Abstract
The mechanism leading to the formation of antiphospholipid antibodies (aPL) is still unknown. Because an in vitro study suggested that aPL may derive from pro-oxidant conditions, we sought a relationship between aPL and isoprostanes, indices of lipid peroxidation in vivo. Thirty patients with systemic lupus erythematosus have been studied. Seventeen (56.6%) were positive for aPL because they had lupus anticoagulant and/or high titer of anticardiolipin antibodies (aCL). Plasma levels of tumor necrosis factor (TNF ) and urinary excretion of two isoprostanes, 8-epi-PGF2α and IPF2α -I, free radical catalyzed oxidation products of arachidonic acid, were measured. Patients with systemic lupus erythematosus had higher urinary excretion of 8-epi-PGF2α and IPF2α -I than controls; urinary excretion of the two isoprostanes was highly correlated (Rho = 0.74, P < .0001). Urinary 8-epi-PGF2α was highly correlated with both aCL titer (Rho = 0.70, P < .0001) and TNF (Rho = 0.84, P < .0001), a measure of disease severity. Excretion of this isoprostane was also higher in those patients who exhibited aPL (P < .0001). Comparable correlations were observed with the isoprostane IPF2α -I. No difference of 8-epi-PGF2α was observed between patients with and without previous history of thrombosis. This study, showing the existence of a close association between aPL and increased in vivo lipid peroxidation, supports the hypothesis that these antibodies may result from pro-oxidative conditions and suggests that inflammation may play an important role.
ANTIPHOSPHOLIPID ANTIBODIES (aPL) are circulating autoantibodies that react with negatively charged phospholipids, such as cardiolipin.1 Patients with aPL are at high risk of venous and arterial thrombosis as well as fetal loss.2 These antibodies have been detected in a variety of inflammatory conditions including autoimmune diseases, infections, chronic liver disease, and atherosclerosis,3-6 but the mechanism of their formation is still unknown. An interesting hypothesis has been proposed recently suggesting that antibodies against cardiolipin (aCL) bind exclusively to the peroxidized molecule, indicating that these antibodies recognize epitopes derived from phospholipid oxidation.7 Should this be true, aPL might be more liable to detection in clinical conditions characterized by elevated lipid peroxidation.
Recently, urinary excretion of F2 -isoprostanes, isomers of PGF2α , formed by a free radical catalyzed oxidation of arachidonic acid, have shown promise as indices of lipid peroxidation.8 F2 -isoprostanes are initially formed in situ, esterified on phospholipids, then released in free forms by phospholipases, which circulate in plasma and are excreted in urine.9,10 We initially focused on one of these compounds, 8-epi-PGF2α , for which we have developed specific and sensitive methods of analysis using gas chromatography/mass spectrometry (GC/MS) assay.11 To date we have observed increments in urinary 8-epi-PGF2α in chronic cigarette smokers, during coronary reperfusion, and in certain poisonings,11-13 syndromes putatively associated with oxidant stress.14,15 Furthermore, we have shown that therapy with antioxidant vitamins may depress urinary 8-epi-PGF2α excretion.12 Although 8-epi-PGF2α (now known as IPF2α -IV;16 ), unlike other F2 -isoprostanes, may also be formed in a cyclo-oxygenase (COX)-dependent manner,17,18 this does not appear to confound its measurement in urine as an index of oxidant stress.12 More recently, we have established an assay for a more abundant F2 -isoprostane, IPF2α -I, which is not subjected to COX-dependent formation.16 In the present study, we have used these indices of lipid peroxidation to address the hypothesis that the presence of aPL in patients with systemic lupus erythematosus (SLE) is likely to reflect oxidant modification of lipids in vivo.
MATERIALS AND METHODS
Study population. Between September 1995 and July 1996 we studied 30 consecutive patients (28 women and 2 men, ages 17 to 58) diagnosed as having SLE in accordance with the criteria of the American College of Rheumatology, formerly the American Rheumatism Association,19 and 20 healthy subjects selected from the hospital personnel (19 women and 1 man, ages 18 to 58) as controls. The duration of disease averaged 8 ± 5 years (range 2 to 16) in the patients. Twenty-two patients were being treated with corticosteroids (prednisone 5 to 25 mg/d or methylprednisolone 4 to 24 mg/d) and/or methotrexate (0.25 to 0.30 mg/kg intravenously once a week). Nine patients were considered hypertensive having values of blood pressure >140/90 mm Hg in at least two different occasions; 8 were treated with diuretics, angiotensin-converting enzyme inhibitors, or calcium antagonists. Four patients had diabetes mellitus, 2 of whom were treated with insulin.
Eleven (36%) patients (10 women and 1 man, ages 31 to 58) had had a history of thrombosis and/or fetal loss in the previous 8 to 32 months; 7 had deep venous thrombosis, 2 had deep venous thrombosis and thromboembolic stroke, 1 had retinal thrombosis, and 1 had recurrent fetal loss. Deep venous thrombosis was confirmed by venous Doppler ultrasound, and thromboembolic stroke was confirmed by computed tomographic scan.
Eight (73%) out of 11 patients with thrombosis were positive for aPL. At the time, all patients with previous thrombotic events were being treated with anticoagulant therapy (International Normalized Ratio 2.5 to 3.5). Four patients without a history of thrombosis were on treatment with aspirin (325 mg/d). Neither patients nor controls took vitamin supplements before 1 month of the study.
Among laboratory indexes, we measured serum levels of some proteins that were known to change during the acute phase of disease, namely, C3 and C4, C-reactive protein, and clottable fibrinogen, as previously described.20 No patient had had active infections, trauma, surgery, liver diseases, or other factors known to influence isoprostane levels, such as alcohol and acetaminophen abuse, during the previous 3 months. Among the healthy subjects, none had cardiovascular risk factors, but three were smokers.
Laboratory tests. Blood samples were taken into tubes containing 3.8% trisodium citrate and centrifuged at 500g after overnight fasting and supine rest for at least 10 minutes. The plasma was used immediately for measurement of fibrinogen and lupus anticoagulant (LA). Blood samples were also taken to measure serum aCL, C-reactive protein, the complement components C3 and C4, and tumor necrosis factor (TNF ).
LA was measured in platelet poor plasma centrifuged twice at 5,000g using four different coagulation tests, as previously described.3 Patients were considered positive for LA if they had at least two abnormal (prolonged) clotting tests, which returned to normal values after adding 0.05 mmol/L phosphatidylcholine-phosphatidylserine liposomes (confirmatory test).3 An enzyme-linked immunosorbent assay, validated in an international workshop, was used for measurement of aCL. IgG or IgM aCL were considered positive when the activity was greater than 10 GPL or 10 MPL units, respectively.21 Patients were considered positive for aPL if LA and/or aCL were detected in two separate occasions at least 2 months apart.
Serum TNF was assayed in duplicate by an enzyme immunoassay (Biokine tumor necrosis factor alpha test kit, T Cell Diagnostics Inc, Cambridge, MA). The detection limit was calculated to be 10 pg/mL. Intra-assay and interassay coefficients of variation were 8% and 9%, respectively. Among 20 healthy subjects, 2 showed detectable TNF-α serum levels (median <10 pg/mL; range <10 to 34 pg/mL).
The same day, 12-hour urine specimens were collected from each patient. Urinary 8-epi-PGF2α and IPF2α -I were assayed by GC/MS, as previously described.11 16 The internal standards used were [18O2 ]8-epi-PGF2α and [2H4 ]-IPF2α -I. The intra-assay and interassay variability in urine obtained from healthy volunteers was ±3% and ±4% for 8-epi-PGF2α and ±4% and ±5% for IPF2α -I, respectively.
Statistical analysis. Statistical analysis was performed by χ2 statistic or Fisher's exact test (if n < 5) for independence and by appropriate t-test. When necessary, appropriate nonparametric tests were used. Correlation analysis was carried out by Spearman test. Data were presented as median ± standard deviation (SD). Median and range are given for TNF, 8-epi-PGF2α , and IPF2α -I because they show appreciably skewed distribution. Only P values lower than .05 were regarded as statistically significant. All calculations were made with the computer program STAT-View II (Abacus Concepts, Berkeley, CA).22
RESULTS
Patients with SLE had a higher urinary excretion of 8-epi-PGF2α than controls (median, 166.5; range, 60 to 405 v median, 87.5; range, 26 to 161 pg/mg creatinine; P < .0001; Fig 1). Seventeen patients (57%) had 8-epi-PGF2α values higher than the cut-off point of 154 pg/mg creatinine (mean ± 2 SD of controls). Among SLE patients, 17 were considered aPL positive because they had LA and/or high titer of aCL; 8 had positivity for LA, 16 were positive for aCL with a titer ranging from 20 to 110 GPL, and 7 were positive for both LA and aCL. TNF was significantly higher in SLE patients than in the control population (median, 101.6; range, 26.8 to 290.4 v median <10; range <10 to 34 pg/mL; P < .0001).
Scattergram showing the difference (P < .0001) in 8-epi-PGF2α values in SLE patients and controls and the higher 8-epi-PGF2α values (P < .001) in aPL positive [aPL (+)] patients than in negative [aPL (−)] ones.
Scattergram showing the difference (P < .0001) in 8-epi-PGF2α values in SLE patients and controls and the higher 8-epi-PGF2α values (P < .001) in aPL positive [aPL (+)] patients than in negative [aPL (−)] ones.
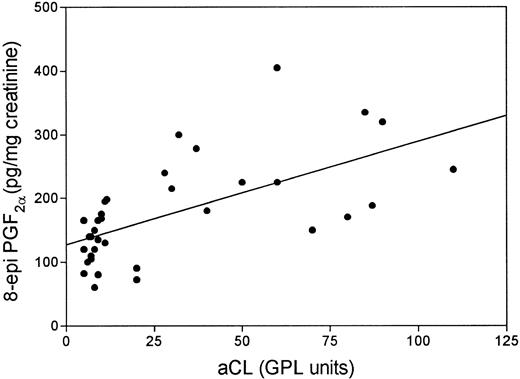
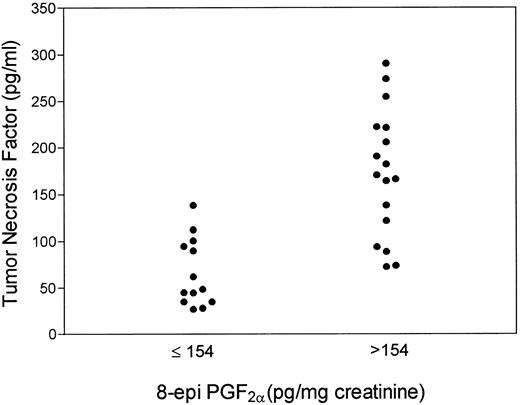
Grouping patients according to the positivity for aPL, we found that positive patients had higher 8-epi-PGF2α than negative ones (median, 225; range, 72 to 405 v median, 130; range, 60 to 175 pg/mg creatinine; P < .001; Fig 1). Fourteen (82%) aPL-positive patients had 8-epi-PGF2α values higher than 154 pg/mg creatinine. Table 1 reports on clinical and laboratory characteristics of aPL-positive and -negative patients. No significant differences in urinary 8-epi-PGF2α were noticed as a function of sex, age, or cardiovascular risk factors, such as hypertension, dyslipidemia, or smoking. Also, they did not show differences in renal function and acute phase reactant proteins, such as C-reactive protein, C3 and C4, and fibrinogen (not shown). Conversely, aPL-positive patients had higher values of TNF than aPL-negative patients; median values of TNF were 170.5 pg/mL (range, 26.8 to 294.4) for aPL-positive patients and 74.1 pg/mL (range, 27.8 to 138.2) for aPL-negative patients (P < .003). Among patients with 8-epi-PGF2α values higher than 154 pg/mg creatinine, 14 were aPL positive and 3 were negative. A significant correlation was observed between aCL titer and 8-epi-PGF2α (Rho = 0.70, P < .0001; Fig 2). Patients taking aspirin (3 with normal and 1 with 20 GPL aCL titer) had 8-epi-PGF2α values similar to those of the remaining SLE population (median, 175; range, 90 to 168 v median, 172.5; range, 60 to 405 pg/mg creatinine; P > .05). This lack of difference persisted when patients taking aspirin were matched for sex, age, aCL titer, and disease activity (median, 133; range, 105 to 180 v median, 143; range, 90 to 168 pg/mg creatinine) with SLE ones not taking aspirin. Patients with 8-epi-PGF2α >154 pg/mg creatinine had higher TNF values than patients with 8-epi-PGF2α <154 pg/mg creatinine (median, 170.5; range, 72.5 to 290.4 v median, 48.5; range, 27.8 to 138.2 pg/mL; P < .001; Fig 3). A strong significant correlation was found between 8-epi-PGF2α and TNF (Rho = 0.84, P < .0001). aPL-positive patients with previous thrombosis had 8-epi-PGF2α values (median, 220; range, 150 to 335 pg/mg creatinine) similar to those of aPL-positive patients without thrombosis (median, 240; range, 75 to 405 pg/mg creatinine; P > .05).
Clinical and Laboratory Characteristics of SLE Patients
| . | . | aPL-Negative Patients . | aPL-Positive Patients . | P Value . |
|---|---|---|---|---|
| . | . | n = 13 . | n = 17 . | . |
| Age (yrs) | 36 ± 10 | 38 ± 13 | ns | |
| range | (24-49) | (17-58) | ||
| Male sex | n (%) | 1 (8) | 1 (6) | ns |
| Diabetes mellitus | n (%) | 2 (15) | 2 (12) | ns |
| Hypertension | n (%) | 4 (31) | 5 (29) | ns |
| Total cholesterol (mg %) | Mean ± SD | 210 ± 40 | 213 ± 45 | ns |
| Smoking | n (%) | 1 (8) | 3 (18) | ns |
| TNF (pg/mL) | Median | 74.1 | 170.5 | P < .003 |
| Range | (27.8-138.2) | (26.8-294.4) | ||
| 8-epi >154 (pg/mg creatinine) | n (%) | 3 (23) | 14 (82) | P < .01 |
| . | . | aPL-Negative Patients . | aPL-Positive Patients . | P Value . |
|---|---|---|---|---|
| . | . | n = 13 . | n = 17 . | . |
| Age (yrs) | 36 ± 10 | 38 ± 13 | ns | |
| range | (24-49) | (17-58) | ||
| Male sex | n (%) | 1 (8) | 1 (6) | ns |
| Diabetes mellitus | n (%) | 2 (15) | 2 (12) | ns |
| Hypertension | n (%) | 4 (31) | 5 (29) | ns |
| Total cholesterol (mg %) | Mean ± SD | 210 ± 40 | 213 ± 45 | ns |
| Smoking | n (%) | 1 (8) | 3 (18) | ns |
| TNF (pg/mL) | Median | 74.1 | 170.5 | P < .003 |
| Range | (27.8-138.2) | (26.8-294.4) | ||
| 8-epi >154 (pg/mg creatinine) | n (%) | 3 (23) | 14 (82) | P < .01 |
Linear regression analysis between urinary excretion of 8-epi-PGF2α and serum aCL titer in patients with systemic lupus erythematosus (Rho = 0.70; P < .0001).
Linear regression analysis between urinary excretion of 8-epi-PGF2α and serum aCL titer in patients with systemic lupus erythematosus (Rho = 0.70; P < .0001).
Scattergram showing TNF value distribution in SLE patients with 8-epi-PGF2α values ≤ or <154 pg/mg creatinine (mean ± SD of controls).
Scattergram showing TNF value distribution in SLE patients with 8-epi-PGF2α values ≤ or <154 pg/mg creatinine (mean ± SD of controls).
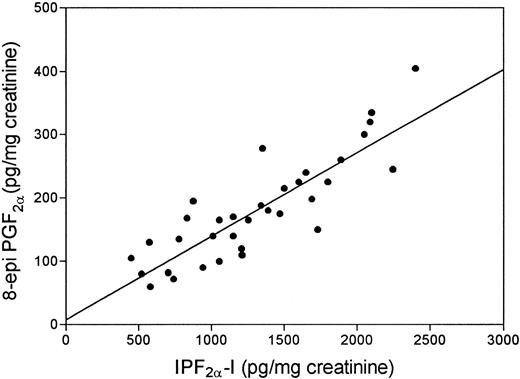
To further confirm that in vivo lipid peroxidation is enhanced in SLE patients, we decided to measure another member of the F2 -isoprostane family, IPF2α -I. Similar to our observations with 8-epi-PGF2α , SLE patients had urinary levels of IPF2α -I, higher than controls (median, 1,252; range, 449 to 2,400 pg/mg creatinine v median, 470; range, 225 to 710 pg/mg creatinine; P < .0001). Excretion of the isoprostanes in patients with SLE was highly correlated (Rho = 0.74; P < .0001; Fig 4). IPF2α -I exhibited the same pattern of the other isoprostane in the respect of aCL, aPL, and TNF (data not shown).
Linear regression analysis of the two isoprostanes excreted in the urine of patients with systemic lupus erythematosus (r = .74).
Linear regression analysis of the two isoprostanes excreted in the urine of patients with systemic lupus erythematosus (r = .74).
DISCUSSION
SLE is an autoimmune disease of unknown cause.23 Coincidence of this condition with the detection of aPL in the circulation confers a striking risk of venous as well as arterial thrombotic events and fetal wastage.1-3,6 It is unknown whether this represents a direct causative effect of aPL or its association with an unknown risk factor. There is some evidence to suggest that aPL may modify procoagulant proteins and/or interfere with the anticoagulant function of endothelium.2 The nature of aPL is currently being explored with the objective of addressing its functional importance in thrombogenesis.
Hörkko et al have recently provided evidence that aPL are directed against epitopes of oxidized phospholipids and suggested that aPL may result from phospholipid oxidation.7
To test this hypothesis in vivo, we measured the urinary excretion of 8-epi-PGF2α in SLE patients with or without aPL positivity with the aim of assessing if there was a relationship between 8-epi-PGF2α and aPL. 8-epi-PGF2α was used as a marker of lipid peroxidation because it is elevated in clinical settings associated with oxidant stress11-13 and is generated during low density lipoproteins oxidation in vitro in temporal correlation with formation of lipid peroxides.18 24
We found that urinary 8-epi-PGF2α excretion was higher in patients with SLE than in age- and gender-matched controls. However, within the patients, those positive for aPL had higher levels of the isoprostane. Indeed, whereas 82% of the SLE patients who were aPL positive had levels of urinary 8-epi-PGF2α above the upper bound of 95% confidence interval for its excretion in healthy individuals (154 pg/mg creatinine), only 16% of the aPL-negative SLE patients fell into this category. Urinary excretion of the compound also correlated closely with the absolute levels of aCL. This observation, given the mechanism of formation of isoprostanes,8,9,25 is consistent with the hypothesis that aPL is directed against oxidized epitopes in phospholipids. Oxidant stress may characterize inflammatory episodes in autoimmune diseases such as SLE.26 Furthermore, we have shown that monocytes may generate 8-epi-PGF2α in response to inflammatory stimuli in vitro and have immunolocalized the compound to these cells in situ in human atherosclerotic plaque.18,27 Thus, it is of interest that the levels of 8-epi-PGF2α excretion correlated with circulating TNF, which is generated by activated monocytes and is elevated in the active phase of the disease.28 29
We have previously shown that COX enzymes exhibit a minor capacity to generate 8-epi-PGF2α , but no other isoprostanes.17,18 However, this pathway appears to make a trivial contribution of overall biosynthesis of the compound as reflected by its excretion in urine even in syndromes of COX activation.11 In the present study we showed increased urinary excretion of IPF2α -I, a second isoprostane that is formed solely in a free radical dependent manner, and the excretion of 8-epi-PGF2α was highly correlated with that of IPF2α -I. Finally, 22 patients were also taking steroids and methotrexate. The possibility that these drugs can influence the level of the isoprostanes measured cannot be excluded, but in a preliminary study of patients with rheumatoid arthritis who were receiving steroids and methotrexate, F2 -isoprostanes excretion did not differ significantly from that of healthy controls.30
We also analyzed whether there was a relationship between lipid peroxidation and thrombosis. We did not find any difference in isoprostane levels in patients with and without previous thrombosis. However, the small cohort investigated did not allow us to reach definitive conclusion. Therefore, further study is necessary to analyze this issue.
Thus, elevated levels of both isoprostanes in the aPL-positive patients are consistent with the hypothesis that lipid peroxidation may underlie the antiphospholipid syndrome. However, further prospective study is necessary to clear-cut establish whether a cause-effect relationship exists between lipid peroxidation and aPL in vivo.
Supported in part by the Consiglio Nazionale delle Ricerche (Grant No. 06152, 95.02298.04 to L.I.) and by the National Institute of Health (Grant No. HL54500 to G.A.F.)
Address reprint requests to Francesco Violi, MD, Institute of Clinical Medicine I, University “La Sapienza,” 00185 Rome, Italy.

![Fig. 1. Scattergram showing the difference (P < .0001) in 8-epi-PGF2α values in SLE patients and controls and the higher 8-epi-PGF2α values (P < .001) in aPL positive [aPL (+)] patients than in negative [aPL (−)] ones.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/10/10.1182_blood.v90.10.3931/3/m_bl_0031f1.jpeg?Expires=1769136499&Signature=BxEUc081BKsG~lwspQEzA2VkjX2w6wtkTE6I7s5B2Fl7aJEhdd5B~J8-~4EiT6EhsBP05CvqvHr9TX61L2WXa9uJTmQJbEW~eHtIR52Z12B1~FrR3euOxWkEYsCKk~rEHcdy~BZlE3FsS2O82lVwnY0Pi4avrMfU1hvVEEkq9zOgi15Sox0-VheaPDPmCX5HSP8Jzn2dDfn6vXSNQVoRUAzKKMvMjGMuPOhWLrinWL2wmd~Zkk4wOfI4MyOWMoTf164qW50mqUjuuJVN3eLhi-A5FxLb7d-E099kOTeelcS5sfaYqLHlglGItnbgGSAVazvCo8SVq05Jb5XfSMmJEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal