Abstract
Intensive chemotherapy followed by autologous bone marrow transplantation (ABMT) may provide an alternative therapy for young patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia following MDS (sAML) lacking a suitable donor. We report the results for 79 patients with MDS/sAML transplanted with autologous marrow in first complete remission (CR). Within the total group of 79, a cohort of 55 patients for whom the duration of first CR was known were compared with a matched control group of 110 patients with de novo AML. The 2-year survival, disease-free survival (DFS), and relapse rates for the 79 patients transplanted in first CR were 39%, 34%, and 64%, respectively. The relapse risk was greater than 55% for all stages and all disease categories. Patients younger than 40 years had a significantly (P = .04) better DFS (39%) than patients older than 40 years (25%). The DFS at 2 years was 28% for the cohort of 55 patients transplanted for MDS/sAML and 51% for those transplanted for de novo AML (P = .025). Relapse rates were 69% for patients with MDS/sAML and 40% for those with de novo AML (P = .007). ABMT for MDS or secondary leukemia results in a lower DFS when compared with similarly treated patients with de novo AML due to a higher relapse rate. The DFS of 28% for these patients suggests that autotransplantation may be a valuable therapy for this disease. The low treatment-related mortality rate of less than 10% supports the view that sufficient numbers of hematopoietic stem cells are present in patients with MDS to allow adequate repopulation after autologous stem-cell transplantation.
THE MYELODYSPLASTIC syndromes (MDSs) are a heterogeneous group of disorders with a variable prognosis. The outlook of patients with refractory anemia with excess of blasts (RAEB), refractory anemia with excess of blasts in transformation (RAEBt), therapy-related MDS, or secondary acute myeloblastic leukemia (sAML; secondary leukemia) is poor, with median survival times of less than 12 months.1,2 If patients with these disorders are treated with intensive chemotherapy, approximately 50% to 60% will achieve complete remission (CR), but the median remission duration is less than 8 months despite maintenance chemotherapy.1,3 Young patients can be treated successfully by allogeneic bone marrow transplantation (BMT), if a histocompatible sibling donor is available.4-7 Intensive chemotherapy followed by autologous BMT (ABMT) may provide an alternative therapy for patients who lack a suitable donor. To date, ABMT has been reported for a small number of patients.8-11
The Chronic Leukemia Registry of the European Group for Blood and Marrow Transplantation contained 114 reports of patients autografted for MDS or secondary leukemia. We wished to use this data to determine the feasibility of ABMT in these patients by assessing overall survival, disease-free survival (DFS), transplant-related mortality, and relapse risk for patients treated by ABMT as consolidation treatment in first CR. In addition, we studied the effect of various prognostic factors, eg, stage and nature of disease and age of patient, on outcome of transplant.
In a second analysis, we wished to compare the outcomes of ABMT for MDS and secondary leukemia with that seen in patients who receive autologous transplants for de novo AML. The interval between attainment of CR and transplant may differ in these two groups of patients, with the physician less likely to proceed to early transplant in patients with MDS. The interval between first CR and BMT is known to be a prognostic factor in AML,12 but this information was not available for all patients reported to the registry. We therefore restricted this analysis to the 55 patients for whom this information was provided, and compared their outcome with that of 110 patients transplanted in first CR for de novo AML.
MATERIALS AND METHODS
Data were retrieved from the registries of the Chronic and Acute Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. These registries continuously collect data related to patients transplanted by member teams. Reports were available for 114 patients from 41 centers. Seventy-nine of these patients were transplanted in first CR: 19 for RAEB or RAEBt, 39 for secondary leukemia, and 21 for MDS or secondary leukemia due to previous cytotoxic therapy. The median age for the 79 patients was 39 years (range, 0 to 63). Forty-five of 79 patients were aged less than 40 at the time of transplant (Table 1). The median interval between diagnosis and BMT was 9 months (range, 2 to 42) for 19 patients with MDS and 7 months (range, 3 to 23) for patients transplanted for sAML or therapy-related MDS/AML. Cytogenetic data were only available for 18 patients. Seventy-three patients received bone marrow only, three patients were treated with collected peripheral stem cells, and three patients received both. None of the stem-cell grafts was subjected to purging procedures.
Patients With MDS, sAML, or Therapy-Related MDS/Leukemia Treated With ABMT in First CR
| Patients transplanted in first CR | 79 |
| Disease category | |
| MDS | 19 |
| sAML | 39 |
| Therapy-related MDS/leukemia | 21 |
| Age (yr) | |
| <40 | 45 |
| ≥40 | 34 |
| Status | |
| Alive | 36 |
| Relapse-free alive | 32 |
| Relapse | 40 |
| Dead | 43 |
| Treatment-related death | 7 |
| Patients transplanted in first CR | 79 |
| Disease category | |
| MDS | 19 |
| sAML | 39 |
| Therapy-related MDS/leukemia | 21 |
| Age (yr) | |
| <40 | 45 |
| ≥40 | 34 |
| Status | |
| Alive | 36 |
| Relapse-free alive | 32 |
| Relapse | 40 |
| Dead | 43 |
| Treatment-related death | 7 |
For the matched case-control analysis, we selected 55 patients with MDS or secondary leukemia for whom information was available on the interval from CR to transplant, and compared them with 110 patients treated by ABMT in first CR for de novo AML, and reported to the Acute Leukemia Working Party Registry. This registry contains data on approximately 1,000 patients treated in this way. Patients were matched for age (<40 v ≥40 years), interval from first CR (±1 month), and year of transplant.
Definitions.The classification of MDS and AML was performed according to the criteria of the French-American-British (FAB) working group.13 AML that develops after antecedent MDS with a duration of at least 3 months is defined as sAML (secondary leukemia). MDS or AML occurring after chemotherapy or radiotherapy is defined as therapy-related MDS or AML.
CR is defined as a normocellular marrow with less than 5% blast cells, including monocytoid cells, and less than 10% blast cells and promyelocytes. Peripheral blood counts should be in the normal range.
Statistics.The intervals for survival, DFS, relapse rate, and risk of transplant-related mortality were calculated from BMT. Relapsed patients were censored for transplant-related mortality from time of relapse. The prognostic value of covariables was studied by Kaplan-Meier curves and the log-rank test. The matched control study was analyzed with the stratified log-rank test.
RESULTS
Seventy-nine patients transplanted in first CR were assessable (Table 1). Thirty-two patients (41%) are alive and disease-free. The median follow-up duration of 36 surviving patients was 10 months (range, 0 to 89 months) and 12 months (range, 0 to 89) for patients remaining in CR. Only seven patients died as a consequence of treatment and 36 after disease recurrence. Forty patients relapsed (51%). Fifteen patients are disease-free survivors more than 2 years after transplant. At 2 years post-ABMT, the overall survival, DFS, and relapse rates were 39%, 34%, and 64%, respectively.
The 2-year DFS rates for patients transplanted with MDS, secondary leukemia, and therapy-related MDS/sAML were 40%, 30%, and 36%, respectively. The relapse rates for these groups are 58%, 68%, and 60%, respectively. The treatment-related mortality rate in all three groups was less than 10% (Table 2). The overall survival, DFS, and relapse risk were not statistically significant different among these three groups of patients (Table 2). In contrast, the DFS of patients treated below the age of 40 years was significantly (P = .04) better (39%) than that for older patients (25%). This difference was mainly due to a significantly higher relapse rate (72%) in patients transplanted over the age 40 years than in younger patients (59%, P = .05).
Actuarial 2-Year Survival, DFS, Relapse Rate, and Transplant-Related Mortality of 79 Patients Transplanted in First CR With ABMT
| . | N . | Survival . | . | DFS . | . | Relapse . | . | TRM . | |
|---|---|---|---|---|---|---|---|---|---|
| Disease category | |||||||||
| MDS | 19 | 46% | 40% | 58% | 5% | ||||
| sAML | 39 | 34% | .68* | 30% | .61* | 68% | .60* | 5% | .77* |
| Postcytotoxic MDS or sAML | 21 | 41% | 36% | 60% | 10% | ||||
| Age (yr) | |||||||||
| <40 | 45 | 46% | 39% | 59% | 5% | ||||
| ≥40 | 34 | 27% | .09* | 25% | .04* | 72% | .05* | 9% | .46* |
| . | N . | Survival . | . | DFS . | . | Relapse . | . | TRM . | |
|---|---|---|---|---|---|---|---|---|---|
| Disease category | |||||||||
| MDS | 19 | 46% | 40% | 58% | 5% | ||||
| sAML | 39 | 34% | .68* | 30% | .61* | 68% | .60* | 5% | .77* |
| Postcytotoxic MDS or sAML | 21 | 41% | 36% | 60% | 10% | ||||
| Age (yr) | |||||||||
| <40 | 45 | 46% | 39% | 59% | 5% | ||||
| ≥40 | 34 | 27% | .09* | 25% | .04* | 72% | .05* | 9% | .46* |
Abbreviation: TRM, treatment-related mortality.
P value, log-rank test.
Matched case-control study.Fifty-five patients transplanted in first CR of MDS/sAML were compared with 110 patients transplanted in first CR of de novo AML. Patients were matched for age, year of transplant, and interval from CR to transplant (Table 3). Eighteen patients are disease-free survivors following treatment for MDS/sAML and 58 patients survive without leukemia relapse after transplant for de novo AML.
Comparison of MDS/sAML Study Group With Matched Control Group of Patients With De Novo AML
| . | Study Group . | Control Group . |
|---|---|---|
| No. of patients | 55 | 110 |
| Age (yr) | ||
| <40 | 28 | 56 |
| ≥40 | 27 | 54 |
| Sex | ||
| Male | 23 | 52 |
| Female | 32 | 58 |
| Year of transplant | ||
| 83-87 | 8 | 16 |
| 88-90 | 20 | 40 |
| 91-94 | 27 | 54 Interval, first CR to transplantation (mo) |
| Median | 4 | 4 |
| Range | 0-20 | 0-13 |
| Alive (%) | 20 (36) | 63 (57) |
| Relapse-free alive (%) | 18 (33) | 58 (53) |
| Relapse (%) | 31 (56) | 37 (34) |
| Dead (%) | 35 (64) | 47 (43) |
| Treatment-related death (%) | 6 (11) | 15 (14) |
| . | Study Group . | Control Group . |
|---|---|---|
| No. of patients | 55 | 110 |
| Age (yr) | ||
| <40 | 28 | 56 |
| ≥40 | 27 | 54 |
| Sex | ||
| Male | 23 | 52 |
| Female | 32 | 58 |
| Year of transplant | ||
| 83-87 | 8 | 16 |
| 88-90 | 20 | 40 |
| 91-94 | 27 | 54 Interval, first CR to transplantation (mo) |
| Median | 4 | 4 |
| Range | 0-20 | 0-13 |
| Alive (%) | 20 (36) | 63 (57) |
| Relapse-free alive (%) | 18 (33) | 58 (53) |
| Relapse (%) | 31 (56) | 37 (34) |
| Dead (%) | 35 (64) | 47 (43) |
| Treatment-related death (%) | 6 (11) | 15 (14) |
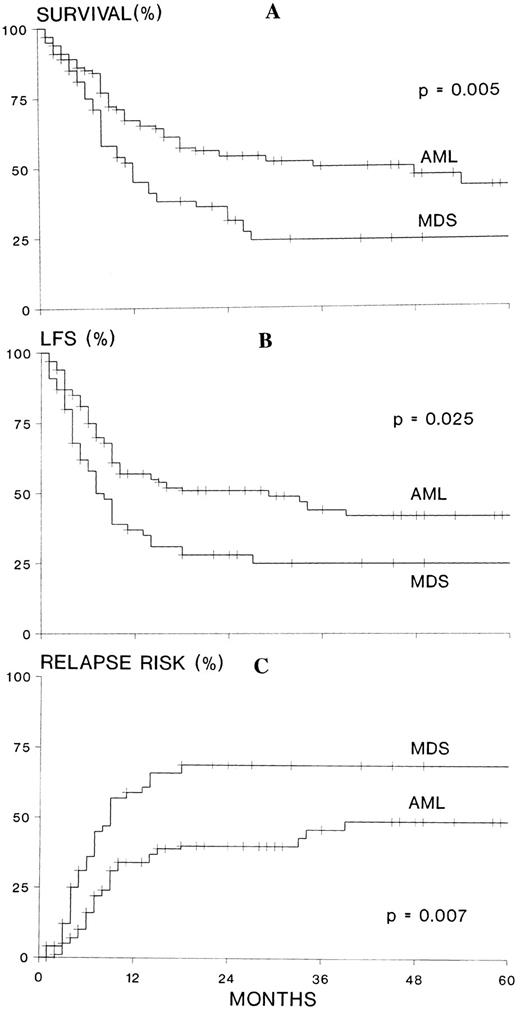
The survival at 2 years after ABMT was 31% for 55 patients with MDS/sAML and 54% for 110 patients with de novo AML (P = .005). The DFS was 28% and 51%, respectively (P = .025). The relapse rate was 69% for patients treated for MDS or secondary leukemia, and 40% for those transplanted for de novo AML (P = .007; Fig 1A through C). The treatment-related mortality rate was 9% for 55 patients transplanted for MDS/sAML and 14% for 110 patients transplanted for de novo AML (P = .99).
(A) Actuarial survival, (B) leukemia-free survival (LFS), and (C) relapse risk of 55 patients transplanted in first CR for MDS, sAML, or therapy-related MDS/leukemia compared with 110 matched patients transplanted in first CR for de novo AML. MDS refers to myelodysplastic syndromes, secondary AML, and therapy related MDS/AML; AML refers to de novo AML.
(A) Actuarial survival, (B) leukemia-free survival (LFS), and (C) relapse risk of 55 patients transplanted in first CR for MDS, sAML, or therapy-related MDS/leukemia compared with 110 matched patients transplanted in first CR for de novo AML. MDS refers to myelodysplastic syndromes, secondary AML, and therapy related MDS/AML; AML refers to de novo AML.
DISCUSSION
Allogeneic BMT is the treatment of choice in the majority of young patients with MDS or secondary leukemias. The results are gratifying, but the majority of patients lack a suitable donor.4-8 Patients with poor risk features who are below the age of 65 years may be candidates for treatment with combination chemotherapy.
Chemotherapy, such as that applied to induce CR in de novo AML, showed CR rates varying from 15% to 64%.1-3,14,15 The overall CR rates of patients with MDS or secondary leukemia are usually lower than those of patients with de novo AML treated with similar chemotherapy regimens. However, patients with RAEB, RAEBt, and de novo AML showed no difference in treatment outcome when matched for prognostic factors such as a history of cytopenias and cytogenetics.16-18 This may reflect a conflict in defining the distinction between MDS and de novo AML. A patient with the morphologic picture of RAEBt, but without cytogenetic abnormalities and a short history of cytopenias, may respond to intensive antileukemic therapy as an average patient with de novo AML. On the other hand, a patient with de novo AML, trilineage dysplasia, and a monosomy-7 may respond as an average patient with MDS or even worse.
Maintaining remission after remission-induction chemotherapy is a difficult issue. Patients who are not eligible for allogeneic BMT could be treated with postremission chemotherapy. Some patients may achieve prolonged DFS19,20 with this approach, but the overall median remission duration was usually less than 12 months.3,21,22 Patients without cytogenetic abnormalities appeared to have a better outcome after antileukemic chemotherapy compared with patients with cytogenetic abnormalities.1
The experience with ABMT in patients with MDS or leukemia secondary to MDS is limited and the literature contains only case reports.8,9 From a large series of 82 adult patients with AML, six patients with a known preceding myelodysplastic state received ABMT in first remission. Three patients relapsed after transplant, and the overall leukemia-free survival was worse than that of the group without an antecedent hematologic disorder.11 A preliminary analysis from the European Group for Blood and Marrow Transplantation reported the results of ABMT in 17 patients with MDS. Engraftment occurred in 15 of 16 assessable patients. The median relapse-free survival duration was 11 months.10 Transplant-related mortality and death due to regeneration failure did not appear to occur more often than after ABMT for de novo AML. Hematopoietic engraftment was slow despite the sufficient number of colony-forming units–granulocyte, macrophage (CFU-GM) being collected per kilogram body weight (5 × 104 kg).10 Laporte et al11 reported the results of ABMT with mafosfamide-treated marrow in patients with AML following MDS. The hematopoietic engraftment was also slow in these seven patients, but all patients engrafted except for one who died of treatment-related causes before engrafting.
MDSs are clonal stem-cell disorders. This may raise concern about the presence of sufficient numbers of residual normal stem cells to perform autologous stem-cell transplantation. However, chemotherapy is capable to induce a cytogenetically normal CR in patients with a cytogenetic marker of the malignant clone. Seven of eight patients with cytogenetic abnormalities in CR after chemotherapy appeared to have a cytogenetically normal bone marrow.1 In addition, polymerase chain reaction techniques based on X-chromosome inactivation patterns demonstrated polyclonality in the peripheral stem-cell harvests of three patients with MDS.23 In this retrospective analysis from the European Group for Blood and Marrow Transplantation registries, only seven of 79 patients transplanted in first CR died due to complications of the transplant. Moreover, the transplant-related mortality was not higher than the transplant-related mortality of the matched control group of patients transplanted for de novo AML in first CR. This would suggest that the engraftment capabilities of stem cells derived from patients with MDS are usually sufficient to restore hematopoiesis to levels that prevent fatal infectious and hemorrhagic complications. The administration of a higher number of stem cells, obtained by mobilization of the stem cells into the peripheral blood, may improve the speed of engraftment. Pilot studies showed the feasibility of collecting peripheral stem cells in patients in CR of MDS or sAML.24 25 The engraftment appeared to be much faster, but this approach is not likely to improve substantially the treatment-related mortality of autologous transplantation in MDS, since the mortality is already less than 10% after the use of autologous bone marrow stem cells.
The main reason for treatment failure was a high relapse risk after the autologous stem-cell procedure. The relapse risk was higher than 55% for all stages and all disease categories. Late relapses beyond 2 years were rare events, although only 15 patients were at risk beyond this time. Age less than 40 years at the time of transplant was associated with a significantly better prognosis, with a DFS of 39%, mainly due to a lower relapse risk in the younger age group. The lower relapse risk in the younger age group may be due to several factors, such as the duration of disease before treatment and the absence of cytogenetic abnormalities, which are associated with a poor outcome. The limited number of patients in the present study and the lack of cytogenetic data precluded an analysis on cytogenetic prognostic criteria. A recently completed joint study of the Leukemia Co-operative Group of the European Organization for Treatment and Research in Cancer and the European Group for Blood and Marrow Transplantation evaluated prospectively the role of autologous stem-cell transplantation in 185 patients with MDS and secondary leukemia.24 Preliminary results showed that cytogenetic characteristics had a major impact on treatment outcome. The actuarial 2-year survival of patients with good risk or intermediate risk was 52% versus 28% in the poor-risk group.24
ABMT for MDS and secondary leukemia resulted in a lower DFS than ABMT for de novo AML. This difference was mainly due to a higher relapse rate in the MDS/sAML group, since the mortality rate was low in both patient groups. The higher relapse rate in patients treated for MDS or secondary leukemia suggests a higher burden of residual disease in these patients. For that reason, it is important to monitor carefully residual disease in future studies both by cytogenetic techniques and by molecular techniques.
ABMT in first CR may be the treatment of choice for patients with MDS or sAML if a histocompatible sibling donor is lacking. Allogeneic BMT with a matched unrelated donor is associated with a substantially higher treatment-related mortality. Anderson et al26 described the results of 52 patients with MDS and sAML transplanted with an unrelated donor. The 2-year DFS, relapse, and nonrelapse mortality rates were 38%, 28%, and 48%, respectively. The incidence of the nonrelapse mortality was higher compared with HLA-identical related recipients. Increasing age was significantly associated with increased risk of death from nonrelapse causes. The nonrelapse mortality was 16% in patients younger than 20 years, 66% and in patients between 21 and 40 years, and 53% in patients older than 40 years.26 A substantial number of patients may not reach the autologous stem-cell transplant procedure due to failure to induce remission or failure to collect sufficient number of stem cells. Careful clinical evaluation of the prognostic factors, such as age, chance to achieve CR, and availability of a matched unrelated donor, should guide the treating physician in advising the patient of the available treatment options. Further analyses and prospective studies may identify patients with MDS who will benefit from intensive antileukemic therapy followed by autologous stem-cell transplantation.
ACKNOWLEDGMENT
The authors acknowledge the valuable contribution of all the transplant centers that reported their patients to the registries of the European Group for Blood and Marrow Transplantation.
Address reprint requests to Prof Theo De Witte, MD, Department of Hematology, University Hospital St Radboud, Nijmegen, Geert Grooteplein 8, 6525 GA Nijmegen, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal