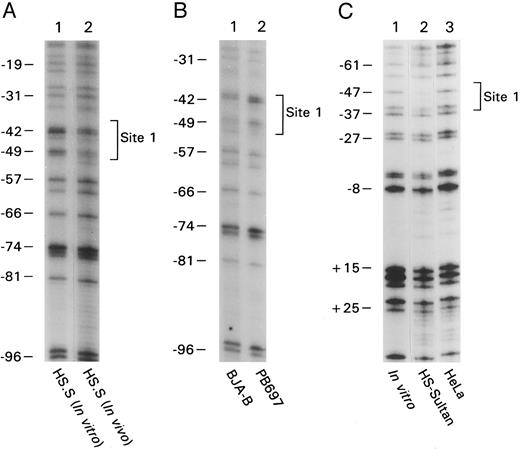
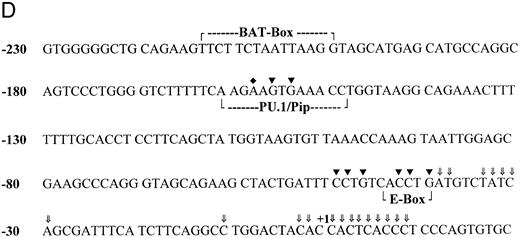
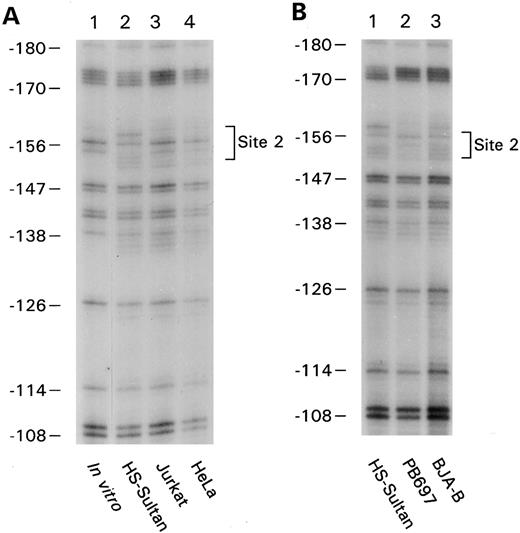
B-cell–specific DNA protein interactions in the proximal CD20 gene promoter. To identify cis-elements likely important in lineage-specific expression of CD20, we analyzed the proximal promoter region of the CD20 gene by in vivo footprinting using either in vitro or in vivo methylated DNA. By comparing in vivo and in vitro methylated DNA from the CD20-positive human B-cell line, HS-Sultan, a human plasmacytoma cell line, we found an in vivo protected region on both strands located at approximately bp −45 (Fig 1A, lane 1 versus 2, lower strand of the promoter amplified and Fig 1C, lane 1 versus 2, upper strand amplified). We failed to detect a similar footprinted region with in vivo methylated DNA prepared from HeLa cells (Fig 1C, lane 3) or from a CD20-negative pre–B-cell line (Fig 1B, lane 2). In vivo methylated DNA from another CD20-positive B-cell line, BJA-B, also contained a footprint at this location (Fig 1B, lane 1). The protected region at −45 will be referred to as site 1 and spanned the nucleotide sequence CCTGTCACCTGA (Fig 1D). We identified another in vivo protected region located at −160 by comparing in vivo and in vitro methylated DNA from HS-Sultan cells (Fig 2A, compare lanes 1 and 2) with in vivo methylated DNA from BJA-B cells (Fig 2B, lane 3). The second in vivo footprint again appeared B-cell specific because no footprint was seen using DNA prepared from the T-lymphocyte cell line Jurkat or HeLa cells (Fig 2A, lanes 3 and 4). Furthermore, we did not detect the footprinted region in DNA prepared from the CD20-negative pre–B-cell line PB697 (Fig 2B, lane 2). The protected region at −160 will be referred to as site 2 and spanned the nucleotide sequence GAAGTG (Fig 1D).
In vivo footprinting of the proximal CD20 promoter by amplifying either the coding or the noncoding strand. (A) Naked genomic DNA (lane 1) or intact cells from the B-cell line HS-Sultan (lane 2) were treated with DMS and in vitro or in vivo footprints were visualized by ligation mediated PCR using primer set 1. The numbers on the left correspond to the sequence of the CD20 promoter as shown in panel D. The in vivo protected residues are designated site 1 and are surrounded by a bracket. (B) In vivo footprinting using primer set 1 over the region indicated was performed using in vivo methylated DNA from the following cell lines: lane 1, CD20positive B-cell line BJA-B; lane 2, CD20 negative pre–B-cell line PB697. (C) In vivo footprinting on the opposing strand of the CD20 promoter was performed using primer set #2. Naked DNA (lane 1) or intact cells from the B-cell line HS-Sultan (lane 2) or the cervical carcinoma cell line HeLa (lane 3) were used. (D) Nucleotide sequence of the first 230 base pairs of the CD20 promoter. Known transcriptional start sites are marked with an arrow (⇓), a nucleotide residue within the most 3′ cluster of transcriptional start sites is designated +1. Binding sites for regulatory proteins as determined by EMSA and in vivo footprinting are surrounded by a bracket. In vivo protected nucleotides are indicated by a triangle (▾), nucleotides rendered hypersensitive by in vivo methylation by a diamond (♦).
In vivo footprinting of the proximal CD20 promoter by amplifying either the coding or the noncoding strand. (A) Naked genomic DNA (lane 1) or intact cells from the B-cell line HS-Sultan (lane 2) were treated with DMS and in vitro or in vivo footprints were visualized by ligation mediated PCR using primer set 1. The numbers on the left correspond to the sequence of the CD20 promoter as shown in panel D. The in vivo protected residues are designated site 1 and are surrounded by a bracket. (B) In vivo footprinting using primer set 1 over the region indicated was performed using in vivo methylated DNA from the following cell lines: lane 1, CD20positive B-cell line BJA-B; lane 2, CD20 negative pre–B-cell line PB697. (C) In vivo footprinting on the opposing strand of the CD20 promoter was performed using primer set #2. Naked DNA (lane 1) or intact cells from the B-cell line HS-Sultan (lane 2) or the cervical carcinoma cell line HeLa (lane 3) were used. (D) Nucleotide sequence of the first 230 base pairs of the CD20 promoter. Known transcriptional start sites are marked with an arrow (⇓), a nucleotide residue within the most 3′ cluster of transcriptional start sites is designated +1. Binding sites for regulatory proteins as determined by EMSA and in vivo footprinting are surrounded by a bracket. In vivo protected nucleotides are indicated by a triangle (▾), nucleotides rendered hypersensitive by in vivo methylation by a diamond (♦).
In vivo footprinting of bases −108 to −180 of the CD20 promoter. (A) Naked genomic DNA (lane 1) or intact cells from the B-cell line HS-Sultan (lane 2), T-cell line Jurkat (lane 3), and cervival carcinoma cell line HeLa (lane 4) were exposed to DMS, and in vivo footprints were visualized by ligation mediated PCR using primer set 3. The numbers on the left correspond to the sequence of the CD20 promoter shown in Fig 1D. The protected residues were designated as site 2 and are surrounded by a bracket. (B) In vivo footprinting was performed in an identical manner using the following cell lines: lane 1, CD20-positive plasmacytoma cell line HS-Sultan; lane 2, CD20-negative pre–B-cell line PB697; and lane 3, CD20-positive B-cell line BJA-B.
In vivo footprinting of bases −108 to −180 of the CD20 promoter. (A) Naked genomic DNA (lane 1) or intact cells from the B-cell line HS-Sultan (lane 2), T-cell line Jurkat (lane 3), and cervival carcinoma cell line HeLa (lane 4) were exposed to DMS, and in vivo footprints were visualized by ligation mediated PCR using primer set 3. The numbers on the left correspond to the sequence of the CD20 promoter shown in Fig 1D. The protected residues were designated as site 2 and are surrounded by a bracket. (B) In vivo footprinting was performed in an identical manner using the following cell lines: lane 1, CD20-positive plasmacytoma cell line HS-Sultan; lane 2, CD20-negative pre–B-cell line PB697; and lane 3, CD20-positive B-cell line BJA-B.
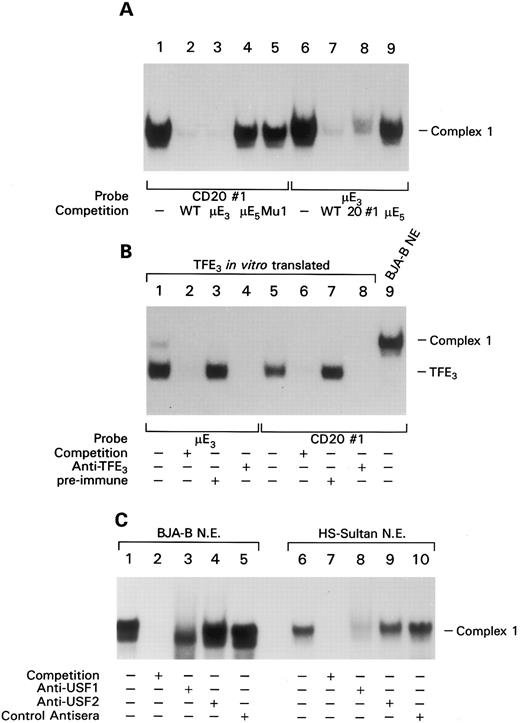
The proximal cis-element of the CD20 promoter binds members of the bHLHZ family of transcription factors. To identify the transcription factor(s) binding to the promoter site 1, we generated a double stranded oligonucleotide probe termed CD20#1 that encompassed the presumptive protein binding site localized by in vivo footprinting. EMSA with nuclear extracts prepared from B-cell lines visualized a DNA-protein doublet consisting of an intense slow migrating band and a less intense faster migrating band (together referred to as complex 1). Adding an excess amount of the unlabeled probe effectively competed detection of the complex (Fig 3A, lanes 1 and 2). Complex 1 is not unique to B-cell nuclear extracts as we observed a complex of similar mobility using nuclear extracts prepared from a variety of cell lines (data not shown). Because the sequence of probe CD20#1 contained a potential E-Box element, we performed further EMSA by using competitors as unlabeled probes containing a mutation in the potential E-Box and probes containing the μE3 site or the μE5 element from the IgH chain intronic enhancer. The μE3 probe, but not the μE5 probe, competed binding (Fig 3A, lanes 3 and 4). In addition, a CD20#1 probe with a mutation in the potential E-Box element failed to compete formation of complex 1 (Fig 3A, lane 5). Performing the EMSA with a labeled μE3 probe yielded a complex with an identical mobility to that of complex 1 and formation of the complex was competed by the CD20 probe, but not by the μE5 element (Fig 3A, lanes 6 to 9). It has been shown that the μE3 and the μE5 element of the IgH intronic enhancer bind distinct subfamilies of basic helix-loop-helix (bHLH) proteins.20 The μE3 element binds bHLHZ DNA-binding proteins such as TFE3, TFEB, and USF, whereas the μE5 site interacts with members of class I bHLH family, such as the products of the E2A and E2-2 genes. To test whether bHLHZ proteins bound the CD20 E Box we translated TFE3 in vitro and performed an EMSA with the CD20#1 probe and the probe containing the μE3 element of the IgH gene enhancer. We detected a complex of identical mobility with either probe that was effectively competed by the respective unlabeled probe (Fig 3B, lanes 1, 2, 5, and 6). The addition of a TFE3-specific antiserum, but not a preimmune serum, abolished the formation of the complex (Fig 3B, lanes 3, 4, 7, and 8). These results show that the CD20 probe binds a member of the bHLHZ protein family; however, complex 1 generated by incubating probe CD20#1 with BJA-B nuclear extract (Fig 3B, lane 9) displayed a slower mobility than the TFE3 complex suggesting that complex 1 contained other members of the bHLHZ protein family. Because it has been shown that USF is the predominant protein binding to μE3 sites in B-cell nuclear extracts2, we examined whether antiserum to USF1 or USF2 impaired detection of complex 1 using extracts from either BJA-B or HS-Sultan cells. With BJA-B the addition of a USF1 antiserum markedly impaired detection of the upper band of complex 1, but not the lower band. The USF2 antiserum had no effect on detection of either band (Fig 3C). With HS-Sultan cells complex 1 contained a single band whose detection was blocked by the USF1 antibody. Thus, USF1 is a major component of complex 1 and likely interacts with the proximal portion of the CD20 promoter. In addition, there may be another bHLHZ family member that is present in BJA-B cells but absent from HS-Sultan cells.
EMSA shows binding of basic helix-loop-helix-zipper transcription factors to CD20 promoter site 1. (A) A total of 0.5 ng of labeled probe CD20#1 (lanes 1 to 5) or a μE3 probe (6 to 9) were incubated with BJA-B nuclear extracts and the indicated unlabeled competitor probe (200-fold excess). The resultant complexes were separated on a 5% native polyacrylamide gel. (B) The same probes as in panel A were either incubated with in vitro translated TFE3 (lanes 1 to 8) or BJA-B nuclear extracts (lane 9) in the absence (−) or presence (+) of 200-fold excess of unlabeled competitor probe. In vitro translated TFE3 was either incubated with preimmune serum (lanes 3 and 7) or anti-TFE3 antiserum (lanes 4 and 8) before addition of the indicated probe. EMSA was performed as in panel A. (C) USF1 is a major component of complex 1. EMSA in which nuclear extracts from the indicated source have been preincubated with an antiserum to USF1 or USF2 or with preimmune control. Competition with a 200-fold excess of cold probe.
EMSA shows binding of basic helix-loop-helix-zipper transcription factors to CD20 promoter site 1. (A) A total of 0.5 ng of labeled probe CD20#1 (lanes 1 to 5) or a μE3 probe (6 to 9) were incubated with BJA-B nuclear extracts and the indicated unlabeled competitor probe (200-fold excess). The resultant complexes were separated on a 5% native polyacrylamide gel. (B) The same probes as in panel A were either incubated with in vitro translated TFE3 (lanes 1 to 8) or BJA-B nuclear extracts (lane 9) in the absence (−) or presence (+) of 200-fold excess of unlabeled competitor probe. In vitro translated TFE3 was either incubated with preimmune serum (lanes 3 and 7) or anti-TFE3 antiserum (lanes 4 and 8) before addition of the indicated probe. EMSA was performed as in panel A. (C) USF1 is a major component of complex 1. EMSA in which nuclear extracts from the indicated source have been preincubated with an antiserum to USF1 or USF2 or with preimmune control. Competition with a 200-fold excess of cold probe.
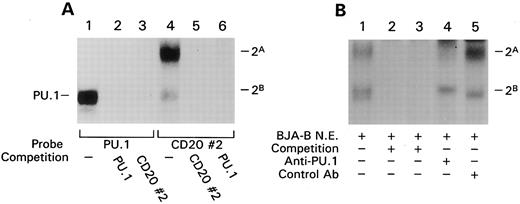
A protein complex containing the transcription factor PU.1 binds the CD20 promoter. The protein binding site identified by in vivo footprinting at bp −160 of the CD20 promoter was also analyzed by EMSA using an oligonucleotide probe (CD20#2) spanning the site. With this probe we observed two complexes, a more intense band with a slower mobility, called complex 2A, and a weaker complex with a higher mobility, termed 2B. The addition of unlabeled probe effectively competed the binding of both complexes (Fig 4A, lanes 4 and 5). Both complexes generated with this probe appeared tissue restricted because we detected them in nuclear extracts prepared from different B-cell lines but not from T-cell lines or HeLa cells (data not shown). The nucleotide sequence spanned by CD20#2 contains an AGAA sequence reminiscent of an Ets binding site, although this does not match the Ets consensus sequence. Because the B-cell and macrophage-specific Ets family member PU.1 binds a purine rich sequence containing an AGAA sequence in the J-chain promoter21 and the CD11b promoter,22 we tested whether or not PU.1 bound site 2. We found that an SV40 PU.1 probe23 efficiently competed the formation of complexes 2A and 2B (Fig 4A, lanes 4 and 6), and conversely, the CD20 #2 probe competed the binding of PU.1 to the SV40 PU.1 probe (Fig 4A, lanes 1 and 3). Although the weaker 2B complex comigrated with the band generated with the SV40 PU.1 probe (lanes 1 and 4 of Fig 4A), the stronger 2A complex migrated more slowly. These observations indicated that the CD20#2 probe not only binds PU.1 but, in addition, another protein. The fact that the SV40 PU.1 probe inhibited formation of both complexes suggested that PU.1 was required for detection of the second protein. To address this possibility we immunoprecipitated PU.1 from a B-cell nuclear extract before a gel shift assay with the CD20#2 probe. This resulted in a marked reduction in the 2A complex and a decrease in the 2B complex (Fig 4B). The 2B complex appears to be composed of two bands, one that vanished with the PU.1 antibody preclearance and another that did not. The failure to completely reduce the 2B complex may result from incomplete clearance of PU.1 or the presence of another DNA binding protein in the complex. These experiments show that PU.1 and PU.1 in conjunction with another protein bind the CD20 promoter. Closer inspection of the sequence surrounding the GA-rich region showed that the nucleotide sequence of probe CD20#2 is homologous to a composite binding site described in the κ3′ enhancer and to the λB element of the λ2 to 4 and λ3 to 1 enhancers. The first 8 bp after the GA-rich region are identical to those of the λB element and similar to those of the κ3′ enhancer (6 of 8 match). The protein that interacts with PU.1 at these sites and binds to the conserved sequence immediately 3′ of the PU.1 binding site has been cloned and termed Pip for PU.1 interacting partner.24 Pip expression is restricted to lymphocytes with higher levels in B cells than in T cells. Because the protein interacting with PU.1 on the CD20 promoter also appeared lymphocyte restricted, Pip was an attractive candidate for the additional factor.
EMSA shows a protein complex containing PU.1 binds CD20 promoter site 2. (A) EMSA with either the PU.1 site of the SV-40 enhancer (PU.1) or the in vivo protected site 2 of the CD20 promoter (CD20#2) were incubated with BJA-B nuclear extract and the indicated unlabeled competitor probe (200-fold excess). The complexes detected with the CD20#2 probe are designated complex 2A and 2B. (B) EMSA with BJA-B nuclear extracts depleted of PU.1 or not using the CD20#2 probe. As in panel A, binding was competed with unlabeled CD20#2 probe (lane 2) or SV-40 PU.1 probe (lane 3). BJA-B nuclear extracts were incubated with a rabbit PU.1 antiserum (lane 4) or a control antiserum (lane 5) and cleared with goat antirabbit Ig coupled to magnetic beads before the EMSA.
EMSA shows a protein complex containing PU.1 binds CD20 promoter site 2. (A) EMSA with either the PU.1 site of the SV-40 enhancer (PU.1) or the in vivo protected site 2 of the CD20 promoter (CD20#2) were incubated with BJA-B nuclear extract and the indicated unlabeled competitor probe (200-fold excess). The complexes detected with the CD20#2 probe are designated complex 2A and 2B. (B) EMSA with BJA-B nuclear extracts depleted of PU.1 or not using the CD20#2 probe. As in panel A, binding was competed with unlabeled CD20#2 probe (lane 2) or SV-40 PU.1 probe (lane 3). BJA-B nuclear extracts were incubated with a rabbit PU.1 antiserum (lane 4) or a control antiserum (lane 5) and cleared with goat antirabbit Ig coupled to magnetic beads before the EMSA.
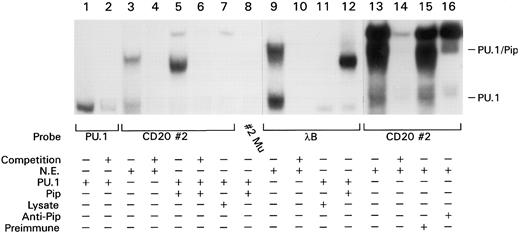
PU.1 and Pip interact to bind the CD20 promoter. To test whether PU.1 and Pip could bind the CD20 promoter, we translated both proteins in vitro and used them in an EMSA with the CD20 promoter probe 20#2. The in vitro–translated PU.1 bound a PU.1 probe but failed to bind the CD20#2 probe (Fig 5, lanes 1 and 7). We observed a specific complex only when the EMSA reaction contained both proteins (Fig 5, lanes 5 to 7). The complex had a slightly different mobility compared with the complex generated with BJA-B nuclear extracts (complex 2A; Fig 5, compare lane 3 with lane 5). This may be because of post-translational modification of the proteins in intact cells. To show that the PU.1/Pip interaction on the CD20 promoter probe depended on the binding of Pip to the sequence 3′ of the GA-rich PU.1 binding site, we introduced a mutation into the Pip binding site leaving the PU.1 binding site intact. No complex was observed when this probe was used in an EMSA along with in vitro translated PU.1 and Pip (Fig 5, lane 8). We next compared binding of the PU.1/Pip complex to the CD20#2 probe with that of the λB element. Incubating in vitro translated PU.1 and Pip with the λB probe generated a complex of identical mobility (Fig 5, lanes 5 and 12). As we had observed with the CD20#2 probe, the complex generated with nuclear extracts and the λB probe had a slightly different mobility than the complex generated with in vitro translated proteins (Fig 5, lanes 9 and 12). The two elements are distinguished by the ability of in vitro translated PU.1 to bind to the λB element, but not to the CD20 promoter probe (Fig 5, lanes 7 and 11). To verify that Pip was actually a component of complex 2A, we added a Pip-specific antiserum to the EMSA reaction using nuclear extracts from normal tonsil B cells. The addition of the Pip-specific antiserum, but not a control antiserum, markedly reduced the detection of the complex (Fig 5, lanes 13 to 15). The PU.1/Pip complex migrates faster than a nonspecific band that we commonly detect in nuclear extracts. In summary, these experiments identify a novel composite binding site for PU.1 and its interaction partner Pip on the CD20 promoter. Formation of the PU.1/Pip complex depends on binding of both PU.1 and Pip to their adjacent binding sites with Pip apparently stabilizing the binding of PU.1 to a GA-rich sequence that lacks an Ets consensus sequence.
PU.1 and Pip bind cooperatively to the CD20 promoter and the λB element. A total of 0.5 ng of the indicated labeled probe were incubated either with BJA-B nuclear extract (lanes 3, 4, 9, and 10), in vitro translated PU.1 (lanes 1 and 2), a combination of in vitro translated PU.1 and either in vitro translated Pip (lanes 5, 6, 8, and 12), unprogrammed reticulocyte lysate (lanes 7 and 11), or a tonsil B nuclear extract (lanes 13 to 16) in the presence of a preimmune antisera (lane 15) or anti-Pip antiserum (lane 16). The PU.1/Pip complex migrates slightly faster than a nonspecific complex that can be partially competed by any DNA probe. A 200-fold excess of respective unlabeled competitor probe was added in the indicated lanes (+). EMSA was performed as described in Fig 3. Lanes 1 to 8, lanes 9 to 12, and lanes 13 to 16 are from three separate experiments so the migration of PU.1 and PU.1/Pip are slightly different.
PU.1 and Pip bind cooperatively to the CD20 promoter and the λB element. A total of 0.5 ng of the indicated labeled probe were incubated either with BJA-B nuclear extract (lanes 3, 4, 9, and 10), in vitro translated PU.1 (lanes 1 and 2), a combination of in vitro translated PU.1 and either in vitro translated Pip (lanes 5, 6, 8, and 12), unprogrammed reticulocyte lysate (lanes 7 and 11), or a tonsil B nuclear extract (lanes 13 to 16) in the presence of a preimmune antisera (lane 15) or anti-Pip antiserum (lane 16). The PU.1/Pip complex migrates slightly faster than a nonspecific complex that can be partially competed by any DNA probe. A 200-fold excess of respective unlabeled competitor probe was added in the indicated lanes (+). EMSA was performed as described in Fig 3. Lanes 1 to 8, lanes 9 to 12, and lanes 13 to 16 are from three separate experiments so the migration of PU.1 and PU.1/Pip are slightly different.
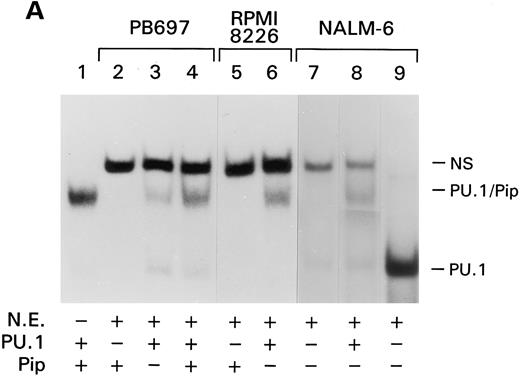
CD20 expression correlates with the presence of the PU.1/Pip complex during B-cell development. Because the PU.1/Pip complex is unique to B cells, the PU.1/Pip site in the CD20 promoter may account for the lineage specificity of the CD20 gene. To see if PU.1/Pip expression correlated with CD20 expression during B-cell development and differentiation, we examined a variety of B-cell lines for CD20 and PU.1/Pip expression. The pre–B-cell lines, PB697 and NALM-6, as well as the multiple myeloma cell line, RPMI 8226, are CD20 negative. Nuclear extracts from these cell lines were prepared and examined for PU.1/Pip via EMSA using the CD20#2 probe. As shown in Fig 6 the PU.1/Pip complex could not be detected by the presence of EMSA in them (Fig 6, lanes 2, 5, and 7). In contrast, the PU.1/Pip complex was found to be present in all CD20-positive cell lines tested (data not shown).
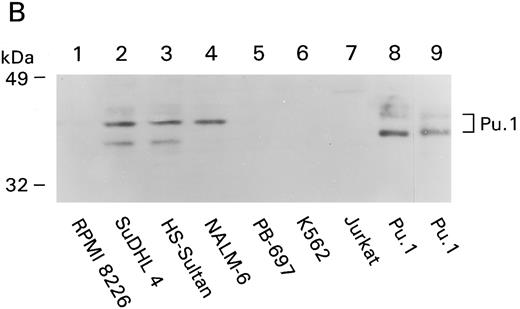
Absence of the PU.1/Pip complex in CD20-negative cell lines. (A) EMSA was performed with 0.5 ng of the CD20#2 probe or the SV40 PU.1 probe (lane 9) and in vitro translated PU.1 and Pip (lane 1) or nuclear extracts from B-cell lines PB697 (lanes 2,3, and 4), RPMI 8226 (lanes 5 and 6), or NALM-6. In vitro translated proteins were added to the gel shift reactions as indicated. NS denotes a nonspecific band. (B) PU.1 immunoblot. A total of 100 μg of whole cell lysate of the indicated cell line (lanes 1 to 7) or 6 λ of 35S-labeled in vitro translated mouse PU.1 (lane 8) were fractionated by SDS-PAGE and immunoblotted with a rabbit PU.1 antiserum. Autoradiography was performed with the same membrane to verify the position of 35S-labeled PU.1 (lane 9).
Absence of the PU.1/Pip complex in CD20-negative cell lines. (A) EMSA was performed with 0.5 ng of the CD20#2 probe or the SV40 PU.1 probe (lane 9) and in vitro translated PU.1 and Pip (lane 1) or nuclear extracts from B-cell lines PB697 (lanes 2,3, and 4), RPMI 8226 (lanes 5 and 6), or NALM-6. In vitro translated proteins were added to the gel shift reactions as indicated. NS denotes a nonspecific band. (B) PU.1 immunoblot. A total of 100 μg of whole cell lysate of the indicated cell line (lanes 1 to 7) or 6 λ of 35S-labeled in vitro translated mouse PU.1 (lane 8) were fractionated by SDS-PAGE and immunoblotted with a rabbit PU.1 antiserum. Autoradiography was performed with the same membrane to verify the position of 35S-labeled PU.1 (lane 9).
We next wanted to know which partner of the PU.1/Pip complex was absent or functionally impaired in the CD20-negative cell lines. We approached this by adding either in vitro translated PU.1 or Pip to the nuclear extracts from the CD20-negative cell lines. Addition of in vitro translated PU.1 reconstituted the PU.1/Pip complex in the PB697, NALM-6, and RPMI 8226 nuclear extracts whereas addition of Pip had no effect (Fig 6A, Lanes 2,3,5, 6, and 8). These results indicate that the absence of the PU.1/Pip complex in these three CD20-negative cell lines is because of low levels of PU.1 or a functional impairment of PU.1 that prevents it from interacting with Pip.
Analysis of PU.1 protein expression in CD20-negative and -positive B-cell lines. As a first approach to distinguish the above mentioned possibilities, we determined PU.1 protein levels in the various CD20-negative and -positive B-cell lines. Both PB697 cells and RPMI 8226 lacked PU.1 by EMSA (data not shown); however, NALM-6 contained levels comparable with mature B cells (Fig 6A, lane 9). We also checked PU.1 levels by immunoblotting cellular extracts. As a positive control we used 35S-labeled PU.1, in vitro transcribed and translated from a mouse cDNA. Identical bands (a major band and a minor band of slower mobility) were visualized by immunodetection with the PU.1 antiserum (Fig 6B, lane 8) or by autoradiography of the same membrane (Fig 6B, lane 9). Consistent with the gel shift results, PU.1 levels were low or undetectable in RPMI 8226, PB697, and in addition, Jurkat and K562 (Fig 6B, lanes 4 to 7). In contrast, lysates from NALM-6 cells contained nearly equivalent levels of PU.1 to those found in the mature B-cell lines HS-Sultan and SuDHL4 (Fig 6B, lanes 2 to 4). Of note, the NALM-6 cells lacked several minor bands detected in the mature B cells. These results explain the absence of the PU.1/Pip complex in PB697 and RPMI 8226 cells and raise the possibility that PU.1 is unable to interact with Pip in NALM-6 cells.
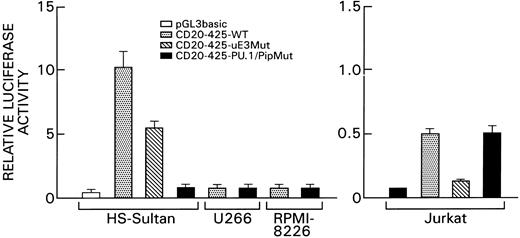
Functional analysis of the PU.1/Pip and the μE3 binding sites. To define the functional significance of the PU.1/Pip site and the μE3 element of the CD20 promoter, we transiently transfected various cell lines with wild type and mutant C20 promoter constructs. The wild-type reporter construct consisted of the first 425 bp of the CD20 promoter (CD20-425 WT) placed immediately upstream of the luciferase gene. This construct is very active in the CD20-positive B-cell line HS-Sultan but considerably less so in the T-cell line, Jurkat, and in the CD20-negative myeloma cell lines, U266 and RPMI-8226 (Fig 7). Mutations were introduced into the PU.1/Pip binding site or into the μE3 element of the CD20-425 WT construct by site-directed mutagenesis that resulted in the loss of binding of the PU.1/Pip complex or of bHLHZ proteins to their respective binding sites as assessed by EMSA. To normalize for differences in transfection efficiency a β-Gal control plasmid was cotransfected. A mutation that abolished the binding of the PU.1/Pip complex reduced CD20 promoter activity in HS-Sultan cells nearly to background levels (14-fold reduction; Fig 7). In contrast, the mutation failed to alter the low levels of promoter activity in Jurkat cells. A mutation in the μE3 binding site had a mild effect on the promoter activity in B cells (2-fold reduction) but dramatically reduced the activity of the promoter construct in Jurkat cells. These results show a requirement for an intact PU.1/Pip binding site for CD20 promoter function in B cells. Although the μE3 site is less critical, it can significantly contribute to CD20 promoter activity. The μE3 site in the promoter is largely responsible for the low activity of the promoter construct in T cells.
Effect of mutations in the PU.1/Pip or μE3 site on CD20 promoter activity in B cells or T cells. The promoterless luciferase vector pGL3 basic, the wild-type CD20 promoter-luciferase gene construct (CD20-425-WT), the μE3 site mutated construct (CD20-425-μE3 Mut: CACCTG → AGCCGA) and the PU.1/Pip site-mutated construct (CD20-425-PU.1/Pip Mut: AAGAAGT → ACTCCGT) were transiently transfected into the B-cell lines, HS-Sultan, U266, and RPMI 8226, or the T-cell line, Jurkat. Experiments were performed in triplicates and repeated a minimum of three times. Mean and standard deviation values shown are derived from a representative experiment. Luciferase activities were normalized for transfection efficiency by cotransfection of a β-Gal control plasmid. Note the different scales used for the B-cell lines and Jurkat cells.
Effect of mutations in the PU.1/Pip or μE3 site on CD20 promoter activity in B cells or T cells. The promoterless luciferase vector pGL3 basic, the wild-type CD20 promoter-luciferase gene construct (CD20-425-WT), the μE3 site mutated construct (CD20-425-μE3 Mut: CACCTG → AGCCGA) and the PU.1/Pip site-mutated construct (CD20-425-PU.1/Pip Mut: AAGAAGT → ACTCCGT) were transiently transfected into the B-cell lines, HS-Sultan, U266, and RPMI 8226, or the T-cell line, Jurkat. Experiments were performed in triplicates and repeated a minimum of three times. Mean and standard deviation values shown are derived from a representative experiment. Luciferase activities were normalized for transfection efficiency by cotransfection of a β-Gal control plasmid. Note the different scales used for the B-cell lines and Jurkat cells.
Transactivation of the CD20 promoter. The previous experiments had shown the binding of PU.1/Pip, TFE3, and USF to two different elements in the CD20 promoter. The interaction of Oct-2 with the CD20 promoter has been previously described.16 We examined the ability of PU.1, Pip, TFE3, USF, and Oct-2 to transactivate the CD20 promoter either alone or in various combinations. We cotransfected the CD20-425 WT construct and various expression vectors into the fibroblast cell line NIH3T3 that lacks PU.1, Pip, and Oct-2. Previous studies have shown that overexpression of TFE3 or USF in these cells can transactivate reporter constructs that have functional binding sites for these proteins despite endogenous levels in NIH3T3 cells. A β-Gal control plasmid was cotransfected to normalize for differences in transfection efficiency, and various amounts of an empty expression vector were added to keep the amount of transfected DNA constant. Control experiments were performed with reporter constructs containing mutations in either the PU.1/Pip or the μE3 site. The results of these experiments are summarized in Table 1. As expected neither PU.1 nor Pip transactivated the CD20 promoter. The simultaneous expression of PU.1 and Pip in 3T3 cells resulted in a low but reproducible 2.3-fold increase in reporter gene activity. We observed similar levels of transactivation when either Oct-2 or TFE3 were cotransfected (2- to 2.2-fold induction). The transcription factor USF was found to be a potent transactivator of the CD20 gene promoter, a 6- to 7-fold induction of reporter gene activity was consistently observed. In cotransfection experiments using the respective mutated promoter-reporter constructs a minimal (1.6-fold) transactivation by USF could be observed. This may indicate the presence of an additional functional μE3 binding site.
Transactivation of the CD20 Gene Promoter
| Transcription Factor(s) . | Induction Wild Type* . | Induction of Mutated Construct† . |
|---|---|---|
| PU.1 | 1 | 1 |
| Pip | 1 | 1 |
| PU.1 + Pip | 2.3 | 1 |
| Oct-2 | 2.2 | NT |
| TFE3 | 2 | 1 |
| USF | 6.5 | 1.6 |
| PU.1 + Pip + Oct-2 | 4.4 | NT |
| PU.1 + Pip + TFE3 | 4 | NT |
| PU.1 + Pip + USF | 6.5 | NT |
| USF + Oct-2 | 9.3 | NT |
| Transcription Factor(s) . | Induction Wild Type* . | Induction of Mutated Construct† . |
|---|---|---|
| PU.1 | 1 | 1 |
| Pip | 1 | 1 |
| PU.1 + Pip | 2.3 | 1 |
| Oct-2 | 2.2 | NT |
| TFE3 | 2 | 1 |
| USF | 6.5 | 1.6 |
| PU.1 + Pip + Oct-2 | 4.4 | NT |
| PU.1 + Pip + TFE3 | 4 | NT |
| PU.1 + Pip + USF | 6.5 | NT |
| USF + Oct-2 | 9.3 | NT |
A total of 6 μg of construct pGL3-CD20-425 were transiently cotransfected with 2.5 μg of each indicated expression vector into NIH-3T3 fibroblasts. The total amount of transfected DNA was kept constant with empty expression vector pC-CMV and a β-Gal control plasmid was cotransfected to normalize for transfection efficiency. Cells were harvested and luciferase assay performed after 16 hours.
The Luciferase Activity of the reporter construct alone has been set at 1.0. Induction refers to fold increase of luciferase activity observed with cotransfection. Results reported are an average of 3 to 5 independent transfections.
For control experiments a promoter-reporter construct with a mutation of the PU.1/Pip or μE3 binding site was used.
We next tested whether the various transcription factors that transactivated the CD20 promoter could cooperate in this function. Coexpression of PU.1/Pip and Oct-2 in 3T3 cells resulted in a 4.4-fold increase in reporter gene activity, which corresponds to the sum of activation by PU.1/Pip or Oct-2 alone. Likewise, cotransfection of PU.1/Pip and TFE3 led to a transactivation of the reporter gene by 4-fold, which again indicates an additive effect. Transactivation by USF could be further increased by coexpressing USF along with Oct-2 (up to 9.3-fold) but not by cotransfecting USF along with PU.1 and Pip (Table 1). In summary, these experiments show that the CD20 promoter can be transactivated in an additive fashion by the B-cell–specific factors Oct-2 and PU.1/Pip, but no evidence of a functional synergy was found (except for the PU.1/Pip cooperation). In addition, we found that the ubiquitous factor USF was the strongest activator of the CD20 promoter.
DISCUSSION
We report the identification of two cis-acting elements in the promoter of the CD20 gene that are important for the tissue-specific activity of this promoter. One element is homologous to a DNA element in the Ig κ 3′ enhancer and the λB motif described in the Ig λ enhancers; although in contrast to these sites the CD20 promoter site lacks a consensus Ets sequence. Analogous to these elements a B-cell–specific ternary complex forms on the CD20 promoter element that contains PU.1 and a recently cloned interacting protein termed Pip. PU.1 and Pip bind to the CD20 promoter in a cooperative manner and the PU.1/Pip element appears critical for promoter activity. The other element is a μE3 site located proximally in the CD20 promoter, which binds members of the bHLHZ family. Although these factors are expressed ubiquitously, in vivo footprinting experiments indicate that this element is only occupied in B cells. The bHLHZ protein USF strongly transactivates the CD20 promoter through the μE3 site.
PU.1 is the product of the Spi-1 proto-oncogene and a member of the Ets family of transcription factors.25,26 The PU.1 gene is expressed specifically in hematopoietic tissues with high levels in the monocytic and B-lymphoid lineage.25,27 In accordance with its expression pattern, gene targeting in mouse embryonic stem cells showed a requirement for PU.1 for multiple hematopoietic lineages.10 Among the various Ets family members, PU.1 has the weakest homology to the consensus Ets domain, which mediates sequence-specific DNA binding. Consistent with this is the observation that PU.1 binds a purine-rich region containing the minimal sequence AGAA and does not require the Ets consensus sequence GGAA/T.28 As with other Ets proteins the sequence surrounding this minimal AGAA sequence is important for the affinity of PU.1 for a particular binding site. Numerous presumptive PU.1 target genes have been identified in the B lymphoid and the monocytic lineages.28 Furthermore, PU.1 has been shown to interact with multiple proteins to exert its function as a transcriptional regulator.29-32
A particularly interesting protein-protein interaction for understanding the role of PU.1 in the regulation of B-cell–specific genes is its interaction with Pip, initially called NF-EM5, on a conserved tissue-specific composite element present in the Ig light chain enhancers κ 3′, λ2-4, and λ3-1.29,32 Participation of Pip in this complex depends on DNA-bound PU.1 and phosphorylation of PU.1 at serine residue 148.33 Competition experiments showed that PU.1 and Pip bound their composite element in a cooperative fashion. Pip is expressed in both B cells and T cells, and based on sequence homology it is a member of the interferon regulatory factor (IRF ) family. Among the IRF family members Pip is most related to interferon consensus sequence-binding protein (ICSBP) because they share 83% amino acid identity within their respective DNA binding domains.24 ICSBP is a lymphoid-myeloid restrictive repressor of interferon-induced transcription and, like Pip, is recruited to the Ig light chain composite element by PU.1.34,35 In contrast to Pip, ICSBP did not enhance PU.1-induced transcription of a reporter plasmid containing a minimal promoter linked to a tetramer of the λB site.36 Using a Pip-specific antiserum, Pip was found to be the major component associating with PU.1 on the λB site in the murine myeloma cell line J55L.36 In preliminary experiments, we have found that PU.1/ICSBP also binds the CD20 PU.1/Pip site, and that together they weakly transactivate the CD20 promoter. Furthermore, the addition of ICSBP to PU.1 and Pip more potently transactivated the CD20 promoter construct than did PU.1 and Pip alone (A. Riva, unpublished observations). Thus, whereas Pip and PU.1 appear to be the major components of complex 2A, ICSBP may also in conjunction with PU.1 bind to the CD20 promoter and contribute to its regulation.
The PU.1/Pip binding site in the CD20 promoter is homologous to the element described in the Ig light chain enhancers, but it lacks an Ets consensus sequence. Thus, distinguishing the CD20 element is the fact that PU.1 binds it very poorly unless Pip or ICSBP is present. This may account for why the addition of ICSBP to PU.1 transactivated the CD20 promoter construct, but failed to alter PU.1-induced activation of the λB tetramer.36 Together PU.1 and Pip bound the CD20 element with high affinity, consistent with the earlier observation of cooperative binding of the two proteins. Because B lymphocytes express both PU.1 and Pip, PU.1 will bind the CD20 promoter element with high affinity in B cells. Evidence for the critical importance of the PU.1/Pip binding site for the B-cell–specific expression of the CD20 gene was provided by several different experiments. First, this element was occupied in vivo only in CD20-expressing B cells. Second, mutation of the element in a reporter plasmid nearly completely abolished promoter function in B cells. Third, the PU.1/Pip complex detected with the CD20 probe seems to be developmentally regulated in a manner similar to CD20. Mature CD20-positive B-cell lines contained a PU.1/Pip complex whereas the immature CD20-negative B-cell lines and a CD20-negative myeloma cell line lacked it.
The PU.1/Pip complex negative cell lines PB697 and RPMI 8226 contained very low levels of PU.1 and adding in vitro translated PU.1 to nuclear extracts reconstituted the PU.1/Pip complex. The undetectable level of PU.1 in PB697 cells was surprising because PU.1 has been found in other pre-B and pro-B cell lines, whereas the undetectable level in the plasmacytoma cell line, RPMI 8226, is consistent with a recent report that showed low or absent PU.1 levels in a panel of human multiple myeloma cell lines.37 This observation suggests that PU.1 is downregulated during the terminal stages of B-cell differentiation, thus, perhaps accounting for the lack of CD20 gene expression in plasma cells. However, these results differ from findings in the murine system in which PU.1 is present and active in most myeloma cell lines.29 38
In contrast to the pre–B-cell line PB697 cells, the pre-B cell line NALM-6 contained high PU.1 levels both by EMSA and immunoblotting. Interestingly, the lysate from this cell line lacked several faint bands of slower mobility that were detected with the lysates from the mature B-cell lines and with the in vitro translated PU.1. These faint bands may represent different phosphorylated isoforms of PU.1. Because phosphorylation of serine 148 in PU.1 is necessary for the PU.1/Pip interaction,33 the lack of such a phosphorylation may account for the lack of a PU.1/Pip complex in these cells. Also consistent with this possibility is the observation that in vitro translated PU.1, which is phosphorylated at serine 148,33 rescues the PU.1/Pip complex in these cells. Further experiments are in progress to determine whether the lack of serine 148 phosphorylation is responsible for the failure to detect a PU.1/Pip interaction in NALM-6 cells.
Although these data define a critical function of the PU.1/Pip interaction for CD20 gene expression, we found only a weak (2- to 3-fold) transactivation of the CD20 promoter by PU.1 and Pip in NIH 3T3 cells. This may be secondary to the lack of another factor in the NIH 3T3 cells. Several experiments have indicated that the Ets proteins in general are poor transcriptional activators and often function as part of multiprotein complexes.39 Given the apparent weak transactivation potential of PU.1 it has been hypothesized that the primary function of PU.1 is to recruit other transcription factors to a gene regulatory element. This function could be carried out either through direct protein-protein interactions or through indirect mechanisms. For example, the cooperative activation of a reporter construct containing the μA and μB site of the IgH intronic enhancer by PU.1 and Ets-1 has been described that required only the DNA binding domain of PU.1.6 38
Similarly, it is conceivable that formation of the PU.1/Pip complex recruits other transcription factors to the CD20 promoter. Our finding that a μE3 site in the CD20 promoter is occupied only in B cells raises the possibility that the PU.1κPip complex regulates the accessibility of the ubiquitous μE3 binding proteins to this site in the CD20 promoter. Although expression of the μE3 binding proteins is not restricted to B cells, there is evidence that these transcription factors can participate in B-cell–specific gene transcription. The μE3 site was initially defined by in vivo genomic footprinting as an element of the IgH intronic enhancer that interacted with binding proteins only in B cells40,41 comparable with the situation in the CD20 promoter. Mutational analysis of the IgH intronic enhancer has shown that the μE3 element is responsible for about 30% of the enhancer activity in B cells.42 A number of transcription factors that bind to the μE3 site have been isolated, among them TFE3,43,44 USF,45 and TFEB.46 In addition to the Ig genes, these factors have been shown to play a role in the regulation of a number of genes,47-49 but CD20 is the only other B-cell–specific gene. Our in vivo footprinting data, gel shift data, along with the strong transactivation of the CD20 promoter by USF1 support the notion that the μE3 element and USF1 play an important role in regulating the B-cell–specific expression of the CD20 gene.
Recently, a novel function of tethering promoter and enhancer elements that both have μE3 sites has been described for the bHLHZ protein, TFE3.50 We have made CD20 promoter-reporter constructs that contain a tetramer of μE3 sites in an enhancer position. In cotransfection experiments with TFE3 we have shown that the function of this artificial enhancer depended on an intact μE3 site in the CD20 promoter, supporting the model that TFE3 can mediate interactions between promoter and enhancer elements (data not shown). This observation might also indicate that the function of the μE3 site in the CD20 promoter is to facilitate an interaction with a CD20 enhancer.
Finally, it is interesting to observe that three important B-cell–specific genes that are first transcriptionally activated during the pre–B-cell stage of B-cell development, the two light chain genes and the CD20 gene, all have a critical PU.1/Pip site in their gene regulatory elements. This observation could indicate an important role for this complex in the expression of genes at the pre–B-cell stage of B-cell development.
ACKNOWLEDGMENT
The authors thank Dr Anthony Fauci for his support and critical reading of the manuscript and Ms Mary Rust for her excellent editorial assistance.
Supported in part by a Swiss National Foundation Grant to A.H.
Address reprint requests to John H. Kehrl, MD, National Institutes of Health, Building 10, Room 11B-13, 10 Center Dr MSC 1876, Bethesda, MD 20892-1876.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal