Abstract
The feasibility of transplanting peripheral blood mononuclear cells (PBMC) from granulocyte colony-stimulating factor (G-CSF)–treated normal human donors to myeloablated allogeneic hosts has been demonstrated recently. The current work examined the ability of recombinant G-CSF to alter peripheral blood T-cell function and graft-versus-host disease (GVHD) in a murine model of allogeneic G-CSF–mobilized PBMC transplantation. Administration of recombinant G-CSF to C57BL/Ka mice markedly increased the capacity of PBMC to reconstitute lethally irradiated syngeneic hosts. T- and B-lineage lymphocytes were depleted about 10-fold in the bone marrow of the treated mice, and the T-cell yield in the blood was increased about fourfold. The ability of PBMC or purified CD4+ and CD8+ T cells to induce acute lethal GVHD in irradiated BALB/c mice was reduced after the administration of G-CSF. This was associated with decreased secretion of interferonγ and interleukin-2 (IL-2) and an increased secretion of IL-4. The donor cell inoculum, which was most successful in the rescue of irradiated allogeneic hosts, was the low-density fraction of PBMC from G-CSF–treated mice. These low-density cells were enriched for CD4−CD8−NK1.1+ T cells and secreted about 10-fold more IL-4 than the unfractionated cells from the G-CSF–treated donors.
RECOMBINANT human granulocyte colony-stimulating factor (G-CSF) has been used extensively to mobilize hematopoietic progenitors into the blood of humans with a variety of malignancies for purposes of autologous stem cell transplantation after myeloablative therapy.1-3 The G-CSF–mobilized autologous blood mononuclear cells engraft more rapidly than bone marrow transplants as judged by the rapidity of reconstitution of neutrophils and platelets in the blood of the hosts.1-3 In recent studies, G-CSF–mobilized blood mononuclear cells from normal donors have been used as a substitute for bone marrow cells in HLA-matched transplantation for the treatment of lymphoid and other malignancies.4-6 Engraftment after allogeneic blood mononuclear cell transplantation was more rapid than that observed with bone marrow cells.4-6 Surprisingly, the incidence and severity of acute graft-versus-host disease (GVHD) was reported to be no greater with blood mononuclear cells than with marrow cells despite a 10-fold increase in the T-cell content of the former grafts. The results suggested that administration of G-CSF may alter the function of T cells.
Recombinant human G-CSF has been shown to mobilize hematopoietic progenitors into the blood of mice.7,8 The T cells in the spleen of G-CSF–treated mice were reported to have an altered pattern of cytokine secretion in which secretion of the Th2-type cytokines (ie, interleukin-4 [IL-4]) is increased and of the Th1-type cytokines (ie, interferon-γ [IFN-γ]) is decreased.8 In the current study, a model of allogeneic transplantation of blood mononuclear cells was developed using C57BL/Ka (H-2b) donors treated with G-CSF and lethally irradiated BALB/c (H-2d) hosts. The feasibility of reconstituting the irradiated hosts with donor low-density mononuclear cells was shown, but reconstitution with unfractionated mononuclear cells was unsuccessful over a 40-fold dose range. The T cells obtained from the blood of G-CSF–treated donors had a reduced capacity to induce lethal GVHD and an altered cytokine pattern (increased IL-4 and deceased IFN-γ) as compared with that from untreated donors. The balance of T cells and hematopoietic progenitors in the blood of these donors required ex vivo manipulation to augment progenitors and reduce T cells to rescue lethally irradiated allogeneic hosts for at least 100 days. This was accomplished by obtaining low-density fractions of the donor blood cells.
MATERIALS AND METHODS
Mice.BALB/c (H-2d) and C57BL/Ka (H-2b) mice were purchased from and maintained in the Department of Comparative Medicine, Stanford University School of Medicine (Stanford, CA). All mice were male and used at 8 to 12 weeks of age for recipients and at 6 to 8 weeks of age for donors.
Treatment of donor mice with recombinant G-CSF.C57BL/Ka donor mice were injected subcutaneously with recombinant human G-CSF (Amgen, Inc, Thousand Oaks, CA) in phosphate-buffered saline (PBS) at a dose of 125 μg/kg twice daily for 5 days. Three hours after the last injection, heparinized peripheral blood was collected.
Preparation of spleen and bone marrow cells.Spleen cells were removed aseptically, and single-cell suspensions were prepared by gently pressing the spleen fragments through a nylon fiber mesh (Tetko Inc, Elmsford, NY) into cold RPMI 1640 medium (GIBCO, Grand Island, NY). Bone marrow cells were prepared by flushing the femora and tibiae using a 25-gauge needle. Bone marrow plugs were then gently resuspended. The cells were washed twice and counted in 0.04% trypan blue solution to determine the concentration of viable cells.
Preparation of Ficoll gradients.Ficoll gradients were made with Lympholyte-M (Accurate, San Diego, CA). Lympholyte-M was placed in 15-mL polystyrene conical tubes, and diluted blood (blood:RPMI = 1:4) was gently layered on top. The gradients were centrifuged at 2,000 rpm (520g) for 20 minutes at room temperature, and the cells in the interface were harvested by aspiration.
Preparation of Percoll gradients.Percoll (Pharmacia, Uppsala, Sweden) was added to calcium- and magnesium-free, 10× Dulbecco's PBS (DPBS) to make up a stock solution of Percoll in a ratio of 1 part (vol/vol) DPBS to 9 parts (vol/vol) Percoll (starting density, 1.130 g/mL). For subsequent use, this working stock solution was progressively diluted in RPMI 1640 medium to obtain solutions containing 40%, 50%, 60%, and 70% of Percoll. Corresponding densities were 1.050, 1.060, 1.075, and 1.090 g/mL, respectively. Two-milliliter volumes of each of the Percoll solutions, starting with the 70% concentration and continuing with the decreasing concentrations, were layered in 15-mL clear polystyrene conical tubes. Diluted blood (2.5 mL) was added to the top of the Percoll gradients. The gradients were centrifuged at 2,000 rpm (520g) for 30 minutes at room temperature, and the cells at each density interface were aspirated with a Pasteur pipette and washed with RPMI 1640.
Immunofluorescent staining, flow cytometry analysis, and sorting.Single-cell suspensions of peripheral blood mononuclear cells (PBMC) and bone marrow cells were stained with fluorochrome-conjugated monoclonal antibodies (MoAbs) and analyzed on a FACstar (Becton Dickinson Immunocytometry System, Mountain View, CA) using FACS-DESK software.9,10 Phycoerythrin (PE)-conjugated anti-CD4 (L3T4), anti-CD8 (Lyt-2), anti–MAC-1 (M1-70.15) MoAbs, and control rat IgG2a and fluorescein isothiocyanate (FITC)-conjugated control hamster-IgG were obtained from Caltag (South San Francisco, CA). FITC-conjugated anti-CD4 and anti-CD8 MoAbs were gifts from Dr I. L. Weissman (Department of Pathology, Stanford University). Allophycocyanin (APC)-conjugated anti-TCR αβ (H57-597), FITC-conjugated anti-TCR αβ, and anti-B220 (RA3-6B2) MoAbs; PE-conjugated anti–Gr-1 (RB6-8C5), anti-B220, and anti-NK1.1 (PK136); and unconjugated anti-CD16/32 (2.4G2) MoAbs were obtained from PharMingen (San Diego, CA). Propidium iodide was obtained from Sigma (St Louis, MO). All experimental staining reagents were used at saturation, and control reagents were used at concentrations identical to the latter with similar fluorochrome conjugation ratios. All stainings were performed in the presence of saturating concentrations of rat monoclonal anti-Fc receptor antibodies (anti-CD16/32) to reduce nonspecific binding. Dead cells were excluded by propidium iodide staining. Cell sorting was performed with FACstar as described in detail previously.10 Cells within a 20-channel interval between positive and negative cells were discarded. Light scatter gating excluded only erythrocytes and dead cells. The sorted Mac-1−Gr-1−B220− PBMC were restained with anti-CD4 and anti-CD8 MoAb, and more than 95% of the sorted cells were CD4+ or CD8+ cells.
Whole body irradiation.Animals were placed in lucite containers and received a single dose of whole body irradiation with 800 cGy. X-rays were delivered from a 200-kV (20 mA) source (Philips Medical System, Inc, Shelton, CT) at a rate of 72.5 cGy/min. The source to the skin distance was 65 cm, and a 0.5-mm copper filter was used.
Monitoring of mice after transplantation.The signs of GVHD such as body weight loss, hair loss, hunched back, swollen facies, diarrhea, and death were monitored daily.
Cytokine assay.For determination of cytokine production, 0.1 to 0.4 × 106 of PBMC from normal or G-CSF–mobilized C57BL/Ka mice were stimulated with 20 ng/mL phorbol myristate acetate (PMA) plus 1 mmol/L Ionomycin (Calbiochem, San Diego, CA) in 10% fetal calf serum and RPMI complete medium in 96-well round-bottom plates. At 48 hours, supernatants were collected and stored at −80°C. IL-2 production was measured by the HT-2 cell assay in the presence of 10 mg/mL rat antimouse IL-4R MoAb (Genzyme, Cambridge, MA). Culture supernatants were added in serial dilutions to HT-2 cells (2 × 104 cells/well) that had been incubated with antireceptor antibody at room temperature for 30 minutes in round-bottom plates. After 20 hours of culture at 37°C in 5% CO2, cells were pulsed with 1 μCi/well of 3H-TdR. Four hours later, cells were harvested onto filter papers and counted in a liquid scintillation counter. The IL-2 concentration was calculated by comparison with a titration of a standard recombinant IL-2. Secretion of IFN-γ and IL-4 were measured with enzyme-linked immunosorbant assay kits (BioSource, Camarillo, CA). Assays were performed according to the manufacturer's protocol. Briefly, IFN-γ or IL-4 in samples was captured by the specific primary MoAb and detected by the biotin-labeled secondary MoAb. The assays were developed by avidin-peroxidase and its substrate, and plates were read at 450 nm using a microplate reader (Model S/N 03856; Molecular Devices, Sunnyvale, CA).
Statistical analysis.Comparison of survival curves of different groups of mice was made using the log rank test. All P values were calculated using the day of death for individual mice in each group. Replicate experiments were performed either concomitantly or within an interval of 2 to 3 months. Comparison of cytokine production of different groups was made using the Wilcoxon rank sums test.
RESULTS
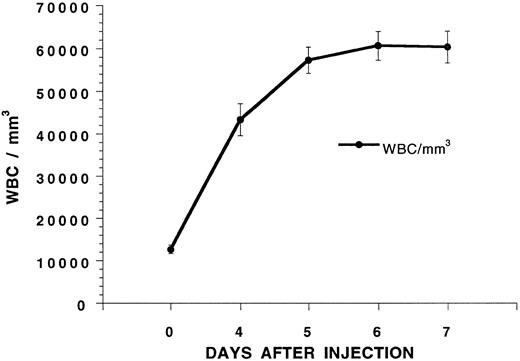
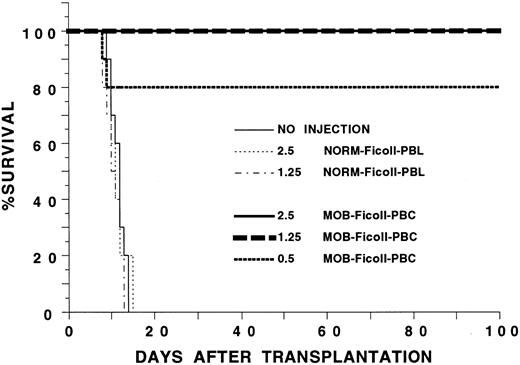
Mobilization of hematopoietic progenitors in the blood with G-CSF.G-CSF was administered twice a day at 125 μg/kg subcutaneously to six C57BL/Ka mice, and the mean white blood count is shown in Fig 1 over a 7-day interval. The white blood cell count increased about fivefold and reached a plateau on day 5. To determine whether hematopoietic progenitors were mobilized into the blood on day 5, C57BL/Ka hosts received a single dose of 800 cGy whole body irradiation and an intravenous injection of blood mononuclear cells obtained from a Ficoll-Hypaque density gradient from G-CSF–treated syngeneic donors 4 to 6 hours later. Figure 2 shows that control hosts receiving no cell injections all died by day 14 after irradiation. Injection of 1.25 × 106 or 2.5 × 106 blood mononuclear cells from C57BL/Ka donors receiving no G-CSF failed to improve the survival, and all died by day 16. However, when blood mononuclear cells were obtained from donors receiving G-CSF for 5 days, the injection of 0.5 × 106 cells allowed 80% of the hosts to survive for at least 100 days and the injection of 1.25 and 2.5 × 106 cells allowed 100% of the hosts to survive (Fig 2). Reduction of the dose of mononuclear cells to 0.25 × 106 from G-CSF donors allowed about 15% of hosts to survive 100 days (see below). Thus, the concentration of hematopoietic progenitor cells that rescue the irradiated hosts is increased at least 10-fold in the blood after the administration of G-CSF, because 0.25 × 106 G-CSF–treated cells provide significantly increased survival (P < .001) as compared with 2.5 × 106 untreated cells.
Changes in the white blood cell (WBC) count of C57BL/Ka mice receiving twice daily injections of G-CSF (125 μg/kg) subcutaneously. Each point represents the mean of a group of six mice, and the brackets show the standard error.
Changes in the white blood cell (WBC) count of C57BL/Ka mice receiving twice daily injections of G-CSF (125 μg/kg) subcutaneously. Each point represents the mean of a group of six mice, and the brackets show the standard error.
Survival of lethally irradiated C57BL/Ka mice receiving an intravenous injection of blood mononuclear cells from G-CSF–treated or untreated syngeneic donors. Irradiation and cell injections were performed on day 0. Hosts received either no cells; 2.5 × 106 or 1.25 × 106 Ficoll-purified cells from untreated donors (NORM-Ficoll-PBL); or 2.5, 1.25, or 0.5 × 106 Ficoll-purified cells from G-CSF–treated donors (MOB-Ficoll-PBC). There were 10 mice in each group of hosts. Two experiments were combined.
Survival of lethally irradiated C57BL/Ka mice receiving an intravenous injection of blood mononuclear cells from G-CSF–treated or untreated syngeneic donors. Irradiation and cell injections were performed on day 0. Hosts received either no cells; 2.5 × 106 or 1.25 × 106 Ficoll-purified cells from untreated donors (NORM-Ficoll-PBL); or 2.5, 1.25, or 0.5 × 106 Ficoll-purified cells from G-CSF–treated donors (MOB-Ficoll-PBC). There were 10 mice in each group of hosts. Two experiments were combined.
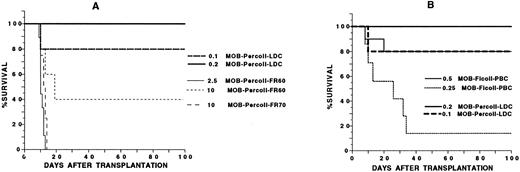
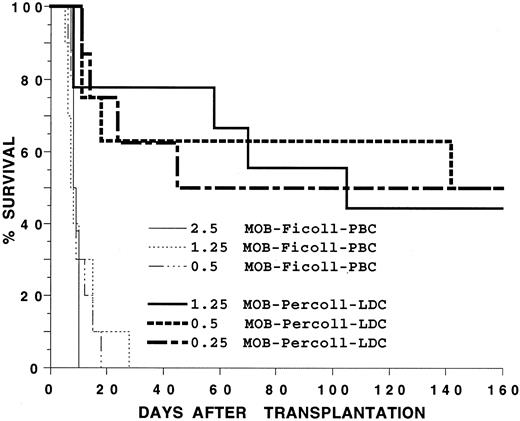
Previous studies have shown that stem cells that reconstitute irradiated allogeneic hosts are found in the low-density fractions of marrow and spleen cells separated on discontinuous Percoll density gradients.9 In the current study, blood mononuclear cells from C57BL/Ka mice receiving G-CSF for 5 days were separated on gradients with 40% to 70% Percoll in steps of 10% each. The low-density cell (LDC) fraction obtained from the 50% to 60% Percoll interface, two high-density fractions obtained from the 60% to 70% interface, and the cell pellet were injected into lethally irradiated hosts. The mean yields of cells in the low-density and two high-density fractions were 12%, 66%, and 22%, respectively (based on total cell output). Figure 3A shows that as few as 0.1 × 106 cells from the LDC fraction from C57BL/Ka donors allowed 80% of irradiated syngeneic hosts to survive at least 100 days and that 0.2 × 106 cells rescued 100% of the hosts. The high-density cell fractions were less effective, and 2.5 × 106 cells from the 60% to 70% interface and 10 × 106 cells from the pellet failed to prolong survival beyond 16 days (P < .001). Increasing the dose of cells from the 50% to 60% interface to 10 × 106 (100-fold higher than that used in the LDC fraction) rescued 40% of irradiated hosts (Fig 3A).
Survival of lethally irradiated C57BL/Ka mice receiving an intravenous injection of G-CSF–treated blood mononuclear cells separated on a discontinuous Percoll density gradient. In (A), hosts received 0.1 or 0.2 × 106 cells from the low-density fraction (MOB-Percoll-LDC), 2.5 or 10 × 106 cells from the 60% to 70% interface (MOB-Percoll-FR60), or 10 × 106 cells from the pellet at the bottom of the 70% Percoll solution (MOB-Percoll-FR70). In (B), hosts received 0.1 or 0.2 × 106 cells from the low-density fraction or 0.25 or 0.5 × 106 unfractionated cells. There were 10 to 12 mice in each group. Two experiments were combined.
Survival of lethally irradiated C57BL/Ka mice receiving an intravenous injection of G-CSF–treated blood mononuclear cells separated on a discontinuous Percoll density gradient. In (A), hosts received 0.1 or 0.2 × 106 cells from the low-density fraction (MOB-Percoll-LDC), 2.5 or 10 × 106 cells from the 60% to 70% interface (MOB-Percoll-FR60), or 10 × 106 cells from the pellet at the bottom of the 70% Percoll solution (MOB-Percoll-FR70). In (B), hosts received 0.1 or 0.2 × 106 cells from the low-density fraction or 0.25 or 0.5 × 106 unfractionated cells. There were 10 to 12 mice in each group. Two experiments were combined.
Figure 3B compares the restorative capacity of the LDC fraction of donors receiving G-CSF with that of the unfractionated mononuclear cells. The LDC fraction was about fivefold more effective than the unfractionated cells, because the survival of hosts receiving 0.1 × 106 LDC was similar to that of hosts receiving 0.5 × 106 unfractionated cells. All hosts receiving 0.2 × 106 LDC survived 100 days, but about 15% of hosts receiving 0.25 × 106 unfractionated cells survived during the same period (P < .01).
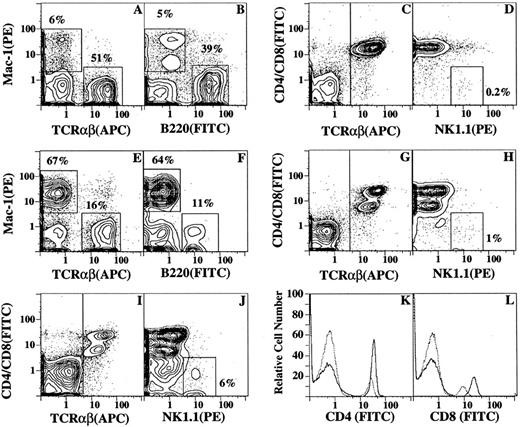
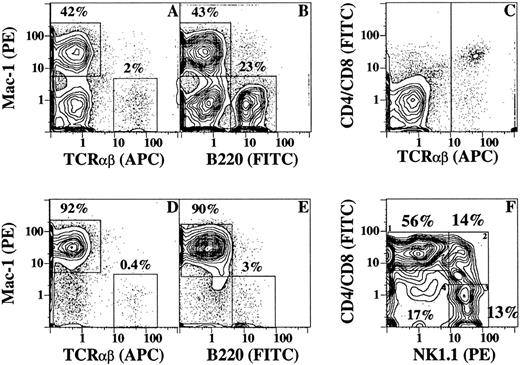
Changes in T and B cells in blood mononuclear cells and bone marrow after the injection of G-CSF.Figure 4 shows the changes in T cells (TCRαβ+), B cells (B220+), and monocyte and granulocyte lineage cells (Mac-1+) in the blood of C57BL/Ka mice before and after the administration of G-CSF. Immunofluorescent staining with a variety of fluorochrome-conjugated MoAbs was analyzed by flow cytometry using multiple color channels. Figure 4A and B show the percentages of Mac-1+ (5% to 6%), TCRαβ+ (51%), and B220+ (39%) cells in a representative sample of blood mononuclear cells before the administration of G-CSF. After the administration of G-CSF, the percentage of Mac-1+ cells increased markedly (64% to 67%), and the percentages of TCRαβ+ (16%) and B220+ (11%) cells were reduced (Fig 4E and F). Almost all T cells were CD4+ or CD8+ before and after the injection of G-CSF (Fig 4C and G, respectively), and the ratio of CD4:CD8 T cells (2:1) was similar in the treated and untreated mice (data not shown). However, the intensity of staining for the CD8 marker was considerably reduced after the administration of G-CSF (Fig 4L), but that for the CD4 marker was slightly reduced (Fig 4K). The change in the CD8 staining intensity resulted in a pattern of two discrete concentric contours when G-CSF–treated cells were stained with a combination of PE-conjugated anti-CD4 and CD8 antibodies and counterstained with APC-conjugated anti-TCRαβ antibodies (Fig 4G), as compared with the single group of contours observed with the untreated cells (Fig 4C). The mean percentages of CD4+ and CD8+ T cells in the blood mononuclear cells before and after G-CSF administration are shown in Table 1. Although the mean percentage of T cells was reduced by about twofold after G-CSF administration, the yield of T cells per milliliter of blood was increased threefold to fourfold due to the sevenfold to eightfold increase in the yield of blood mononuclear cells. The mean percentage of T cells in the LDC fraction was similar to that of the unfractionated mobilized mononuclear cells (27% v 25%). However, the yield of T cells in the LDC fraction was reduced more than 10-fold as compared with the unfractionated cells (Table 1).
Flow cytometric analysis of immunoflourescent staining of blood mononuclear cells from untreated C57BL/Ka mice or from mice receiving G-CSF for 5 days. (A) through (D) show two-color analyses of mononuclear cells from untreated mice stained with conjugated MoAbs: PE — anti–Mac-1 versus APC–anti-TCRαβ (A), PE — anti–Mac-1 versus FITC–anti-B220 (B), FITC–anti-CD4 and FITC–anti-CD8 versus APC–anti-TCRαβ (C), and FITC–anti-CD4 and FITC–anti-CD8 versus PE–anti-NK1.1 after gating on TCR αβ+ cells (D). Boxes enclose either Mac-1+, TCR αβ+, or B220+ cells, and the percentage of cells in each box is shown. The vertical line in (C) shows the threshold for gating TCRαβ+ cells. Conjugated isotype-matched irrelevant antibodies stained less than 2% of cells. (E) through (H) show the same staining analyses for mononuclear cells obtained from G-CSF–treated mice. (I) shows the two-color analysis of low-density cells from G-CSF–treated mice after staining for CD4 and CD8 versus TCRαβ receptors. (J) shows the analysis of CD4 and CD8 versus NK1.1 receptors after gating on TCRαβ+ cells from (I). (K) and (L) show one-color analysis of unfractionated mononuclear cells from untreated (solid line) or treated (dashed line) mice stained with FITC–anti-CD4 or FITC–anti-CD8 antibodies, respectively.
Flow cytometric analysis of immunoflourescent staining of blood mononuclear cells from untreated C57BL/Ka mice or from mice receiving G-CSF for 5 days. (A) through (D) show two-color analyses of mononuclear cells from untreated mice stained with conjugated MoAbs: PE — anti–Mac-1 versus APC–anti-TCRαβ (A), PE — anti–Mac-1 versus FITC–anti-B220 (B), FITC–anti-CD4 and FITC–anti-CD8 versus APC–anti-TCRαβ (C), and FITC–anti-CD4 and FITC–anti-CD8 versus PE–anti-NK1.1 after gating on TCR αβ+ cells (D). Boxes enclose either Mac-1+, TCR αβ+, or B220+ cells, and the percentage of cells in each box is shown. The vertical line in (C) shows the threshold for gating TCRαβ+ cells. Conjugated isotype-matched irrelevant antibodies stained less than 2% of cells. (E) through (H) show the same staining analyses for mononuclear cells obtained from G-CSF–treated mice. (I) shows the two-color analysis of low-density cells from G-CSF–treated mice after staining for CD4 and CD8 versus TCRαβ receptors. (J) shows the analysis of CD4 and CD8 versus NK1.1 receptors after gating on TCRαβ+ cells from (I). (K) and (L) show one-color analysis of unfractionated mononuclear cells from untreated (solid line) or treated (dashed line) mice stained with FITC–anti-CD4 or FITC–anti-CD8 antibodies, respectively.
Yield of CD4+/CD8+ TCRαβ+ T Cells in the Normal and G-CSF–Mobilized C57BL/Ka Mouse Blood
| . | White Blood Cell Count . | Yield of Mononuclear Cells . | % CD4+CD8+ αβ+ T Cells . | Yield of CD4+/CD8+ αβ+ T Cells . |
|---|---|---|---|---|
| NORM-PBL (n = 9) | 13 ± 2 | 3.9 ± 0.8 | 56 ± 10 | 2.2 ± 0.4 |
| MOB-PBC (n = 8) | 61 ± 6 | 30 ± 5 | 25 ± 8 | 8 ± 2 |
| MOB-LDC (n = 8) | 61 ± 6 | 2.5 ± 0.7 | 27 ± 9 | 0.6 ± 0.2 |
| . | White Blood Cell Count . | Yield of Mononuclear Cells . | % CD4+CD8+ αβ+ T Cells . | Yield of CD4+/CD8+ αβ+ T Cells . |
|---|---|---|---|---|
| NORM-PBL (n = 9) | 13 ± 2 | 3.9 ± 0.8 | 56 ± 10 | 2.2 ± 0.4 |
| MOB-PBC (n = 8) | 61 ± 6 | 30 ± 5 | 25 ± 8 | 8 ± 2 |
| MOB-LDC (n = 8) | 61 ± 6 | 2.5 ± 0.7 | 27 ± 9 | 0.6 ± 0.2 |
Values are the number of cells × 10−6 per milliliter of blood (mean ± SE).
The G-CSF–treated and –untreated blood mononuclear cells were analyzed for the presence of NK1.1+ T cells by gating on the TCRαβ+ cells in Fig 4C and G and analyzing the staining of CD4 and CD8 versus NK1.1 receptors on the gated cells. CD4−CD8−NK1.1+ T cells accounted for about 0.2% of cells in untreated blood and about 1% in mobilized blood (Fig 4D and H). A discrete population of CD4−CD8−NK1.1+ cells was not detected in either population of gated cells. A similar analysis was performed on the low-density fraction of G-CSF–treated mononuclear cells. Figure 4I shows that almost all low-density T cells were CD4+ or CD8+, but after gating on the TCRαβ+ cells, a discrete population of CD4−CD8− NK1.1+ cells (enclosed in box in Fig 4J) was observed and accounted for about 6% of the TCRαβ+ cells. The low-density cells from untreated mice were not analyzed for T-cell subsets, because the yield of low-density cells was too low (<1 × 105/mouse) to perform immunofluorescent staining.
Changes in the content of T and B cells in the bone marrow were studied before and after the administration of G-CSF also. As shown in Fig 5A, B, D, and E, the percentage of TCRαβ+ and B220+ cells were about fivefold and eightfold, respectively, reduced in the bone marrow after the administration of G-CSF, such that more than 90% of the residual cells were Mac-1+ and few Mac-1− cells remained. Thus, most marrow cells in the T- and B-cell lineages were depleted by the G-CSF regimen. Analysis of T-cell subsets in untreated mice showed that the majority of the bright TCRαβ+ cells were CD4+ or CD8+ (Fig 5C). After gating on the bright TCRαβ+ cells and analyzing the staining for CD4 and CD8 markers versus the NK1.1 marker, four subsets of cells were observed (Fig 5F). The majority of cells (56%) were CD4+ or CD8+ and NK1.1− (box 1), and only a single group of concentric contours was observed indicating similar staining intensities for the CD4 and CD8 markers. This was confirmed by single-color analysis after staining for CD4 and CD8 markers individually (data not shown). Two populations of NK1.1+ cells were observed; one stained positively for CD4 or CD8 receptors (box 2) and the other was CD4−CD8− (box 3). Thus, the surface phenotype of the latter subset (TCRαβ+ NK1.1+ CD4−CD8−) was similar to that in the low-density cells in the blood enclosed in the box in Fig 4J. The fourth bone marrow subset of T cells was CD4−CD8− NK1.1− (box 4) and represented 17% of the bright TCRαβ+ cells. Too few T cells remained in the marrow after the administration of G-CSF to perform accurate cell subset analyses.
Flow cytometric analysis of immunofluorescent staining of bone marrow cells from untreated and G-CSF–treated mice. (A) and (B) show the analyses of staining for Mac-1 versus TCRαβ receptors and Mac-1 versus B220 receptors in untreated mice. (D) and (E) show the analyses in treated mice. (C) shows the staining pattern of untreated cells for CD4 and CD8 versus TCRαβ receptors. Cells in (C) were gated for TCRαβ+ cells and staining for CD4 and CD8 versus NK1.1 receptors is shown in (F). The four boxes in (F) show the percentage of CD4+ or CD8+ NK1.1− (1), CD4+ or CD8+ NK1.1+ (2), CD4−CD8− NK1.1+ (3), and CD4−CD8− NK1.1− (4) αβ T cells.
Flow cytometric analysis of immunofluorescent staining of bone marrow cells from untreated and G-CSF–treated mice. (A) and (B) show the analyses of staining for Mac-1 versus TCRαβ receptors and Mac-1 versus B220 receptors in untreated mice. (D) and (E) show the analyses in treated mice. (C) shows the staining pattern of untreated cells for CD4 and CD8 versus TCRαβ receptors. Cells in (C) were gated for TCRαβ+ cells and staining for CD4 and CD8 versus NK1.1 receptors is shown in (F). The four boxes in (F) show the percentage of CD4+ or CD8+ NK1.1− (1), CD4+ or CD8+ NK1.1+ (2), CD4−CD8− NK1.1+ (3), and CD4−CD8− NK1.1− (4) αβ T cells.
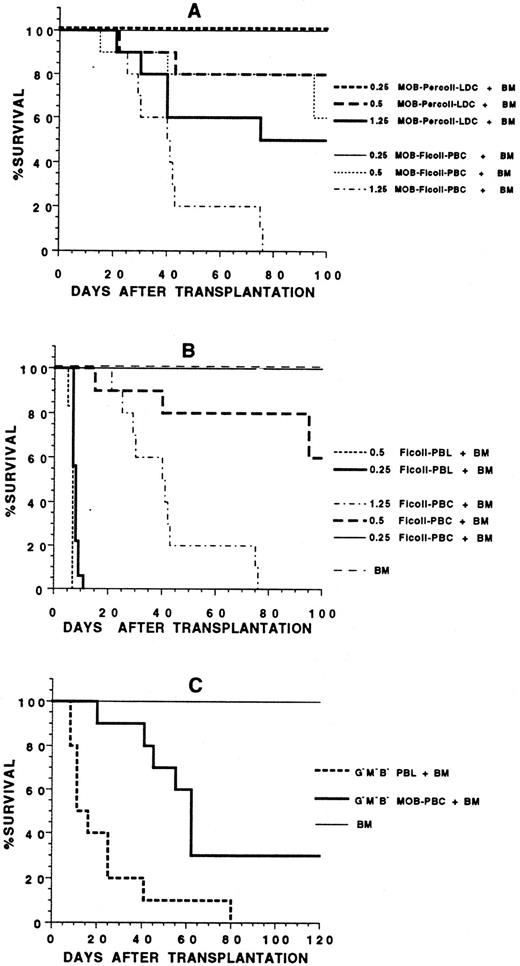
Transplantation of blood mononuclear cells to allogeneic hosts.In several experiments, G-CSF–treated blood mononuclear cells from C57BL/Ka (H-2b) mice were transplanted to lethally irradiated BALB/c (H-2d) hosts using a dose range of cells capable of rescuing irradiated syngeneic recipients for more than 100 days. Hosts were observed daily for evidence of GVHD such as weight loss, diarrhea, hair loss, hunched back, and swollen facies. Based on these parameters, GVHD was graded as mild, moderate, or severe. Figure 6 shows that 0.5 × 106, 1.25 × 106, and 2.5 × 106 G-CSF–treated C57BL/Ka mononuclear cells failed to significantly prolong the survival of the irradiated BALB/c hosts after transplantation, and all hosts died between 14 to 28 days after the injection of cells (P > .05). Almost all of these hosts showed evidence of marked (>20%) weight loss and diarrhea, and GVHD was graded as severe. Diarrhea was not observed in hosts receiving only irradiation (data not shown). Six of six hosts receiving 10 × 106 G-CSF–treated mononuclear cells died by day 10 (data not shown). In contrast, the LDC fraction of the G-CSF–treated blood mononuclear cells rescued about 50% of the hosts for more than 160 days in the dose range of 0.25 to 1.25 × 106 cells. The difference in survival of hosts receiving 1.25 × 106 unfractionated cells or the LDC fraction was significant (P < .001). Almost all hosts receiving the LDC fraction and that died after 20 days showed moderate to severe GVHD. The long-term surviving hosts (>160 days) had either no clinical signs of GVHD or had mild GVHD with hair loss, hunched back, and mild diarrhea.
Survival of irradiated BALB/c hosts receiving an intravenous injection of blood mononuclear cells from C57BL/Ka donors receiving G-CSF. Hosts received 2.5, 1.25, or 0.5 × 106 unfractionated mononuclear cells or 0.25, 0.5, or 1.25 × 106 cells from the low-density fraction of a Percoll gradient. There were 12 mice in each group. Two experiments were combined.
Survival of irradiated BALB/c hosts receiving an intravenous injection of blood mononuclear cells from C57BL/Ka donors receiving G-CSF. Hosts received 2.5, 1.25, or 0.5 × 106 unfractionated mononuclear cells or 0.25, 0.5, or 1.25 × 106 cells from the low-density fraction of a Percoll gradient. There were 12 mice in each group. Two experiments were combined.
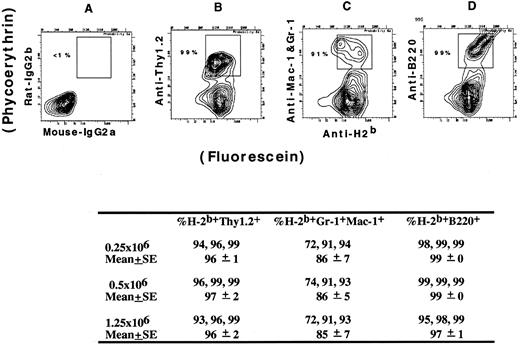
Groups of three hosts receiving either 0.25 × 106, 0.5 × 106, or 1.25 × 106 cells from the LDC fraction were tested for chimerism more than 160 days after transplantation. Figure 7B, C,and D show examples of the two-color flow cytometric analysis for donor-type cells (stained with FITC-conjugated anti-H-2b antibodies) in the T cells, macrophages and granulocytes, and B cells (stained with PE-conjugated anti-Thy 1.2, anti–Mac-1 and anti–Gr-1, and anti-B220 antibodies, respectively) in the host spleen. The upper boxes enclose the donor-type cells, and the percentages of T cells, macrophages and granulocytes, and B cells, which are of donor type, are shown adjacent to the boxes. Figure 7A shows background staining with control reagents. The table in the bottom part of Fig 7 summarizes the mean percentages of donor-type cells in each group of three hosts. Between 85% and 99% of all three cell lineages were of donor-type.
Flow cytometric analysis of donor-type cells in the blood of BALB/c hosts receiving C57BL/Ka low-density blood mononuclear cells. Donors were treated with G-CSF, and host blood cells were examined more than 160 days after the injection of donor cells. Host mononuclear cells were stained with FITC-conjugated anti–H-2b antibodies and PE-conjugated anti-Thy 1.2, anti–Mac-1 and anti–Gr-1, or anti-B220 antibodies (B, C, and D, respectively). Control staining is shown in (A). Boxes enclose donor-type T cells, macrophages and granulocytes, or B cells. The mean percentage of donor type cells in each category is summarized for groups of three mice receiving three different dosages of cells.
Flow cytometric analysis of donor-type cells in the blood of BALB/c hosts receiving C57BL/Ka low-density blood mononuclear cells. Donors were treated with G-CSF, and host blood cells were examined more than 160 days after the injection of donor cells. Host mononuclear cells were stained with FITC-conjugated anti–H-2b antibodies and PE-conjugated anti-Thy 1.2, anti–Mac-1 and anti–Gr-1, or anti-B220 antibodies (B, C, and D, respectively). Control staining is shown in (A). Boxes enclose donor-type T cells, macrophages and granulocytes, or B cells. The mean percentage of donor type cells in each category is summarized for groups of three mice receiving three different dosages of cells.
The inability of the unfractionated G-CSF–treated donor mononuclear cells to rescue the BALB/c hosts may have been due to lack of engraftment of donor hematopoietic progenitor cells, GVHD, or a combination of the two. To determine the contribution from GVHD, several experiments were performed in which irradiated BALB/c hosts were injected with sufficient hematopoietic progenitors from the C57BL/Ka bone marrow to reconstitute the hosts without GVHD, and C57BL/Ka blood mononuclear cells were added to the marrow cell inoculum. Figure 8 shows that 2.5 × 106 C57BL/Ka bone marrow cells from untreated donors rescued all BALB/c hosts for at least 100 days (Fig 8B and C). None of the hosts showed signs of GVHD. However, the addition of 1.25 × 106 G-CSF–treated blood mononuclear cells to the marrow cell inoculum resulted in moderate to severe GVHD in all hosts, and none survived for more than 76 days (P < .01; Fig 8A). About 60% of hosts receiving the marrow cells plus 0.5 × 106 G-CSF–treated mononuclear cells survived 100 days, and 100% of hosts receiving marrow cells plus 0.25 × 106 G-CSF–treated cells survived the same period. The survival of hosts receiving marrow cells plus 0.25 or 0.5 × 106 of the LDC fraction of G-CSF–treated mononuclear cells was not statistically significantly different from that receiving marrow cells plus the unfractionated mononuclear cells (P > .05). However, at the highest cell dose (1.25 × 106), the survival of the group receiving the LDC fraction was significantly increased (P < .01). A comparison of Fig 8A with Fig 6 indicates that the rapid death at less than 30 days with diarrhea and weight loss in hosts receiving 0.5 and 1.25 × 106 G-CSF–treated mononuclear cells alone was due in part to an insufficient numbers of hematopoietic progenitors associated with slow blood cell reconstitution, because the addition of donor bone marrow cells significantly prolonged the survival of the hosts, especially in the group receiving marrow cells plus 0.5 × 106 blood mononuclear cells (P < .01).
Survival of irradiated BALB/c hosts receiving 2.5 × 106 C57BL/Ka bone marrow cells plus blood mononuclear cells from C57BL/Ka donors. In (A), hosts received bone marrow (BM) cells plus 0.25, 0.5, or 1.25 × 106 unfractionated blood mononuclear cells or low-density cells from donors receiving G-CSF. There were 10 to 12 mice in each group. In (B), hosts received marrow cells alone or with 0.25 or 0.5 × 106 mononuclear cells from untreated donors or marrow cells with 0.25, 0.5, or 1.25 × 106 mononuclear cells from donors receiving G-CSF. There were 10 to 12 mice in each group. In (C), hosts received marrow cells plus 0.1 to 0.25 × 106 purified CD4+ and CD8+ T cells from untreated donors (G−M−B− PBL) or 0.1 to 0.25 × 106 purified CD4+ and CD8+ T cells from G-CSF–treated donors (G−M−B− MOB-PBC). There were 10 mice in each group.
Survival of irradiated BALB/c hosts receiving 2.5 × 106 C57BL/Ka bone marrow cells plus blood mononuclear cells from C57BL/Ka donors. In (A), hosts received bone marrow (BM) cells plus 0.25, 0.5, or 1.25 × 106 unfractionated blood mononuclear cells or low-density cells from donors receiving G-CSF. There were 10 to 12 mice in each group. In (B), hosts received marrow cells alone or with 0.25 or 0.5 × 106 mononuclear cells from untreated donors or marrow cells with 0.25, 0.5, or 1.25 × 106 mononuclear cells from donors receiving G-CSF. There were 10 to 12 mice in each group. In (C), hosts received marrow cells plus 0.1 to 0.25 × 106 purified CD4+ and CD8+ T cells from untreated donors (G−M−B− PBL) or 0.1 to 0.25 × 106 purified CD4+ and CD8+ T cells from G-CSF–treated donors (G−M−B− MOB-PBC). There were 10 mice in each group.
The ability of the G-CSF–treated blood mononuclear cells to induce GVHD after addition to the marrow inoculum was compared with that of untreated blood mononuclear cells after addition to marrow cells. Although all hosts receiving 0.25 × 106 of the G-CSF–treated mononuclear cells survived at least 100 days, none receiving the same dose of untreated mononuclear cells survived beyond 11 days (P < .001; Fig 8B). When 0.5 × 106 cells were compared, 60% of hosts receiving G-CSF–treated cells survived at least 100 days, but none receiving untreated cells survived beyond 8 days (P < .01). Survival of hosts receiving five times the number of G-CSF–treated cells (1.25 × 106) as compared with untreated cells (0.25 × 106) was still significantly increased (P < .01). Thus, the G-CSF–treated mononuclear cells had a markedly reduced capacity to induce acute lethal GVHD as compared with the untreated cells.
The relative inability of the G-CSF–treated cells to induce GVHD as compared with the untreated cells could be due to changes in regulatory non-T cells in the blood, to the balance of T-cell subsets in the blood after administration of G-CSF, or to changes in the function of the subsets of T cells (CD4+ and CD8+) that induce GVHD. To examine whether the function of the CD4+ and CD8+ T cells changed, purified CD4+ and CD8+ T cells were obtained by flow cytometry from the two sources of blood mononuclear cells. To minimize the possibility that MoAbs bound to the cell surface of these T cells could alter their function, the CD4+ and CD8+ T cells were obtained by negative selection. The blood mononuclear cells were stained with a combination of PE-conjugated anti-B220, anti–Mac-1, and anti–Gr-1 MoAbs, and the B220−, Mac-1−, Gr-1− cells contained in the lymphocyte light scatter gate were obtained by cell sorting. These cells contained at least 95% CD4+ and CD8+ T cells and less than 1% CD4−CD8− T cells on reanalysis.
The purified CD4+ and CD8+ T cells were added to untreated bone marrow cells (2.5 × 106), and the inoculum of C57BL/Ka bone marrow and T cells was transplanted to irradiated BALB/c hosts. Results with 0.1 and 0.25 × 106 T cells were similar, such that data from two experiments are combined. Figure 8C shows that 30% of hosts receiving 0.1 to 0.25 × 106 of the purified T cells from G-CSF–treated donors survived at least 100 days, but none of the hosts given the same number of purified T cells from untreated donors survived more than 80 days (P < .01). Hosts surviving at least 100 days showed minimal signs of GVHD. Thus, changes in the function of the CD4+ and CD8+ T cells occurred after the administration of G-CSF such that they developed a reduced capacity to induce rapid and lethal GVHD. However, the more marked differences in the survival of hosts receiving unfractionated mononuclear cells from G-CSF–treated or untreated donors shown in Fig 8B indicate that changes in the percentage of T cells as well as the presence of regulatory non-CD4 or non-CD8 T cells contribute to these differences also.
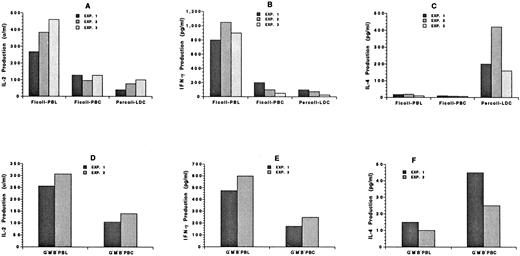
Changes in cytokine secretion of blood mononuclear cells after the administration of G-CSF.To understand the mechanisms by which GVHD is reduced after the administration of G-CSF, the cytokine secretion patterns of unfractionated blood mononuclear cells from untreated donors and the unfractionated and low-density mononuclear cells from treated donors were studied. The different cells were incubated in vitro with calcium ionophore and PMA for 48 hours. Supernatants were collected thereafter and assayed for the presence of IL-2, IFN-γ, and IL-4. Figure 9A through C show the results of three independent experiments using 0.2 × 106 cells. The percentage of T cells in the samples was in the range shown in Table 1. The secretion of IL-2 was reduced about twofold to fourfold in the unfractionated and low-density cells from treated donors as compared with untreated donors (P < .05). The secretion of IFN-γ was reduced fivefold to 10-fold (P < .01). Surprisingly, neither the unfractionated untreated nor the treated mononuclear cells secreted more than 15 pg/mL of IL-4, but the low-density treated cells secreted 10- to 20-fold higher levels (P < .01). This may be related in part to the increased percentage of NK1.1+ T cells in this fraction (Fig 4J), because the latter cells have been reported to secrete high levels of IL-4.11 Similar results were obtained with 0.4 × 106 unfractionated treated, LDC fraction, or untreated cells (data not shown).
Cytokine secretion patterns of blood mononuclear cells from untreated C57BL/Ka mice or from mice receiving G-CSF. (A), (B), and (C) show the concentration of IL-2, IFN-γ, or IL-4, respectively, in the supernatants from 0.2 × 106 cells cultured for 48 hours in the presence of calcium ionophore and PMA. Each bar shows the average value of two or three replicate determinations of supernatants from one of three independent experiments: Ficoll-PBL (untreated mononuclear cells), Ficoll-PBC (G-CSF–treated mononuclear cells), and Percoll-LDC (G-CSF–treated low-density mononuclear cells). (D), (E), and (F) show the concentrations of IL-2, IFN-γ, or IL-4 from 0.2 × 106 purified CD4+ and CD8+ T cells obtained by flow cytometry in two independent experiments: G−M−B−PBL (sorted Gr-1− Mac-1− B220− mononuclear cells from untreated mice) and G−M−B−PBC (sorted Gr-1− Mac-1− B220− mononuclear cells from treated mice).
Cytokine secretion patterns of blood mononuclear cells from untreated C57BL/Ka mice or from mice receiving G-CSF. (A), (B), and (C) show the concentration of IL-2, IFN-γ, or IL-4, respectively, in the supernatants from 0.2 × 106 cells cultured for 48 hours in the presence of calcium ionophore and PMA. Each bar shows the average value of two or three replicate determinations of supernatants from one of three independent experiments: Ficoll-PBL (untreated mononuclear cells), Ficoll-PBC (G-CSF–treated mononuclear cells), and Percoll-LDC (G-CSF–treated low-density mononuclear cells). (D), (E), and (F) show the concentrations of IL-2, IFN-γ, or IL-4 from 0.2 × 106 purified CD4+ and CD8+ T cells obtained by flow cytometry in two independent experiments: G−M−B−PBL (sorted Gr-1− Mac-1− B220− mononuclear cells from untreated mice) and G−M−B−PBC (sorted Gr-1− Mac-1− B220− mononuclear cells from treated mice).
In two additional experiments (Fig 9D through F), purified CD4+ and CD8+ T cells (0.2 × 106) from untreated and treated donors were incubated in vitro with calcium ionophore and PMA, and the secretion of IL-2, IFN-γ, and IL-4 was measured. As shown in Fig 9, the secretion of IL-2 was reduced about twofold (P < .05) and that of IFN-γ about twofold to threefold (P < .05) after the administration of G-CSF. On the other hand, the secretion of IL-4 was increased about twofold (P < .05). Similar results were obtained with 0.1 × 106 cells (data not shown). Thus, the pattern of reduced IL-2 and IFN-γ secretion and increased IL-4 secretion was similar in the unfractionated mononuclear cells and in the purified cells.
DISCUSSION
As reported by Mollineux et al,7 administration of recombinant human G-CSF to mice increased the white blood cell count and mobilized hematopoietic progenitors into the peripheral blood. Whereas 0.5 × 106 blood mononuclear cells from G-CSF–treated mice were able to rescue about 80% of lethally irradiated syngeneic (C57BL/Ka) mice for more than 100 days, five times that number of mononuclear cells from untreated mice did not prolong survival beyond 14 days. Separation of the G-CSF–treated mononuclear cells on a discontinuous Percoll density gradient showed that the low-density fraction was enriched with progenitor cells at least fivefold as compared with unfractionated cells and at least 100-fold as compared with the highest density cells. Low-density fractions of bone marrow cells and G-CSF–mobilized blood have been reported to be enriched for progenitors in both mice and humans by several laboratories.6,9,10 12-14
Recent studies in humans have shown the feasibility of substituting G-CSF–mobilized PBMC for bone marrow cells in HLA-matched allogeneic transplantation in humans.1-3 Engraftment of neutrophils and platelets were more rapid than with marrow cells, and acute GVHD was reported to be no more severe than with marrow cells, despite the increase of the T-cell dose by about 10-fold.1-3 In the current study, the T-cell subsets in the G-CSF–mobilized blood of mice were identified by immunofluorescent staining and flow cytometry. As expected, the percentage of B cells and of CD4+ and CD8+ T cells in the blood mononuclear cells was reduced in G-CSF–treated mice as compared with untreated mice due to influx of mature and immature granulocytes and monocytes, most of which express the Mac-1 marker. However, the absolute T-cell yields per milliliter of blood were increased threefold to fourfold. The mean percentage of CD4+ and CD8+ T cells in the low-density fraction of the G-CSF–treated mononuclear cells was unexpectedly higher than that of the unfractionated cells. This is in contrast to studies of human bone marrow cells that have shown that low-density fractions are markedly reduced in their content of CD4+ and CD8+ T cells.12 15 It is possible that the administration of G-CSF altered the activation state of the mouse T cells such that they became larger and of lower density, because the functional state of these T cells was altered (see below). The low-density cells contained an increased percentage of CD4−CD8− NK1.1+ T cells as compared with the unfractionated mononuclear cells from G-CSF–treated or untreated mice. It is likely that these unusual T cells were mobilized from the bone marrow, because they were identified in the marrow of untreated mice, and most T and B cells were depleted from the marrow after the administration of G-CSF. The latter result indicates that the efflux of marrow cells induced by G-CSF is not specific for hematopoietic precursors, but includes most cells from the lymphocyte lineage as well. This point may not be easily appreciated in humans, because blood mononuclear cells heavily contaminate marrow aspirates and thus obscure changes in the resident marrow cell populations.
The ability of G-CSF–treated low-density and unfractionated C57BL/Ka (H-2b) blood mononuclear cells to reconstitute lethally irradiated allogeneic (BALB/c) (H-2d) mice was determined in the current study. None of the allogeneic hosts survived beyond 30 days after the injection of 0.5 to 2.5 × 106 G-CSF–treated unfractionated cells despite the fact that at least 80% of syngeneic hosts receiving the same doses of cells survived for more than 100 days. However, about 50% of the allogeneic hosts survived at least 160 days and were chimeric after the injection of 0.25 to 1.25 × 106 of the low-density fraction of G-CSF–treated blood mononuclear cells. The increased survival of the hosts receiving the low-density cells may have been contributed to by an increase in the number of hematopoietic progenitor cells injected and/or a decrease in the number of T cells that induced acute lethal GVHD.
To determine whether administration of G-CSF results in changes in the severity of GVHD induced by blood mononuclear cells, the G-CSF–treated and untreated donor mononuclear cells were injected into allogeneic hosts, along with untreated donor bone marrow cells. The latter cells provided sufficient progenitor cells to reconstitute the allogeneic hosts and insufficient T cells to cause clinical signs of GVHD. The G-CSF–treated unfractionated blood mononuclear cells had a markedly reduced capacity to induce acute lethal GVHD as compared with untreated blood mononuclear cells. The capacity of the unfractionated and low-density G-CSF–treated mononuclear cells to induce acute GVHD was similar in the 0.25 to 0.5 × 106 cell dose range, but the capacity of the low-density cells was significantly reduced as compared with unfractionated cells at the 1.25 × 106 cell dose. The inability of the unfractionated G-CSF–treated cells to rescue allogeneic hosts at the lower dose range was due to reduced hematopoietic progenitor cells rather than increased GVHD as compared with the low-density cells. The hosts receiving low-dose unfractionated cells did not succumb only to marrow aplasia, because severe diarrhea was present. This suggests that marrow aplasia combined with mild gut GVHD resulted in severe diarrhea and early death. As the dose of unfractionated cells was increased to bring the progenitor level to that required for reconstitution, GVHD began to play an important role in host mortality even in the presence of rapid reconstitution. Thus, none of the unfractionated cell doses tested rescued the allogeneic hosts. The low-density cells were effective due to the increased ratio of progenitor to T cells, and the decreased capacity of equal numbers of T cells to induce GVHD in the high-dose range. Human studies of allogeneic transplantation of G-CSF–mobilized blood mononuclear cells differed from the current studies in mice. In addition to the species differences, the former used major histocompatibility complex (MHC)-matched donor-host combinations and posttransplant drugs to suppress GVHD4-6 and the latter studies crossed MHC barriers and used no immunosuppressive drugs.
The marked inability of G-CSF–treated mononuclear cells to induce acute GVHD as compared with untreated cells may have been due to the different concentrations and balances of the different T-cell subsets and to changes in the function of CD4+ and CD8+ T cells. To study the latter possibility, purified CD4+ and CD8+ T cells were obtained by negative selection by flow cytometry. The survival of allogeneic hosts receiving C57BL/Ka marrow cells and G-CSF–treated CD4+ and CD8+ T cells was significantly prolonged as compared with those receiving marrow cells and equal numbers of the untreated T cells. Thus, G-CSF alters the function of the CD4+ and CD8+ T cells such that their capacity to induce lethal GVHD is reduced. This may be due to changes in the pattern of cytokine secretion by T cells, because a previous study showed that splenic T cells in G-CSF–treated mice shifted the cytokine profile to favor Th2 as compared with Th1 cytokines.8
Accordingly, the unfractionated blood mononuclear cells as well as purified blood CD4+ and CD8+ T cells were activated in vitro with calcium ionophore and PMA and the secretion of IL-2, IL-4, and IFN-γ was measured. Comparison of unfractionated cells from treated and untreated mice showed a marked reduction in the secretion of IL-2 and IFN-γ after G-CSF treatment. Although small amounts of IL-4 were secreted by either the untreated or treated unfractionated cells, more than a 10-fold increase in IL-4 secretion was observed in treated low-density cells as compared with treated unfractionated cells. This may be related to the increased percentage of CD4−CD8− NK1.1+ T cells in the low-density cells, because NK1.1 T cells have been reported to secrete large amounts of IL-4.11,16 Decreased IL-2 and IFN-γ secretion and increased IL-4 secretion was observed using the purified CD4+ and CD8+ T cells from treated as compared with untreated mice. The results indicate that G-CSF induces a shift toward Th2 cytokine secretion in CD4+ and CD8+ T cells in the blood, as well as in the spleen.8 However, the levels of IL-4 secretion from the treated T cells were still fivefold to 10-fold lower than that from equal numbers of the whole population of treated low-density cells. This suggests that cells other than CD4+ or CD8+ T cells (ie, CD4−CD8− T cells) in the low-density population are secreting large amounts of IL-4 and contribute to the suppression of GVHD. The latter cells may be similar or identical to low-density CD4−CD8− T cells in the untreated marrow17,18 or to cloned CD4−CD8− T cells from the spleen19 that suppress GVHD.
In conclusion, the reduced capacity of G-CSF–treated blood mononuclear cells to induce acute GVHD appears to be related to the reduced percentage of CD4+ and CD8+ T cells, changes in the cytokine secretion of CD4+ and CD8+ T cells, and the influx of a variety of marrow-derived cell subsets, which may have important immune suppressive/regulatory activities, including the secretion of cytokines such as IL-4.
ACKNOWLEDGMENT
We thank V. Cleaver for preparation of the manuscript, A. Mukhopadyay for technical assistance, M. Garciá-Ojeda for help with preparation of FACS graphics, and Drs B.W. Brown and J. Halpern for biostatistical consultation.
Supported by National Cancer Institute Grant No. R01 55793 and by National Heart, Lung and Blood Institute Grant No. R01 58250. D.Z. was supported in part by funds from Activated Cell Therapy, Inc (Mountain View, CA).
Address reprint requests to Samuel Strober, MD, Division of Immunology and Rheumatology, Room S105B, Stanford University School of Medicine, Stanford, CA 94305-5111.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal