Abstract
Factor VIIIa is a heterotrimer of A1, A2, and A3-C1-C2 subunits, the activity of which is labile due to a weak affinity interaction of the A2 subunit with the A1/A3-C1-C2 dimer. We have used the zero-length cross-linking reagent, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC), to localize regions of interaction within the A1 and A2 subunits. Reaction of factor VIIIa with EDC resulted in the formation of a cross-linked product of approximately 90 kD consisting of the A1 and A2 subunits as judged by Western blotting. Alkaline resistance of this product indicated an amide rather than ester linkage. Factor VIIIa activity decreased as the concentration of cross-linked product increased, suggesting that flexibility in the inter-subunit interaction may be required for proper cofactor function. This product was not formed in the contiguous A1-A2 domains of factor VIII, suggesting that, upon cofactor activation, a conformational change occurs that leads to the formation of a new interdomainal salt bridge(s). Reaction of the EDC-treated factor VIIIa with activated protein C (APC), which cleaves the A1 subunit at Arg336 and bisects the A2 subunit at Arg562, resulted in the formation of an approximately 30 kD product that contains the C-terminus region of A1 covalently linked to the N-terminal half of the A2. The approximately 90 kD cross-linked product was generated after reaction of A2 subunit with A1/A3-C1-C2 dimer but not with A1336/A3-C1-C2, a form of the dimer produced by APC cleavage and lacking the C-terminal acidic region of A1. A synthetic peptide corresponding to this acidic region (Met337-Arg372) was found to covalently cross-link to the isolated A2 subunit in 1:1 stoichiometry, suggesting that this region is both necessary and sufficient for the interaction of the A1 and A2 subunits. Sequence analysis of this product suggested that Glu344 in the A1 peptide may contribute to the cross-linkage. These results indicate that activation of factor VIII results in formation of a new ionic linkage(s) localized to the acidic C-terminal region of A1 and the N-terminal half of A2.
FACTOR VIII FUNCTIONS as a protein cofactor in the intrinsic factor Xase complex. Individuals with a deficiency or defect in factor VIII suffer from the severe bleeding disorder hemophilia A, underscoring its essential role in the process of hemostasis. Factor VIII is synthesized as a single chain precursor of approximately 300 kD1,2 with the domain structure A1-A2-B-A3-C1-C2.3 After posttranslational processing, factor VIII circulates as a series of metal ion-linked heterodimers that result from cleavage at the B-A3 junction as well as additional cleavages within the B domain.4,5 Factor VIII is converted to its active form, factor VIIIa,1 upon proteolytic cleavage by thrombin6 and is a heterotrimer composed of the A1, A2, and A3-C1-C2 subunits.7 The A1 and A3-C1-C2 subunits retain the metal ion linkage forming a stable dimer,8-10 whereas the A2 subunit is weakly associated primarily through electrostatic forces.9 At physiologic pH, factor VIIIa activity is labile and the spontaneous decay is attributed to the dissociation of the A2 subunit from the A1/A3-C1-C2 dimer.8,11 At slightly acidic pH, this dissociation is reversible and factor VIIIa activity can be reconstituted from the A2 subunit and A1/A3-C1-C2 dimer.9
The A1 subunit of factor VIIIa terminates with a 36 residue segment (Met337-Arg372 ) rich in acidic residues.3 This segment is removed after cleavage at Arg336 by activated protein C (APC),2,6 which results in inactivation of the cofactor. Previous work in our laboratory suggested an essential role for this acidic region in retention of the A2 subunit after thrombin activation.10 Furthermore, a synthetic peptide prepared to this region inhibited the reconstitution of factor VIIIa activity from the A2 subunit and A1/A3-C1-C2 dimer.12 Thus, one mechanism proposed for cofactor inactivation after cleavage by APC is a weakened affinity of the A1 and A2 subunits.10 Observations based on activity analyses support a role for the C-terminal region of A1 in the inter-subunit interaction; however, there is little physical data indicating the involvement of this region in A2 interaction and no information exists on the putative complementary region(s) in the A2 subunit.
In this study, we used the zero-length cross-linking reagent, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC), to localize sites of interaction within the A1 and A2 subunits. A primary cross-linked product of approximately 90 kD is obtained with factor VIIIa and consists of the A1 and A2 subunits. This product is not formed in the contiguous A1-A2 domains of factor VIII, suggestive of a conformational change leading to formation of a new interdomainal interaction after cofactor activation. Reaction of the cross-linked product with APC, which cleaves at Arg336 in the A1 subunit and Arg562 in the A2 subunit,10 results in the generation of an approximately 30 kD product that contains the C-terminus of the A1 subunit linked to the NH2 -terminal half of the A2 subunit.
MATERIALS AND METHODS
Reagents. EDC was purchased from Pierce (Rockford, IL) and the ECL Western Blot detection system was obtained from Amersham (Arlington Heights, IL). Phospholipid vesicles composed of 20% phosphatidylserine, 40% phosphatidylcholine, and 40% phosphatidylethanolamine were prepared as previously described.13 The synthetic peptide corresponding to factor VIII residues 337-372 (FVIII337-372 ) was prepared by the Biotechnology Analytical and Synthesis Facility at Cornell University and has been described previously.12
Proteins. Recombinant human factor VIII was a generous gift from Debra Pittman (Genetics Institute, Cambridge, MA) and Dr Jim Brown (Bayer Corp, Berkeley, CA). Factor VIII(a) activity was measured in a one-stage clotting assay using substrate plasma that has been chemically depleted of factor VIII activity.14 Human α-thrombin (Enzyme Research Laboratories, South Bend, IN) and bovine APC (Haematologic Technologies Inc, Essex Junction, VT) were purchased from the indicated vendors.
Preparation of factor VIIIa and factor VIIIa-derived subunits. Factor VIIIa was prepared using the method of Curtis et al.15 The A1/A3-C1-C2 dimer was prepared as described previously.12 The preparation of A1336/A3-C1-C2 was accomplished using minor modification of our previously described method16 that included a single application to the Mono S column.
Preparation of factor VIII A2 subunit. The A2-secreting CHO cell line was a generous gift from Dorothea Scandella (American Red Cross, Rockville, MD). Culture media containing the A2 subunit was made 20 mmol/L Tris, pH 7.2, 0.5 mol/L NaCl and applied to an antibody affinity column (R8B12-Affi-gel)9 equilibrated in 50 mmol/L Tris, pH 7.2, 0.5 mol/L NaCl, 0.02% Tween-20. The column was washed with buffer containing 20 mmol/L morpholinoethanesulfonic acid (Mes), pH 6.5, 0.8 mol/L NaCl, 0.01% Tween-20. An S-Sepharose column (0.5 × 0.5 cm) was attached in series and the two columns were washed with buffer containing 20 mmol/L Mes, pH 6.5, 0.1 mol/L NaCl, 0.01% Tween-20. A2 eluting from the antibody column upon washing with buffer containing 20 mmol/L Mes, pH 6.5, 0.1 mol/L NaCl, 0.01% Tween-20, and 50% ethylene glycol bound directly to the S-Sepharose column. The S-Sepharose column was disconnected and extensively washed with buffer containing 20 mmol/L Mes, pH 6.5, 0.1 mol/L NaCl, 0.01% Tween-20. Purified A2 was eluted from the column with the same buffer containing 0.8 mol/L NaCl. This material was essentially homogeneous (>95% pure) as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Approximately 0.2 mg of A2 was obtained from 500 mL of culture supernatant.
Antibodies. Several monoclonal and polyclonal factor VIII antibodies were used in this study and a schematic indicating the location of the epitopes is shown in Fig 1. The monoclonal antibodies 58.12 and C5 were kind gifts from Dr Jim Brown and Dr Carol Fulcher (Scripps Clinic and Research Institute, La Jolla, CA), respectively. Monoclonal antibody R8B129 and the anti-FVIII337-372 polyclonal antibody12 have been described previously. The anti-FVIII403-427 polyclonal antibody was prepared to a synthetic peptide comprised of factor VIII residues 403 to 427 (obtained from QCB, Inc, Boston, MA) conjugated to rabbit serum albumin using methods described previously.12 The antibody titer was 1/400 using factor VIII by enzyme-linked immunosorbent assay (ELISA). The secondary antibodies, goat antirabbit-horseradish peroxidase (HRP; Bio-Rad Laboratories, Richmond, CA) and goat antimouse-HRP (Boehringer Mannheim, Indianapolis, IN), were purchased from the indicated vendors.
Localization of factor VIII heavy chain-reactive antibodies. The A1 and A2 domains are separated by an acidic region (hatched). Cleavage sites for thrombin3 and APC3,10 are designated by arrowheads. Antibody 58.12 was prepared against a 13 residue peptide composed of the heavy chain amino terminal sequence. The C5 epitope has been mapped to residues 351-36535 and is retained as the C-terminus of the A1 subunit after thrombin cleavage. R8B12 recognizes an epitope near the C-terminus of the A2 subunit.10 Epitopes for the antipeptide polyclonal antibodies are as indicated.
Localization of factor VIII heavy chain-reactive antibodies. The A1 and A2 domains are separated by an acidic region (hatched). Cleavage sites for thrombin3 and APC3,10 are designated by arrowheads. Antibody 58.12 was prepared against a 13 residue peptide composed of the heavy chain amino terminal sequence. The C5 epitope has been mapped to residues 351-36535 and is retained as the C-terminus of the A1 subunit after thrombin cleavage. R8B12 recognizes an epitope near the C-terminus of the A2 subunit.10 Epitopes for the antipeptide polyclonal antibodies are as indicated.
Cross-linking with EDC. Cross-linking reactions contained the indicated concentrations of reactants and were run in buffer containing 20 mmol/L Mes, pH 6, and 0.01% Tween-20 at room temperature for the indicated times. Reactions were terminated by addition of SDS electrophoresis sample buffer and boiling the samples for 3 minutes.
APC cleavage of factor VIIIa or cross-linked factor VIIIa. Bovine APC (0.045 μmol/L) and phospholipid vesicles composed of 20% phosphatidylserine, 40% phosphatidylcholine, and 40% phosphatidylethanolamine (PSPCPE; 100 μg/mL) were added to factor VIIIa or EDC cross-linked factor VIIIa (0.9 μmol/L) and reactions were incubated 16 hours at 37°C. Electrophoresis and Western blot analysis of the samples were performed as described below.
Electrophoresis and Western blotting. SDS-PAGE was performed using the method of Laemmli17 with a Bio-Rad minigel system. Electrophoresis was performed at 150 V for 1 hour. The proteins were transferred to polyvinylidene difluoride (PVDF) membranes (0.2 mm; Bio-Rad) using a Bio-Rad mini-transblot apparatus at 100 V for 1.5 hours in buffer containing 192 mmol/L glycine, 25 mmol/L Tris, and 20% (vol/vol) methanol. Western blotting used indicated primary antibodies followed by the complementary secondary antibody as described.5 The ECL system was used to detect the signal (with luminol as the substrate) and the blots were exposed to film for various times.
Amino acid sequencing of cross-linked FVIII337-372 and A2 subunit. A2 (500 nmol/L) was incubated with FVIII337-372 (50 μmol/L) for 30 minutes at room temperature. EDC (500 μmol/L) was added and incubation was continued for 1 hour at room temperature. The reaction was dialyzed against 20 mmol/L Mes, pH 6, 100 mmol/L NaCl, and 0.01% Tween-20 for 4 hours to remove free peptide. After addition of SDS sample buffer, the reaction mixture was subjected to electrophoresis on a 10% gel. Electrophoresis running buffer contained 0.1 mmol/L thioglycolate. Proteins were transferred to PVDF in 9 mmol/L 3-(cyclohexylamino)-1-propanesulfonic acid (Caps), pH 11, and 10% methanol for 30 minutes at 0.5 A, constant current. The blot was stained with 0.2% Coomassie brilliant blue in 45% methanol and 10% acetic acid for 20 minutes. After destaining (3 × 2 minutes) with 90% methanol and 7% acetic acid, the desired band was cut out and sequenced using an Applied BioSystems Procise Sequenator by the Protein Sequencing Facility at Cornell University.
RESULTS
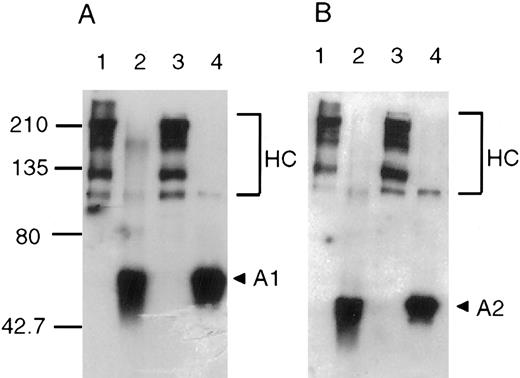
Cross-linking of the A1 and A2 subunits of factor VIIIa. The zero-length cross-linking reagent EDC was used to identify interacting regions within the A1 and A2 subunits of factor VIIIa. This reagent reacts initially with carboxylic groups in the protein to form an unstable O-acylisourea adduct that can subsequently interact with a proximal nucleophilic group, thereby covalently linking residues that likely participated in an ionic bond.18 Purified factor VIIIa (750 nmol/L) was reacted with increasing concentrations of EDC for 2 hours at room temperature. Aliquots were removed and the effects of cross-linking were assessed after factor VIII activity and polypeptide composition analyses. For the latter study, samples were subjected to SDS-PAGE, transferred to PVDF, and immunoblotted with antibody to the A1 subunit (58.12) or the A2 subunit (R8B12). Results (Fig 2A and B) showed a dose-dependent formation of an approximately 90 kD product that is recognized by both antibodies, indicating a covalent cross-link between the A1 and A2 subunits. The minor band seen at 90 kD in the absence of EDC (lane 1 of both panels) likely represents residual intact heavy chain (contiguous A1-A2 domains) resulting from incomplete thrombin activation, ie, cleavage at Arg740 that liberates B domain but not at Arg372. At higher concentration of EDC, we observed the formation of higher molecular weight cross-linked products that also were recognized by anti-A1 and anti-A2 antibodies. The identity of these products is not clear, but may represent either covalent factor VIIIa heterotrimers and/or higher order aggregates of heavy chain-derived subunits. Although their size is consistent with factor VIIIa, we were not able to identify the presence of A3-C1-C2 subunit in this material (data not shown). Furthermore, as EDC concentration increased, an increasing proportion of free A1 subunit exhibited an increased electrophoretic mobility. This result suggested that an intra-A1 cross-link(s) was formed by EDC resulting in a lower apparent molecular weight product. No internal linkages were detected by this criterion in the A2 subunit at all EDC concentrations used. However, at higher EDC concentrations (above 0.75 mmol/L), we observed significant loss of the approximately 40 kD-A2-reactive band. The reason for this is not known but may result from formation of covalent aggregates that fail to resolve in the gel and/or transfer.
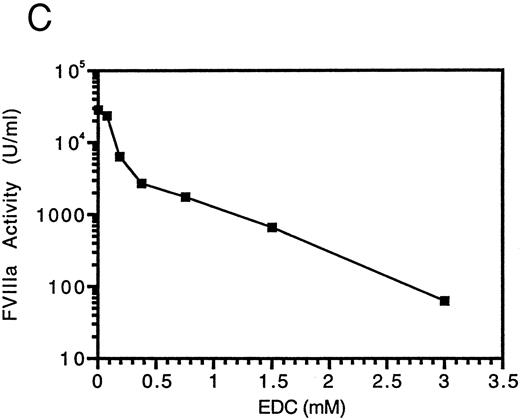
Cross-linking of factor VIIIa with EDC. Factor VIIIa (750 nmol/L) was incubated in the absence (lane 1) and presence of EDC at 75 μmol/L (lane 2), 188 μmol/L (lane 3), 375 μmol/L (lane 4), 750 μmol/L (lane 5), 1.5 mmol/L (lane 6), or 3 mmol/L (lane 7) for 2 hours at room temperature in a reaction (35 μL) containing 25 mmol/L Mes, pH 6, and 0.01% Tween-20. An aliquot was removed from each sample and assayed for factor VIIIa activity (C). SDS sample buffer was added to the remainder of each sample and reactions were subjected to electrophoresis on 6% to 15% gels, transferred to PVDF, and immunoblotted with the anti-A1 subunit antibody, 58.12 (A), or the anti-A2 subunit antibody, R8B12 (B).
Cross-linking of factor VIIIa with EDC. Factor VIIIa (750 nmol/L) was incubated in the absence (lane 1) and presence of EDC at 75 μmol/L (lane 2), 188 μmol/L (lane 3), 375 μmol/L (lane 4), 750 μmol/L (lane 5), 1.5 mmol/L (lane 6), or 3 mmol/L (lane 7) for 2 hours at room temperature in a reaction (35 μL) containing 25 mmol/L Mes, pH 6, and 0.01% Tween-20. An aliquot was removed from each sample and assayed for factor VIIIa activity (C). SDS sample buffer was added to the remainder of each sample and reactions were subjected to electrophoresis on 6% to 15% gels, transferred to PVDF, and immunoblotted with the anti-A1 subunit antibody, 58.12 (A), or the anti-A2 subunit antibody, R8B12 (B).
Factor VIIIa activity was determined in a one-stage clotting assay immediately after the cross-linking reaction (Fig 2C). Factor VIIIa activity decreased as the concentration of EDC and extent of cross-linking increased. At 3 mmol/L EDC, residual factor VIIIa activity is less than 1% of the original level. Control experiments indicated that, at the sample dilution used the resulting concentration of EDC had no effect on the clotting assay. The loss in activity appeared to correlate with the extent of intra- and inter-chain cross-linking. The reason for this inhibition is not known, but one possible explanation is that flexibility in the interaction of the subunits may be required for cofactor function. Thus, formation of a covalent bond between the A1 and A2 subunits may constrain the molecule such that it is unable to properly interact within the factor Xase complex.
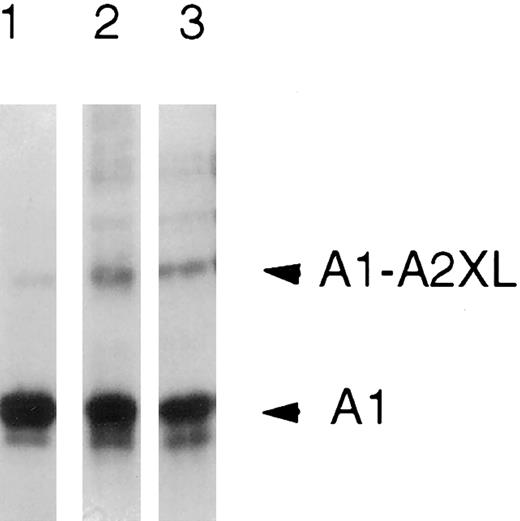
The effect of base on the chemical stability of the inter-A1-A2 cross-link was determined to establish the nature of the ionic bond in the native protein. The observation that the cross-link was resistant to 0.75 mol/L NH4OH (Fig 3) indicated the presence of an isoamide bond between the carboxylic acid and the ε-NH2 of a lysine residue because ester linkages formed by cross-linking other nucleophiles are sensitive to base hydrolysis.19 Neither the number of isoamide bonds nor whether ester linkages are also present in the cross-linked dimer is known.
Alkaline treatment of the cross-linked A1-A2 dimer. Cross-linked factor VIIIa (750 nmol/L) was incubated in the presence of 0.75 mol/L ammonium hydroxide, pH 11, for 15 minutes. Sample buffer was added and reactions (25 μL) were subjected to electrophoresis on 6% to 15% gels, transferred to PVDF, and immunoblotted with the anti-A1 subunit antibody, 58.12. Lane 1, factor VIIIa; lane 2, cross-linked factor VIIIa; and lane 3, cross-linked factor VIIIa plus 0.75 mol/L NH4OH.
Alkaline treatment of the cross-linked A1-A2 dimer. Cross-linked factor VIIIa (750 nmol/L) was incubated in the presence of 0.75 mol/L ammonium hydroxide, pH 11, for 15 minutes. Sample buffer was added and reactions (25 μL) were subjected to electrophoresis on 6% to 15% gels, transferred to PVDF, and immunoblotted with the anti-A1 subunit antibody, 58.12. Lane 1, factor VIIIa; lane 2, cross-linked factor VIIIa; and lane 3, cross-linked factor VIIIa plus 0.75 mol/L NH4OH.
Absence of an A1-A2 cross-link in factor VIII. To determine whether the inter-subunit A1-A2 cross-link existed as an interdomainal linkage in the factor VIII procofactor, the following experiment was performed. Factor VIII was reacted with a level of EDC (250 μmol/L) that results in a high proportion of inter-A1-A2 cross-links in factor VIIIa. The material was then subjected to cleavage by thrombin and products were assessed after SDS-PAGE and Western blotting as performed previously (Fig 4). Reaction of the cross-linked factor VIII with thrombin resulted in near complete conversion of the heavy chains to the A1 and A2 subunits. Although low levels of contiguous A1-A2 were detected in near equivalent amounts in the cross-linked and control lanes, no material corresponding to cross-linked A1-A2 was observed. Because an interdomainal interaction would be independent of protein concentration, the failure to observe the cross-link in factor VIII is strong evidence that the ionic bond(s) cross-linked in factor VIIIa is unique to the active cofactor form.
Cross-linking of factor VIII with EDC. Factor VIII (250 nmol/L) was incubated in the presence (lanes 1 and 2) or absence (lanes 3 and 4) of EDC (250 μmol/L) in a reaction (100 μL) containing 20 mmol/L Mes, pH 6, and 0.01% Tween-20 for 1 hour at room temperature. After cross-linking, the pH of the reactions was raised to 7.2 and thrombin (100 nmol/L) was added (lanes 2 and 4) for 1 hour at room temperature. After addition of SDS sample buffer, reactions were subjected to electrophoresis on 8% gels, transferred to PVDF, and immunoblotted with antibody 58.12 (A) or R8B12 (B).
Cross-linking of factor VIII with EDC. Factor VIII (250 nmol/L) was incubated in the presence (lanes 1 and 2) or absence (lanes 3 and 4) of EDC (250 μmol/L) in a reaction (100 μL) containing 20 mmol/L Mes, pH 6, and 0.01% Tween-20 for 1 hour at room temperature. After cross-linking, the pH of the reactions was raised to 7.2 and thrombin (100 nmol/L) was added (lanes 2 and 4) for 1 hour at room temperature. After addition of SDS sample buffer, reactions were subjected to electrophoresis on 8% gels, transferred to PVDF, and immunoblotted with antibody 58.12 (A) or R8B12 (B).
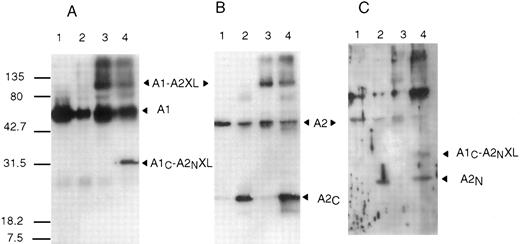
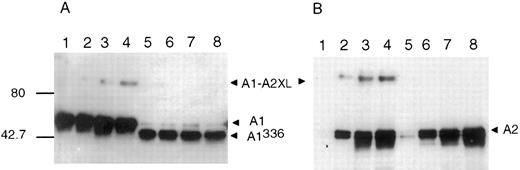
APC cleavage of cross-linked A1-A2. Proteolytic cleavage of factor VIIIa or cross-linked factor VIIIa was performed to further localize the regions of interaction. APC cleaves at two locations within factor VIIIa.10 Cleavage within the A1 subunit at Arg336 releases the C-terminal acidic region previously proposed to be important in the association of A2 with the A1/A3-C1-C2 dimer12 and cleavage at Arg562 bisects the A2 subunit into fragments of similar size that we designate as A2N and A2C . The products of APC cleavage of factor VIIIa and cross-linked factor VIIIa were evaluated by Western blot analysis (Fig 5). Antibodies were used that recognize the A2N fragment (anti-FVIII403-427 ), the A2C fragment (R8B12) or the carboxy terminus of the A1 subunit (C5 and anti-FVIII337-372 ). Cleavage of the non–cross-linked samples by APC (lane 2 of each panel) yielded the expected products based on antibody recognition, ie, the A2C fragment detected by R8B12 (panel B), the slightly larger A2N fragment detected by anti-FVIII403-427 (panel C), and no product detected by C5 (panel A) due to its low molecular weight (approximately 4 kD). Similar to results presented above, the C5, R8B12, and, to a lesser extent, anti-FVIII403-427 antibodies identify an approximately 90 kD product in reactions containing EDC. Analysis of the cleavage products derived from cross-linked factor VIIIa (lane 4 in each panel) showed, in some cases, the appearance of a unique band of approximately 30 kD. This band was detected by the C5 (panel A), anti-FVIII403-427(panel C), and anti-FVIII337-372 (not shown) antibodies but not R8B12 (panel B). Probing of the blots with the anti-A1 N-terminus antibody (58.12) showed no differences when comparing cross-linked and non–cross-linked samples (results not shown). These results indicate that cross-linking of the A1 and A2 subunits occurs within the carboxy terminal residues of the A1 subunit (residues 337-372) and the amino terminal half of the A2 subunit (residues 373-562) and localize an interfactor VIIIa subunit salt bridge to these sites.
APC cleavage of factor VIIIa or EDC cross-linked factor VIIIa. Bovine APC (0.045 μmol/L) and PSPCPE (100 μg/mL) were added to factor VIIIa or EDC cross-linked factor VIIIa (0.9 μmol/L) and reactions (600 μL) were incubated 16 hours at 37°C. After addition of SDS sample buffer, reactions were subjected to electrophoresis on 12% gels, transferred to PVDF, and immunoblotted with the following antibodies: C5 (A), R8B12 (B), or anti-FVIII403-427 (C). Lane 1, factor VIIIa; lane 2, APC-cleaved factor VIIIa; lane 3, cross-linked factor VIIIa; and lane 4, APC-cleaved cross-linked factor VIIIa.
APC cleavage of factor VIIIa or EDC cross-linked factor VIIIa. Bovine APC (0.045 μmol/L) and PSPCPE (100 μg/mL) were added to factor VIIIa or EDC cross-linked factor VIIIa (0.9 μmol/L) and reactions (600 μL) were incubated 16 hours at 37°C. After addition of SDS sample buffer, reactions were subjected to electrophoresis on 12% gels, transferred to PVDF, and immunoblotted with the following antibodies: C5 (A), R8B12 (B), or anti-FVIII403-427 (C). Lane 1, factor VIIIa; lane 2, APC-cleaved factor VIIIa; lane 3, cross-linked factor VIIIa; and lane 4, APC-cleaved cross-linked factor VIIIa.
Role of the C-terminal acidic region of the A1 subunit in the formation of the A1-A2 cross-linked product. Under the appropriate conditions, factor VIIIa activity can be reconstituted from the isolated A1/A3-C1-C2 dimer and the A2 subunit.9 Reconstitution of A2 with the intact A1/A3-C1-C2 or an APC-cleaved dimer lacking the C-terminal acidic region of A1 (designated A1336/A3-C1-C2) was performed followed by addition of EDC (Fig 6). Reaction of the A1/A3-C1-C2 dimer with increasing levels of A2 resulted in the formation of the approximately 90 kD cross-linked product. However, no cross-linked product was formed after reaction of A2 with the A1336/A3-C1-C2 dimer, confirming our earlier observation that the C-terminal acidic region was required for the interaction with A210 and indicating that this interaction was mediated by formation of a salt bridge.
EDC cross-linking of A2 with A1/A3-C1-C2 or A1336/A3-C1-C2. Reactions (40 μL) containing A1/A3-C1-C2 (200 nmol/L) (lanes 1 through 4) or A1336/A3-C1-C2 (200 nmol/L) (lanes 5 through 8) were incubated with the following: no additions (lanes 1 and 5), 100 nmol/L A2 (lanes 2 and 6), 200 nmol/L A2 (lanes 3 and 7), or 400 nmol/L A2 (lanes 4 and 8) for 30 minutes at room temperature. EDC (200 μmol/L) was added and incubation was continued for 1 hour. After addition of SDS sample buffer, reactions were subjected to electrophoresis on 6% to 15% gels, transferred to PVDF, and immunoblotted with 58.12 (A) or R8B12 (B).
EDC cross-linking of A2 with A1/A3-C1-C2 or A1336/A3-C1-C2. Reactions (40 μL) containing A1/A3-C1-C2 (200 nmol/L) (lanes 1 through 4) or A1336/A3-C1-C2 (200 nmol/L) (lanes 5 through 8) were incubated with the following: no additions (lanes 1 and 5), 100 nmol/L A2 (lanes 2 and 6), 200 nmol/L A2 (lanes 3 and 7), or 400 nmol/L A2 (lanes 4 and 8) for 30 minutes at room temperature. EDC (200 μmol/L) was added and incubation was continued for 1 hour. After addition of SDS sample buffer, reactions were subjected to electrophoresis on 6% to 15% gels, transferred to PVDF, and immunoblotted with 58.12 (A) or R8B12 (B).
That this C-terminal acidic region of A1 is necessary and sufficient for interaction of the A2 subunit was determined by experiments showing that a synthetic peptide to this region can specifically cross-link to the A2 subunit (Fig 7). Increasing levels of the peptide, FVIII337-372, were incubated with the A2 subunit, followed by the addition of EDC. Western blot analysis using an antibody prepared against this peptide showed the formation of a cross-linked product that runs at approximately 45 kD, thus suggesting that the site for A2 interaction is contained within this region. The size of the peptide-A2 product formed with EDC was consistent with a 1:1 stoichiometry of reactants, suggesting specificity in the peptide-A2 interaction.
Formation of A2-FVIII337-372 cross-linked product. A2 (200 nmol/L) was incubated with no additions (lane 1), 0.625 μmol/L (lane 2), 1.25 μmol/L (lane 3), 2.5 μmol/L (lane 4), 5 μmol/L (lane 5), 10 μmol/L (lane 6), or 20 μmol/L (lane 7) FVIII337-372 in a reaction volume of 20 μL for 30 minutes at room temperature. EDC (200 μmol/L) was added and incubation was continued for 1 hour. After addition of SDS sample buffer, reactions were subjected to electrophoresis on 12% gels, transferred to PVDF, and immunoblotted with R8B12 (A) or anti-FVIII337-372 (B).
Formation of A2-FVIII337-372 cross-linked product. A2 (200 nmol/L) was incubated with no additions (lane 1), 0.625 μmol/L (lane 2), 1.25 μmol/L (lane 3), 2.5 μmol/L (lane 4), 5 μmol/L (lane 5), 10 μmol/L (lane 6), or 20 μmol/L (lane 7) FVIII337-372 in a reaction volume of 20 μL for 30 minutes at room temperature. EDC (200 μmol/L) was added and incubation was continued for 1 hour. After addition of SDS sample buffer, reactions were subjected to electrophoresis on 12% gels, transferred to PVDF, and immunoblotted with R8B12 (A) or anti-FVIII337-372 (B).
Further evidence that the peptide was interacting at a single site in A2 and the tentative identification of a critical residue in this linkage were obtained after N-terminal sequence analysis of the approximately 45 kD product formed after reaction of A2 (500 nmol/L) with peptide (50 μmol/L) in the presence of EDC (500 μmol/L). Two separate preparations of cross-linked A2-peptide were subjected to this analysis. For each preparation, two residues were identified for each cycle of automated sequence analysis. Residues identified matched the predicted residues for the NH2 -termini of A23 and the peptide. Figure 8 shows the sequence data (in picomole residue recovered) for each of the two analyses with results obtained for peptide in Fig 8A and A2 in Fig 8B. Identification of residues beyond 10 cycles was not possible, likely due to the limited amount of material available for this analysis and/or the increase in background resulting from the simultaneous analysis of the two N-termini. Similar yields of the two residues were obtained at each cycle. This result suggested that a single peptide molecule was bound per A2 subunit. Interestingly, Glu344 in the peptide (expected in sequencing cycle no. 8) was not identified during the sequencing of one preparation of the cross-linked A2-peptide and identified with markedly reduced yield compared with other residues in the other preparation. Sequence analysis of the native peptide (data not shown) indicated that Glu344 was obtained in cycle no. 8 with a yield consistent with other identified residues. Because a residue that participates in a covalent cross-link is not identified by sequence analysis,19 we tentatively identify Glu334 in the peptide as forming the cross-linkage with a yet unidentified Lys residue in A2. The failure to identify Trp at cycle no. 10 of the A2 sequence likely reflects formation of oxidation products from this residue rather than its involvement in a cross-link.
Sequence analysis of the A2-FVIII337-372 cross-linked product. Results are presented as subtraction chromatograms wherein the yield (in picomoles) for a given residue (except the initial residue) has subtracted from it the value for that residue in the previous cycle. This method corrects for background and is useful in analyzing low yield samples. The bar graph shows the residue yield for each cycle for experiment no. 1 () and experiment no. 2 (▪). (A) shows the residue yields attributed to the FVIII337-372 peptide sequence and (B) shows the residue yields attributed to the A2 subunit sequence. For (A), the peptide sequence for 10 cycles, using the single letter code, corresponds to MKNNEEAEDY; for (B), the A2 sequence is SVAKKHPKT(W). Trp (W), is typically recovered in low yield, and this residue was not identified at cycle 10 of the A2 sequence.
Sequence analysis of the A2-FVIII337-372 cross-linked product. Results are presented as subtraction chromatograms wherein the yield (in picomoles) for a given residue (except the initial residue) has subtracted from it the value for that residue in the previous cycle. This method corrects for background and is useful in analyzing low yield samples. The bar graph shows the residue yield for each cycle for experiment no. 1 () and experiment no. 2 (▪). (A) shows the residue yields attributed to the FVIII337-372 peptide sequence and (B) shows the residue yields attributed to the A2 subunit sequence. For (A), the peptide sequence for 10 cycles, using the single letter code, corresponds to MKNNEEAEDY; for (B), the A2 sequence is SVAKKHPKT(W). Trp (W), is typically recovered in low yield, and this residue was not identified at cycle 10 of the A2 sequence.
DISCUSSION
The interaction of A2 subunit with the A1 subunit of the A1/A3-C1-C2 dimer is crucial for maintenance of cofactor function. Recent kinetic studies indicate that, at predicted physiologic reactant concentrations, the decay of factor Xase activity is governed by this interfactor VIIIa subunit affinity and residual factor Xa generating activity approaches a value consistent with this equilibrium.20 In the current study, we show the generation of an interfactor VIIIa subunit salt bridge after cofactor activation that is made covalent by the zero-length cross-linking reagent, EDC. The site of this cross-link localizes to the C-terminal region of the A1 subunit and the N-terminal half of A2 subunit. This observation implies formation of an inter-subunit bond between proximal carboxylic and nucleophilic groups in the factor VIIIa subunits that are not similarly oriented in the procofactor form of the molecule.
Results of these studies support earlier observations characterizing the inter-subunit interaction as primarily electrostatic and implicating the C-terminal acidic region of A1 in the retention of A2 in the factor VIIIa heterotrimer. Reconstitution of factor VIIIa from isolated A1/A3-C1-C2 dimer and A2 subunits is sensitive to increasing ionic strength9 and slightly alkaline pH.8 kd estimates show an order of magnitude decrease in affinity of A2 for dimer when the pH is increased from 6.0 (kd approximately 30 nmol/L) to 7.2 (kD approximately 260 nmol/L).11,21 The reconstitution of factor VIIIa activity could be inhibited by free A1 subunit11 but not the APC-cleaved A1336/A3-C1-C2 dimer,10 suggesting the importance of the C-terminal acidic region of A1 in interaction with A2. Consistent with these observations is the failure to detect binding of A2 subunit to the APC-cleaved dimer by surface plasmon resonance.22 The role of this sequence in maintaining factor VIIIa structure was further supported by experiments showing that the synthetic peptide, designated FVIII337-372, was a potent inhibitor of factor VIIIa reconstitution (Ki approximately 5 mmol/L) and interacted with the A2 subunit as judged by the capacity of A2 subunit to enhance the fluorescence emission of a dansylated peptide.12
The present study extends the above functional observations by providing direct, physical evidence for the generation of a novel ionic interaction(s) between residues within this region of the A1 subunit and the A2 subunit, consistent with the electrostatic nature of this interaction. The alkaline resistance of the cross-link indicated minimally a single isopeptide bond between a carboxylic acid and a Lys residue. These residues would presumably interact as a salt bridge in the native protein. The presence of base-sensitive, ester linkages formed after reaction of EDC with nucleophiles such as Ser or Thr in addition to the isoamide linkage is not precluded. This salt bridge formation likely contributes a significant portion of the binding energy between the A1 and A2 subunits in factor VIIIa. The intersubunit affinity (kd approximately 260 nmol/L11,21), correlates with a thermodynamic stability of approximately 9 kcal⋅mol−1, whereas the free energy of a charged or ion pair hydrogen bond ranges from 3 to 6 kcal⋅mol−1.23
Several years ago we showed that treatment of factor VIII with homo-bifunctional, NHS-active ester cross-linking reagents stabilized the labile factor VIIIa activity.24 Reagents used in that study were reactive to primary amino groups and possessed effective chain lengths of approximately 12 Å. Hence, linkages were likely formed between noninteracting, spatially separated Lys residues. Activity stabilization was the direct consequence of covalent cross-linking of factor VIIIa. This conclusion was based on regeneration of the labile factor VIIIa activity by mild reducing conditions when the factor VIIIa was cross-linked with an internal disulfide-containing reagent. Limitations in the amounts of factor VIII protein available for that study precluded identification of inter-subunit cross-links. In a more recent study performed using similar NHS cross-linking reagents, Persson and Ezban25 confirmed the stabilization of factor VIIIa activity as well as showed covalent linkages between factor VIIIa subunits, yielding product of a size consistent with the intact trimer.
An interesting observation from the present study is that, unlike the results obtained with the long chain length NHS-active ester cross-linking reagents that markedly stabilized factor VIIIa activity, reaction with the zero-length cross-linker to covalently preserve the inter-subunit ionic bond abolished activity. Effects of EDC on activity appear particular to the protein in question and range from activating26 to benign27 to inhibitory.28 For example, reaction of EDC with phospholipase A2 resulted in a several-fold activation that was attributed to stabilization of a loop structure involved in interfacial binding.26 On the other hand, EDC virtually eliminated the surface-dependent enhancement of dynamin GTPase activity.28 The investigators in that study proposed that EDC treatment confers a structural rigidity to the molecule, thereby preventing necessary conformational changes associated with enhanced rates of nucleotide hydrolysis. Titration of factor VIIIa with EDC resulted in the dose-dependent loss of clotting activity that correlated with formation of the inter-A1-A2 linkage(s) as well as an intra-A1 subunit linkage(s). It is not known whether activity loss resulted from either type of covalent linkage alone or in combination. However, this observation suggests that some degree of flexibility, ie, the potential to break and reform this bond, either within the A1 subunit or between A1 and A2 subunits, may be required for cofactor function. Such flexibility may have been unimpaired under the constraints imposed by linking more distal residues with the long chain length NHS-reagents. It is unlikely that loss of activity resulted from modification of a single residue by EDC because the O-acylisourea intermediate is unstable and hydrolyzes to yield the original carboxylic acid in the absence of reaction with a nucleophile.
The cross-linking observed in this study indicates a close spatial relationship between the C-terminus region of the A1 subunit and the N-terminus region of A2. This result, obtained after APC cleavage of the cross-linked A1-A2 adduct, supports recent modeling studies of factor VIII based on homologies to nitrite reductase29 and ceruloplasmin.30 These studies predict each A domain of factor VIII consists of two subdomains, each possessing a single β-barrel unit. According to the models, the orientation of A domains is such that the C-terminal subdomain of A1 (designated D2) juxtaposes the N-terminal subdomain of A2 (D3), whereas the C-terminal subdomain of A2 (D4) is far removed from the A1 domain. It is also noted that APC cleavage at Arg562, which bisects the A2 subunit,10 occurs at the subdomainal (D3-D4) junction separating the β-barrel units of that domain.
Little is known concerning structural events leading to factor VIII activation after cleavage by thrombin. Proteolysis at the A1-A2 junction is essential for activation as judged by naturally occurring31,32 or site-directed33 mutations at Arg372 that result in the marked reduction of cofactor activity. Recently, we showed that both the fluorescence emission properties and the affinity of the hydrophobic probe, bis-ANS, bound to A2 subunit were altered compared with that of the probe bound to intact factor VIII heavy chain,34 suggesting a change in the conformation of A2 after cleavage. Results from the current study offer direct evidence that this proteolytic event leads to a change in the relative orientation of A1 and A2 subunits. Treatment of factor VIIIa with EDC resulted in an inter-A1-A2 cross-link(s). We do not know whether a single or multiple inter-subunit interactions form after factor VIII activation. However, no such linkage was observed with EDC-treatment of the procofactor form. Thus, the ionic bond(s) made covalent by the cross-linker appears to be a novel interaction characteristic of the active cofactor form.
Based on the high concentration of acidic residues in the C-terminal region of A1 (13 Glu plus Asp contained within residues 337-372), we predict that one or more of these residues contributes the carboxylic function to the salt bridge(s). Dallman et al19 observed that, in many cases, the sequence containing the cross-linked acidic residue also contained a high concentration of other acidic residues. These investigators speculated that such sequences often participate in protein-protein interactions and(or) that multiple carboxylic groups may enhance reactivity of the cross-linker. We tentatively identify Glu344 as contributing to the salt bridge. Limitation of material coupled with the increase in background after multiple cycles of sequence analysis of the two N-termini precluded identification of additional residues beyond 10 cycles. Our earlier results showed that a tryptic product of peptide FVIII337-372, containing residues 337 to 359, possessed similar inhibitory activity to the parent peptide in a factor VIIIa reconstitution assay.12 Thus, other potential A2-interactive carboxylic residues are likely contained within this region with the complementary nucleophilic residues contained within the A2 subunit residues 373-562. Based upon the alkaline resistance of the cross-link, we propose that a critical Lys residue in this region serves as a nucleophile in forming the salt bridge.
ACKNOWLEDGMENT
The authors thank Dorothea Scandella for providing the A2 expressing CHO cell line and many helpful discussions on the expression and isolation of recombinant A2, Jim Brown for antibody 58.12 as well as Kogenate used in the preparation of factor VIII, Carol Fulcher for the C5 antibody, and Debra Pittman for recombinant factor VIII. We also thank Ted Tannhauser of the Cornell Biotechnology Center for helpful discussions and performance of the sequence analysis and Autumn Cottom for technical assistance.
Supported by Grants No. HL38199 and HL30616 from the National Institutes of Health and by the American Heart Association, New York State Affiliate Research Fellowship (L.M.O.).
Address reprint requests to Philip J. Fay, PhD, Vascular Medicine Unit, PO Box 610, University of Rochester School of Medicine, 601 Elmwood Ave, Rochester, NY 14642.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal