Abstract
We describe the phenomenon of waning of focal hepatic and/or splenic lesions on abdominal computed tomographic (CT) scan during neutropenia in patients with chronic disseminated candidiasis. After observation of the phenomenon in one patient, a total of five cases were prospectively monitored with serial CT scans. After the diagnosis of disseminated candidiasis, hepatic lesions decreased in size and conspicuousness in three patients, while in two others they disappeared completely during a subsequent chemotherapy-induced neutropenia. After recovery of the neutrophils, the lesions reappeared or increased in conspicuousness in all five patients. Of three patients treated with a second cycle of myeloablative chemotherapy, lesions again decreased in two patients during neutropenia and increased again in one patient after neutrophil recovery. In all five patients, candidiasis eventually resolved after prolonged antifungal treatment. In chronic disseminated candidiasis, hepatic or splenic lesions may transiently disappear during neutropenia. Thus, antifungal therapy should not be discontinued on the basis of radiologic findings alone.
THE SYNDROME OF CHRONIC disseminated candidiasis (synonyms: hepatosplenic candidiasis, hepatic candidiasis, focal hepatic candidiasis) is a well-described complication of prolonged neutropenia in patients treated for hematologic malignancies.1-4 It is well known that focal hepatic and/or splenic lesions become visible only when the neutrophil count recovers.1 3 Here, we report five cases in which these focal lesions not only appeared once neutrophils recovered, but then decreased or disappeared during subsequent phases of neutropenia.
MATERIALS AND METHODS
Between January 1, 1989, and June 30, 1995, a total of 142 patients with acute leukemia have been treated with myeloablative chemotherapy at our institution. In 13 cases, a diagnosis of disseminated candidiasis with hepatic involvement was made when neutropenia had resolved. In five of 13 patients, we were able to follow prospectively the evolution of radiologic signs of hepatic and/or splenic lesions during subsequent phases of neutropenia due to further myeloablative chemotherapy.
Characteristics of these five patients are listed in Table 1. Their age ranged from 18 to 47 years. There were four women and one man. Three patients had acute myelogenous leukemia (AML), while two had acute lymphoblastic leukemia (ALL). Disseminated candidiasis was diagnosed after their first or second cycle of induction chemotherapy and was not considered to be a contraindication to continue myeloablative chemotherapy. After diagnosis of disseminated candidiasis, the patients underwent one or two further cycles of myeloablative treatment (Table 1). Despite this treatment, all patients eventually were cured of candidiasis. Thus, these five cases support the concept that myeloablative chemotherapy can be given safely to patients with hepatosplenic candidiasis.5
Diagnosis and Outcome of Disseminated Candidiasis
| Patient . | Age, Sex, Disease . | CT Scan Dx* . | Smear . | Histology . | No. of Cycles† Before Dx . | No. of Cycles† After Dx . | Treatment and Outcomeρ of Disseminated Candidiasis . |
|---|---|---|---|---|---|---|---|
| A | 42, F, AML | Liver | Neg (3×) | Neg | 1 | 21-155 | Ampho 107 d, Fluc 7 d, Ampho 101 d, Fluc 225 d; no recurrence thereafter for 34+ mo |
| Spleen | — | — | |||||
| B | 18, F, ALL | Liver | Pos | ND | 2 | 1 | Ampho 11 d, Fluc 58 d, Ampho 21 d, Fluc 121 d; no recurrence thereafter for 31+ mo |
| C | 41, F, ALL | Liver | Pos | Consistent | 2 | 1 | Ampho 41 d, Fluc 147 d; no recurrence thereafter for 7 mo |
| Spleen | — | — | |||||
| D | 47, F, AML | Liver | Neg | Consistent | 2 | 2 | Ampho 23 d, Fluc 17 d, Ampho 28 d, Fluc 102 d; no recurrence thereafter for 17+ mo |
| E | 26, M, AML | Liver | Neg | Pos | 1 | 2¶ | Ampho 22 d, Fluc 363 d; no recurrence thereafter for 10+ mo |
| Spleen | — | — |
| Patient . | Age, Sex, Disease . | CT Scan Dx* . | Smear . | Histology . | No. of Cycles† Before Dx . | No. of Cycles† After Dx . | Treatment and Outcomeρ of Disseminated Candidiasis . |
|---|---|---|---|---|---|---|---|
| A | 42, F, AML | Liver | Neg (3×) | Neg | 1 | 21-155 | Ampho 107 d, Fluc 7 d, Ampho 101 d, Fluc 225 d; no recurrence thereafter for 34+ mo |
| Spleen | — | — | |||||
| B | 18, F, ALL | Liver | Pos | ND | 2 | 1 | Ampho 11 d, Fluc 58 d, Ampho 21 d, Fluc 121 d; no recurrence thereafter for 31+ mo |
| C | 41, F, ALL | Liver | Pos | Consistent | 2 | 1 | Ampho 41 d, Fluc 147 d; no recurrence thereafter for 7 mo |
| Spleen | — | — | |||||
| D | 47, F, AML | Liver | Neg | Consistent | 2 | 2 | Ampho 23 d, Fluc 17 d, Ampho 28 d, Fluc 102 d; no recurrence thereafter for 17+ mo |
| E | 26, M, AML | Liver | Neg | Pos | 1 | 2¶ | Ampho 22 d, Fluc 363 d; no recurrence thereafter for 10+ mo |
| Spleen | — | — |
Abbreviations: Dx, diagnosis; F, female; M, male; Neg, negative; Pos, positive; ND, not determined; Ampho, amphotericin B; Fluc, fluconazole.
Diagnosis of disseminated candidiasis was based on clinical, laboratory, radiologic, pathologic, and microbiologic findings (see text).
Number of chemotherapy cycles with prolonged neutropenia before and after the diagnosis of hepatic candidiasis is listed.
ρ Treatment duration in days is listed chronologically for amphotericin B and fluconazole (see text).
In addition to their 2 cycles of chemotherapy, both patients A and E later underwent alloBMT (see text).
Chronic disseminated candidiasis was suspected on the basis of clinical and laboratory findings. Right upper abdominal discomfort was present in one patient, persistent fever after neutrophil recovery in three patients, elevated transaminases in two patients (maximum, 2.7 times upper normal limit), elevated alkaline phosphatase level in four patients (maximum, five times upper normal limit), and elevated C-reactive protein level in all five patients (maximum, 16 times upper normal limit). A tentative diagnosis was made when multiple round lesions in the liver and/or the spleen were seen on a computed tomographic (CT) scan after recovery from prolonged severe neutropenia. In all patients, confirmation of diagnosis was attempted with a biopsy (Table 1). Fine-needle aspiration of liver lesions confirmed the diagnosis of disseminated candidiasis in two cases: patient B had fungal pseudohyphae, while patient C had budding yeast forms visible on the smear. In four patients, laparoscopic biopsy of liver lesions was performed and confirmed the diagnosis in another two patients (patients D and E). In patient E, necrosis and budding yeast forms were found. In patients C and D, inflammatory infiltrates consistent with candidiasis were seen. All aspirate and biopsy specimens were sent for fungal culture, none were found positive.
In summary, three patients (B, C, and E) had pathologic proof of the diagnosis of candidiasis. In these three patients, antifungal therapy was initiated on the day of biopsy. One patient (D) had histologic evidence consistent with candidiasis. In this patient, antifungal therapy had been started 2 days before biopsy. In patient A, empirical antifungal therapy was initiated at a time when no liver lesions were evident, which may have contributed to the negative findings. Specifically, fine-needle aspirations were performed 16, 34, and 72 days and laparoscopic liver biopsy 77 days after amphotericin B had been initiated. Diagnosis of candidiasis in patient A is based on the typical clinical course (see Discussion).
After initial diagnosis, candidiasis was treated with amphotericin B as long as the patient was hospitalized, and with oral fluconazole after recovery from neutropenia and after discharge.6,7 At readmission for subsequent myeloablative chemotherapy, antifungal treatment was switched to amphotericin B again. After discharge from the last cycle of chemotherapy, fluconazole was continued until serum chemistries (C-reactive protein, alkaline phosphatase, and transaminases) had normalized and until lesions on CT scan had completely resolved.5 This requirement led to treatment durations of several months after recovery from the last neutropenic period. Specifically, antifungal treatment was continued for 107 to 152 days for patients B, C, and D, who did not undergo allogeneic bone marrow transplantation (alloBMT) (Table 2).
Evolution of Lesions on CT Scan During and After Neutropenia
| Patient . | Organs* Involved . | First Cycle† . | Second Cycle† . | Long-Termρ . | ||
|---|---|---|---|---|---|---|
| . | . | During Neutropenia‡ . | After Neutropenia‡ . | During Neutropenia‡ . | After Neutropenia‡ . | . |
| A | Liver, spleen | Disappearance (6 d) | Reappearance (46 d) | Decrease (15 d) | Increase (35 d) | Disappearance (230 d, 94 d) |
| B | Liver | Decrease (16 d) | Increase (31 d) | NA | NA | Disappearance (129 d) |
| C | Spleen, liver | Disappearance (9 d) | Reappearance (7 d) | NA | NA | Disappearance (152 d) |
| D | Liver | Decrease (11 d) | Increase (18 d) | Decrease (12 d) | Unchanged (21 d) | Disappearance (107 d) |
| E | Liver, spleen | Decrease (16 d) | Increase (20 d) | Unchanged (11 d) | Decrease (51 d) | Disappearance (135 d, 17 d) |
| Patient . | Organs* Involved . | First Cycle† . | Second Cycle† . | Long-Termρ . | ||
|---|---|---|---|---|---|---|
| . | . | During Neutropenia‡ . | After Neutropenia‡ . | During Neutropenia‡ . | After Neutropenia‡ . | . |
| A | Liver, spleen | Disappearance (6 d) | Reappearance (46 d) | Decrease (15 d) | Increase (35 d) | Disappearance (230 d, 94 d) |
| B | Liver | Decrease (16 d) | Increase (31 d) | NA | NA | Disappearance (129 d) |
| C | Spleen, liver | Disappearance (9 d) | Reappearance (7 d) | NA | NA | Disappearance (152 d) |
| D | Liver | Decrease (11 d) | Increase (18 d) | Decrease (12 d) | Unchanged (21 d) | Disappearance (107 d) |
| E | Liver, spleen | Decrease (16 d) | Increase (20 d) | Unchanged (11 d) | Decrease (51 d) | Disappearance (135 d, 17 d) |
Abbreviation: NA, not applicable.
The organ with more visible lesions is listed first.
“First cycle” and “second cycle” refer to the first cycle and the second cycle after the diagnosis of hepatic candidiasis. The total number of chemotherapy cycles with prolonged neutropenia before and after the diagnosis of hepatic candidiasis is listed in Table 1.
“During neutropenia” refers to lesions on a CT scan performed while the patient was neutropenic. The number of days of severe neutropenia (<500/μL) passed at the time the scan was performed is listed in parentheses. “After neutropenia” refers to lesions on a CT scan performed after recovery from severe neutropenia. The number of days from recovery of severe neutropenia (>500/μL) until the scan was performed is listed in parentheses.
ρ “Long-term” refers to the first CT scan on which all lesions had disappeared. The number of days from recovery of the last period of neutropenia until the scan was performed is listed in parentheses. Patients A and E underwent alloBMT 6 and 3 months after their last chemotherapy. For them, both the duration from the last chemotherapy-induced, as well as from alloBMT-induced neutropenia, is listed. In both patients, hepatic lesions were visible at admission for alloBMT and disappeared later. No CT scans were performed during alloBMT.
Persistent evidence of candidiasis was not considered a contraindication to alloBMT, as described in the literature.8 The two patients (A and E) readmitted for alloBMT were not switched to amphotericin B, but instead continued on fluconazole to avoid excessive renal toxicity. In both patients, fluconazole was continued successfully throughout the procedure and thereafter for 6 months until the discontinuation of all immunosuppressive therapy. At that time, both patients had normal CT scans (Table 2). Thus, these two patients lend support to the concept that disseminated candidiasis under fluconazole treatment is not a contraindication for alloBMT.
The sequence and duration of treatments in individual patients are listed in chronologic order in Table 1. Outcome was excellent, without recurrence of fungal disease in all five cases.
CT examinations were performed using the helical technique (Somatom Plus and Somatom Plus 4; Siemens Medizin Technik, Erlangen, Germany) before and after bolus injection (3 mL/s uniphasic injection) of 150 mL of nonionic contrast medium (Imagopaque [Guerbet, Paris, France] or Ultravist [Schering AG, Berlin, Germany]). Examinations consisted of a precontrast (table speed, 8 mm/s; collimation, 8 mm) and postcontrast (table speed, 5 mm/s; collimation, 5 mm) volume scanning of the entire liver with a scan delay of 45 seconds in order to achieve good visualization of the portal enhancement phase. An oral contrast medium was administered 45 minutes before the examination for better visualization of bowel loops. All CT images were evaluated prospectively by an experienced radiologist (G.P.K.) without knowledge of neutrophil counts.
Oncologic outcome is not the focus of this study, but is briefly mentioned here. Patient A was treated with alloBMT 1 year after diagnosis of AML in first complete remission (CR). Three years later, she is still in continued CR (CCR). Patient B suffered a CNS and systemic relapse 28 months after diagnosis of ALL. She has achieved a second CR, now lasting 8 months. Patient C died of relapsed ALL at 15 months. Patient D is in CCR at 25 months from diagnosis. Patient E underwent alloBMT in first remission 7 months after diagnosis of AML. He is in CCR 17 months later.
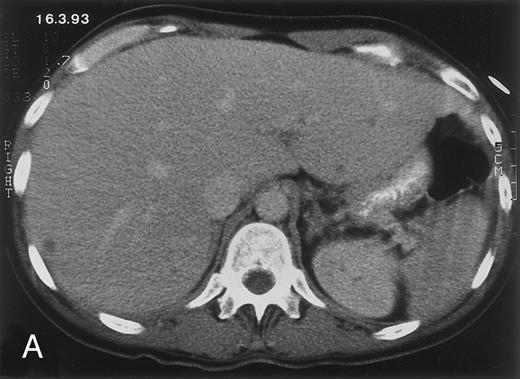
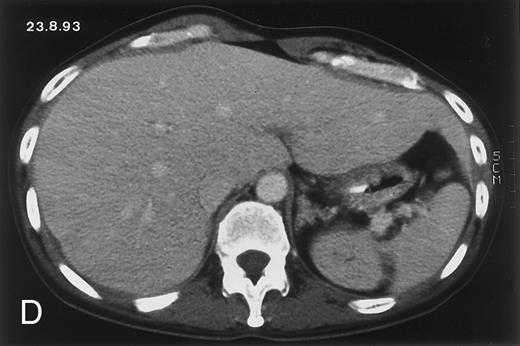
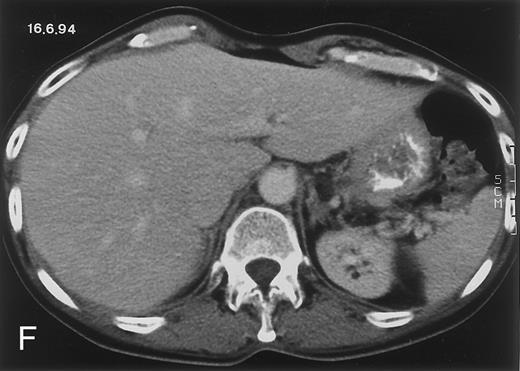
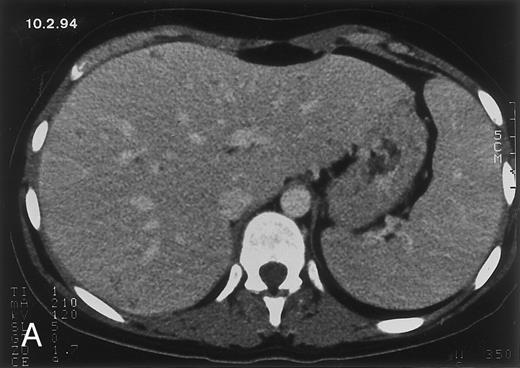
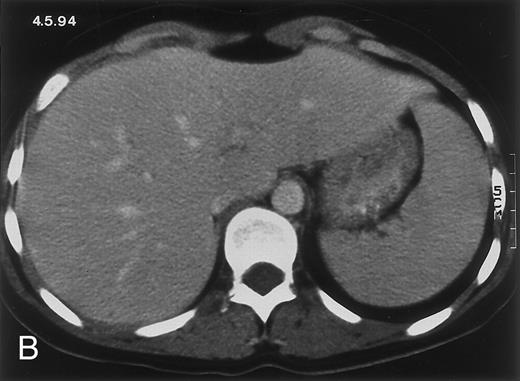
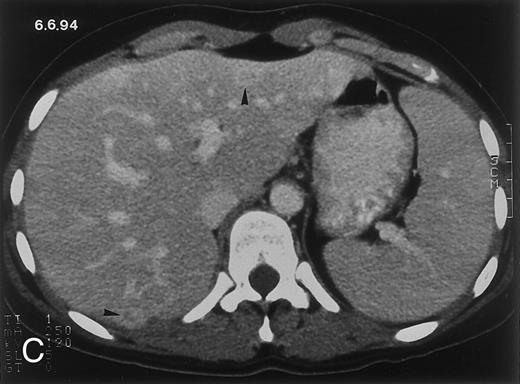
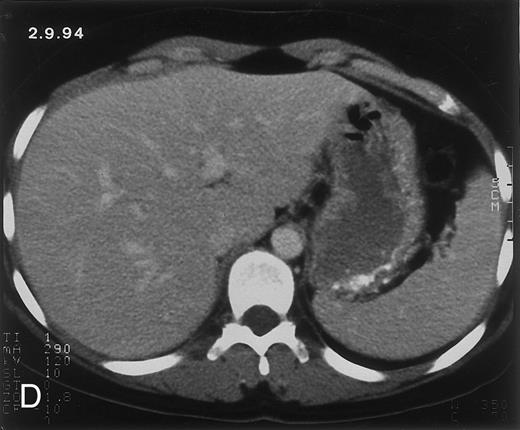
Radiologic evolution in patient A. (A) Postcontrast abdominal CT scan on March 16, 1993 shows multiple small hypodense areas in all segments of the liver, suggestive of hepatic candidiasis. Seven days previously, the patient had recovered from severe neutropenia after the first cycle of chemotherapy for AML. (B) On April 23, 6 days after the neutrophil count had dropped below 500/μL in the second cycle of chemotherapy, there is no evidence of focal lesions on postcontrast CT scan. (C) On June 18, when neutrophils were normalized for 46 days, CT scan again shows multiple small abscesses.
(D) On August 23, 15 days after the neutrophil count had dropped below 500/μL in the third chemotherapy cycle, focal liver lesions clearly decreased in size and number. (E) On September 30, 35 days after neutrophil recovery, multiple hepatic abscesses are seen (arrowhead). To show maximal extent of lesions the scan shown is at a slightly different level. (F) On June 16, 1994, 230 days after neutrophil recovery, the CT scan has normalized.
Radiologic evolution in patient A. (A) Postcontrast abdominal CT scan on March 16, 1993 shows multiple small hypodense areas in all segments of the liver, suggestive of hepatic candidiasis. Seven days previously, the patient had recovered from severe neutropenia after the first cycle of chemotherapy for AML. (B) On April 23, 6 days after the neutrophil count had dropped below 500/μL in the second cycle of chemotherapy, there is no evidence of focal lesions on postcontrast CT scan. (C) On June 18, when neutrophils were normalized for 46 days, CT scan again shows multiple small abscesses.
(D) On August 23, 15 days after the neutrophil count had dropped below 500/μL in the third chemotherapy cycle, focal liver lesions clearly decreased in size and number. (E) On September 30, 35 days after neutrophil recovery, multiple hepatic abscesses are seen (arrowhead). To show maximal extent of lesions the scan shown is at a slightly different level. (F) On June 16, 1994, 230 days after neutrophil recovery, the CT scan has normalized.
Radiologic evolution in patient B. (A) Postcontrast abdominal CT scan on February 10, 1994 shows multiple small hypodense areas in all segments of the liver suggestive of hepatic candidiasis. Twenty-four days previously, the patient had recovered from severe neutropenia after the second cycle of chemotherapy for ALL. (B) On May 4, 16 days after the neutrophil count had dropped below 500/μL in the third chemotherapy cycle, there is clear improvement of the radiologic findings, with only a few hypodense areas in the liver.
(C) On June 6, 31 days after normalization of neutrophils, CT again shows multiple lesions in the liver (arrowheads) with a different presentation: the small lesions show strong peripheral enhancement. (D) On September 2, 129 days after neutrophil recovery, all hepatic lesions have completely resolved.
Radiologic evolution in patient B. (A) Postcontrast abdominal CT scan on February 10, 1994 shows multiple small hypodense areas in all segments of the liver suggestive of hepatic candidiasis. Twenty-four days previously, the patient had recovered from severe neutropenia after the second cycle of chemotherapy for ALL. (B) On May 4, 16 days after the neutrophil count had dropped below 500/μL in the third chemotherapy cycle, there is clear improvement of the radiologic findings, with only a few hypodense areas in the liver.
(C) On June 6, 31 days after normalization of neutrophils, CT again shows multiple lesions in the liver (arrowheads) with a different presentation: the small lesions show strong peripheral enhancement. (D) On September 2, 129 days after neutrophil recovery, all hepatic lesions have completely resolved.
RESULTS
Evolution of fungal lesions on CT scans during and after neutropenia is summarized in Table 2. Figure 1 (patient A) and Fig 2 (patient B) serve to illustrate Table 2.
In three patients, multiple round lesions were seen on postcontrast CT scans of the abdomen in the liver and the spleen, while in two cases, lesions could only be seen in the liver (Table 2). Following the diagnosis of disseminated candidiasis, these lesions decreased in size and visibility during subsequent neutropenia in three patients (compare Fig 2A and B) and disappeared completely in two patients (compare Fig 1A and B). The numbers of days of severe neutropenia (neutrophils <500/μL) at the time the CT scan was performed are listed in Table 2. After recovery from neutropenia and despite continued antifungal therapy, the size and visibility of the lesions increased again (Fig 1C). Similarly, the number of days since recovery from severe neutropenia (neutrophils >500/μL) are listed in Table 2. In two of three patients treated with another cycle of myeloablative chemotherapy, this waxing and waning pattern of radiologic lesions could be seen again (Fig 1D and E). In all patients, lesions eventually disappeared (Figs 1F and 2D) after prolonged antifungal therapy.
Two patients (A and E) underwent alloBMT before resolution of lesions and while still on antifungal treatment. For logistic reasons, no CT scans were performed during the procedure. It is noteworthy that in one patient (E), visible fungal lesions had disappeared at the end of alloBMT, while in the other patient (A), lesions disappeared 3 months later (Table 2).
DISCUSSION
This is the first report to describe the phenomenon of disappearance of hepatic and/or splenic lesions in chronic disseminated candidiasis. It occurs when patients reenter neutropenia in a subsequent cycle of myeloablative therapy. When neutrophils recover, these lesions reappear. Thus, candidiasis is still present and antifungal treatment needs to be continued. Therefore, for correct interpretation of the radiologic findings in disseminated candidiasis, they have to be correlated with the evolution of the neutrophil count.
We have monitored five patients prospectively with repetitive abdominal CT scans during subsequent myeloablative treatments. We found that focal hepatic and/or splenic lesions disappeared or substantially decreased in size and conspicuousness after 6 to 16 days of neutropenia (neutrophils <500/μL). This observation is in concordance with the well-described phenomenon that these focal lesions become visible on imaging only once the neutrophil count recovers.1 3 Similarly, we found that the lesions reappeared or increased again at 7 to 46 days after neutrophil recovery. In two of three patients who underwent another cycle of myeloablative treatment, a waxing and waning pattern of the visibility of radiologic lesions was documented.
It has been shown by biopsy and autopsy studies that a focus of hepatic candidiasis becomes encircled and walled off by neutrophils and other inflammatory cells.3,9 This is associated with better visibility and sometimes the typical target-type, bull's eye, or wheel-within-wheel appearance of lesions on imaging studies.9 To our knowledge, the phenomenon of decreasing visibility or disappearance of lesions during subsequent neutropenia has not been previously described. To explain this observation, one can speculate that the renewed absence of neutrophils might lead to a breakdown of the cellular containment of the fungus and allow for its reactivation and spread into adjacent tissues, thus blurring the boundary with neighbouring tissue. The clinician might be fooled to believe that he has eradicated the fungus, while in reality it is spreading. It is possible that other factors than neutropenia or a combination of host and fungal factors influence the visibility of the radiologic lesions. In our opinion, the observation of an undulating visibility pattern with a clear temporal relationship to the neutrophil count makes this the most probable explanation of this phenomenon.
Patients A and E who underwent alloBMT merit additional discussion. In both of them, hepatic lesions were visible at admission for alloBMT, disappeared at discharge (patient E) or soon thereafter (patient A), and did not recur. This apparently speedier resolution of lesions after alloBMT than after chemotherapy might be related to host factors specific for alloBMT like graft-versus-host disease (GVHD) or venoocclusive disease (VOD). Patient A had only grade 1 acute GVHD, while patient E had no GVHD at all. Neither patient had evidence of VOD. On a purely speculative note, one might think that the graft could have had some antiinfectious effect. A more likely explanation for the disappearance of lesions soon after alloBMT is that it was due to the simple passage of time under continued antifungal treatment.
Diagnosis of disseminated candidiasis is often difficult, because cytologic, histologic, and microbiologic findings may remain negative.1 3 Biopsy confirmation of diagnosis was achieved in four of five cases. The initiation of antifungal treatment before biopsy in patient A may have contributed to the negative pathologic findings. Nevertheless, the typical presentation with right upper abdominal discomfort, persistent fever after resolution of prolonged neutropenia, elevated C-reactive protein level, and typical multiple lesions on CT scan (Fig 1A), as well as the disease course with eventual total disappearance of lesions under antifungal treatment, was sufficient evidence to establish the diagnosis in patient A.
In conclusion, in chronic disseminated candidiasis antifungal therapy should not be discontinued on the basis of imaging studies alone and not before neutrophil recovery has occurred and lasted for a few weeks.
Address reprint requests to Bernhard C. Pestalozzi, MD, Division of Oncology, Department of Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal