Abstract
To investigate erythropoiesis in paroxysmal nocturnal hemoglobinuria (PNH), we studied the expression of glycosylphosphatidylinositol (GPI)-anchored membrane proteins on circulating erythrocytes and erythroblasts obtained by erythropoietic cell culture in nine patients with this disease. One-color and two-color flow cytometric analyses were performed using monoclonal antibodies for decay-accelerating factor (DAF ) and/or CD59/membrane attack complex-inhibitory factor (MACIF). In addition, terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick end-labeling (TUNEL) analysis was performed to assess apoptosis of erythroblasts from six patients. On flow cytometric analysis, cases 1 to 6 had positive and negative erythrocyte populations, case 7 intermediate and negative populations, case 8 positive, intermediate, and negative populations, and case 9 a single double-negative population. In addition, cases 1 to 6 and 8 had positive, intermediate, and negative erythroblast populations, while cases 7 and 9 had intermediate and negative populations. The percentage of double-negative erythrocytes showed a significant correlation with that of double-negative erythroblasts (r = .741, P < .05). In seven of nine patients, more erythroblasts than erythrocytes were negative for the two membrane proteins. Also, some patients with an intermediate population of erythrocytes did not necessarily show an increase of PNH II erythroblasts. Apoptosis of PNH erythroblasts was also detected, but the percentage of apoptotic cells in PNH patients showed no difference from that in healthy volunteers. These findings suggest that the final phenotype of mature erythrocytes in PNH is determined during maturation from erythroblasts to erythrocytes by the disappearance or persistence of PNH II erythroblasts. In addition, PNH erythroblasts in vitro may be partly lost by apoptosis, but apoptosis does not play an important role in determining GPI-linked protein expression.
PAROXYSMAL NOCTURNAL hemoglobinuria (PNH) is an acquired disorder characterized by the increased susceptibility of erythrocytes to complement-mediated hemolysis.1 This increased susceptibility to complement also appears to affect platelets and granulocytes of PNH patients.2-4 Thus, PNH may be a stem cell disorder, since all hematopoietic lineages are affected,5 indicating that the abnormality must occur at least at the level of the pluripotent hematopoietic stem cell. In fact, it has been reported that a decrease of progenitors and/or hypersensitivity of precursors to complement can be detected in PNH, with the latter finding implying some changes of cell characteristics, such as proliferation and differentiation, due to complement-mediated effects on erythropoietic precursor cells in the bone marrow.6-11 In rare cases, PNH has been reported to transform into acute leukemia, suggesting that it is related to the myelodysplastic syndrome.12-14
It was recently reported that the increased susceptibility of PNH erythrocytes to complement-mediated lysis is related to a deficiency of complement regulatory membrane proteins, especially decay-accelerating factor (DAF/CD55),15,16 homologous restriction factor17/C8-binding protein,18 and membrane attack complex–inhibitory factor (MACIF/CD59),19-22 all of which are glycosylphosphatidylinositol (GPI)-anchored membrane proteins.23 Therefore, the phenotype of PNH erythrocytes determined in the complement lysis sensitivity (CLS) test24 is related to the extent of membrane deficiency of DAF and CD59/MACIF.25-27 Recent studies have shown that the number of circulating GPI-deficient neutrophils and reticulocytes in PNH patients is typically higher than the number of affected erythrocytes.15,28-30 In fact, flow cytometric detection of affected granulocytes can be used for early diagnosis of PNH.31-33 Although hypersensitivity of erythroblasts in vivo and in vitro from PNH patients has previously been demonstrated using Ham's test10,34 or the CLS test,8,11,24 the erythroblast phenotypes present in this disease and their membrane expression of DAF and CD59/MACIF have not been clarified. Therefore, the relationship between erythrocyte and erythroblast phenotypes in PNH is still unknown. A recent study showed that hematopoietic cells and stromal cells of the bone marrow undergo apoptosis in most patients with myelodysplastic syndrome.35,36 Although it has been suggested that PNH is related to myelodysplastic syndrome,12-14 apoptosis of erythroblasts in PNH patients has not been investigated either.
To investigate the process of erythropoiesis in PNH, we studied the expression of DAF and CD59/MACIF by circulating erythrocytes and by erythroblasts obtained from erythropoietic cell culture in nine PNH patients using one- and two-color flow cytometric analysis. In addition, we investigated whether the cultured erythroblasts underwent apoptosis using the terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick end-labeling (TUNEL) method37 in six of nine of these patients.
SUBJECTS AND METHODS
PNH patients.Peripheral blood and bone marrow samples from nine patients with PNH were studied. The diagnosis of PNH was made on the basis of medical history, clinical features, laboratory findings, and a positive Ham's test.34 At the time of study, all patients were clinically stable with no episodes of severe hemolysis. Clinical and hematologic data for the nine patients are summarized in Table 1. Case 4 showed transformation from PNH to aplastic anemia via aplastic anemia-PNH syndrome. In this patient, packed red blood cell transfusion to a total volume of 12,800 mL was performed in the 12 months before examination because of severe anemia associated with hypocellular marrow, and the most recent transfusion was given only 3 weeks before the study. As a control, peripheral blood samples were also obtained from 31 healthy volunteers for flow cytometric analysis of erythrocytes and from four volunteers for erythropoietic cell culture. Informed consent was obtained from all subjects.
Hematologic Parameters, Transfusion Requirement, and Two-Color Flow Cytometric Analysis of DAF and CD59/MACIF Expression by Erythrocytes in Nine PNH Patients
| Case No. . | Age/Sex . | Hb (g/dL) . | Reticulocytes (%) . | Bone Marrow Cellularity (× 104/μL) . | Transfusion Requirement* . | Two-Color Analysis (%) . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | Positive . | Intermediate . | Negative . |
| 1† | 50/M | 9.2 | 2.6 | 25.0 | None | 66.0 | ND | 33.8 |
| 2 | 65/M | 15.1 | 2.6 | 19.2 | None | 35.1 | ND | 64.4 |
| 3 | 61/M | 8.8 | 2.5 | 5.0 | None | 88.9 | ND | 10.9 |
| 4† | 24/F | 5.4 | 0.2 | 0.8 | Frequent | 98.1 | ND | 1.6 |
| 5 | 20/M | 11.4 | 2.6 | 10.0 | None | 88.8 | ND | 10.7 |
| 6 | 58/M | 6.7 | 6.8 | 11.0 | None | 54.9 | ND | 45.0 |
| 7 | 77/M | 5.5 | 2.4 | 3.1 | None | ND | 78.1 | 21.8 |
| 8 | 24/F | 6.3 | 7.6 | 34.7 | None | 5.4 | 78.6 | 15.9 |
| 9 | 38/F | 8.8 | 10.4 | 2.3 | None | ND | ND | 95.0 |
| Case No. . | Age/Sex . | Hb (g/dL) . | Reticulocytes (%) . | Bone Marrow Cellularity (× 104/μL) . | Transfusion Requirement* . | Two-Color Analysis (%) . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | Positive . | Intermediate . | Negative . |
| 1† | 50/M | 9.2 | 2.6 | 25.0 | None | 66.0 | ND | 33.8 |
| 2 | 65/M | 15.1 | 2.6 | 19.2 | None | 35.1 | ND | 64.4 |
| 3 | 61/M | 8.8 | 2.5 | 5.0 | None | 88.9 | ND | 10.9 |
| 4† | 24/F | 5.4 | 0.2 | 0.8 | Frequent | 98.1 | ND | 1.6 |
| 5 | 20/M | 11.4 | 2.6 | 10.0 | None | 88.8 | ND | 10.7 |
| 6 | 58/M | 6.7 | 6.8 | 11.0 | None | 54.9 | ND | 45.0 |
| 7 | 77/M | 5.5 | 2.4 | 3.1 | None | ND | 78.1 | 21.8 |
| 8 | 24/F | 6.3 | 7.6 | 34.7 | None | 5.4 | 78.6 | 15.9 |
| 9 | 38/F | 8.8 | 10.4 | 2.3 | None | ND | ND | 95.0 |
Abbreviations: Hb, hemoglobin; ND, not detectable.
The transfusion requirement was judged from the history of transfusion in the 12-month period before each experiment.
Aplastic anemia progressed to PNH.
Monoclonal antibodies.Three mouse monoclonal antibodies to DAF [IA10 (IgG2a), IIH6 (IgG1), and VIIIA7 (IgG1)]28 and a mouse monoclonal antibody to CD59/MACIF (3E1, IgG1)19 were used. Irrelevant monoclonal antibodies of the same subclass were also used as negative controls for flow cytometric analysis.25 27
Erythropoietic cell culture.Bone marrow cells were aspirated from the sternum of each patient into syringes containing preservative-free heparin, and whole blood samples from four healthy volunteers were collected into syringes containing preservative-free heparin. Mononuclear cells were separated from the bone marrow blood of nine PNH patients using Ficoll-Isopaque (Pharmacia, Uppsala, Sweden), and 1 × 105 cells were used in the experiments. Erythropoietic cell culture was performed using a modification38 of the methylcellulose clonal assay developed by Iscove et al.39 After culture for 14 days, well-hemoglobinized erythroid colonies were identified as primitive erythroid progenitors (BFU-E) and counted using an inverted microscope. Recombinant erythropoietin (Kirin Brewery Co, Tokyo, Japan) was used at a concentration of 2.0 U/mL. In control experiments, 3 × 105 peripheral blood mononuclear cells from four healthy volunteers were used. Between 30 and 50 BFU-E colonies were collected from the healthy controls and from cases 1, 2, and 5 to 9, whereas all the BFU-E colonies were harvested from cases 3 and 4. BFU-E colonies obtained from each subject were pooled in a microcentrifuge tube (Sanko Junyaku Co, Tokyo, Japan), and washed three times with RPMI 1640 medium (Flow Laboratories Inc, Rockville, MD) containing 10% heat-inactivated fetal calf serum (Flow Laboratories) and three times with phosphate-buffered saline containing 1% bovine serum albumin and 0.1% sodium azide (PBS-BSA). After cell counting, aliquots of the suspensions were used for flow cytometric analysis. In addition, between 30 and 50 BFU-E colonies were collected from cases 1, 2, 3, 6, 7, and 8, as well as from the same healthy controls (all BFU-E colonies were harvested from case 3) and washed three times with RPMI 1640 medium containing 10% heat-inactivated fetal calf serum. After preparing the pellets by centrifugation, the number of erythroblasts was counted and aliquots of the suspensions were used for TUNEL analysis.
To examine the purity and maturity of erythroblasts obtained from BFU-E, smears were prepared from erythroid cell suspensions using a cytocentrifuge (Shandon Elliott, Runcorn Cheshire, UK) and were stained with May-Grünwald and Giemsa solution. Then, 100 to 200 erythroblasts per smear were subjected to morphologic analysis.
Immunofluorescence staining and flow cytometric analysis.Peripheral blood cells and cultured erythroblasts were used for flow cytometric analysis of erythrocytes and erythroblasts, respectively. Two-color analysis was made by flow cytometry after staining with DAF and CD59/MACIF antibodies.25,27 In brief, 2 × 106 cells in 50 μL PBS-BSA were incubated for 30 minutes on ice with 50 μL CD59/MACIF monoclonal antibody19 (40 μg/mL in PBS-BSA) or an irrelevant monoclonal antibody of the same subclass as a control. The cells were washed three times, resuspended in 50 μL PBS-BSA, and mixed with an equal volume of 1:20-diluted fluorescein isothiocyanate–conjugated goat anti–mouse IgG (Tago Inc, Camarillo, CA). Then, normal mouse serum (50 μL) was added to the resuspended cells (50 μL) and the mixture was incubated for 15 minutes on ice. Next, the cells were stained for DAF by incubation with 50 μL of a mixture of the biotinylated DAF antibodies28 (10 μg/mL each) plus phycoerythrin-conjugated streptavidin (Biomeda Corp, Foster, CA) and analyzed using a FACScan (Becton Dickinson, Mountain View, CA). Cells subjected to negative-control staining were analyzed first to determine the regions positive for DAF only, positive for both antigens, negative for both antigens, and positive for CD59/MACIF only (regions 1 to 4, respectively). To enhance cell purity, gating was performed during analysis of cultured erythroblasts. The number of gated events in each subject was set at 10,000, except for case 4 (1,200 events).
One-color analysis of DAF or CD59/MACIF expression by erythrocytes and erythroblasts was also performed by flow cytometry using the same specimens as for two-color analysis.
In both one-color and two-color analyses, the negative fraction was defined as cells having approximately the same low intensity as the negative control population, and the positive fraction was defined as the cell population with an intensity similar to that of the positive control population. In addition, the intermediate fraction was defined as the population between the negative and positive fractions. The percentage of each fraction was calculated as follows: 100 × (number of cells in each population/total number of cells tested).
TUNEL analysis.Erythroblasts obtained from erythropoietic cell culture were used for TUNEL analysis37 with minor modifications. Briefly, 1 × 105 cells were prepared in 100 μL 4% neutral buffered formaldehyde solution and incubated for 20 minutes at room temperature. Aliquots of the suspensions (1 × 104 cells) were plated onto slides using a cytocentrifuge, followed by air-drying at room temperature. After washing the slides twice with PBS, they were dried and stored at room temperature until TUNEL analysis. The slides were then incubated with 2% H2O2 for 7 minutes for inactivation of endogeneous peroxidase, and then were incubated for 60 minutes at 37°C with 0.3 EU/μL terminal deoxynucleotidyl transferase (Takara Schuzo Co, Kyoto, Japan) and 0.04 nmol/μL biotinylated dUTP (Boehringer, Mannheim, Germany) in terminal deoxynucleotidyl transferase buffer containing 30 mmol/L Tris-HCl (pH 7.2), 140 mmol/L sodium cacodylate, and 1 mmol/L cobalt chloride. The reaction was terminated with buffer containing 30 mmol/L sodium citrate and 300 mmol/L NaCl. The slides were coated with avidin-conjugated peroxidase (Medical and Biological Laboratories Co, Nagoya, Japan), and peroxidase was visualized using the chromogen 3,3′-diaminobenzidine and H2O2 . Counterstaining was performed with methylgreen. Cells with nuclear staining were judged to show apoptosis, and 100 to 200 erythroblasts per smear were counted under a microscope to determine the percentage of apoptotic cells. Duplicate slides for each subject were counted by two hematologists, and the percentage of apoptotic cells was calculated as follows: 100 × (number of apoptotic cells counted in each smear/total number of cells counted in each smear).
Statistical analysis.The relationship between the size of the double-negative population of erythrocytes and that of erythroblasts in nine PNH patients was analyzed with correlation coefficient. In addition, the difference between the percentage of apoptotic erythroblasts in six PNH patients or in individual patients and that in healthy volunteers was assessed for statistical significance using Student's t test.
RESULTS
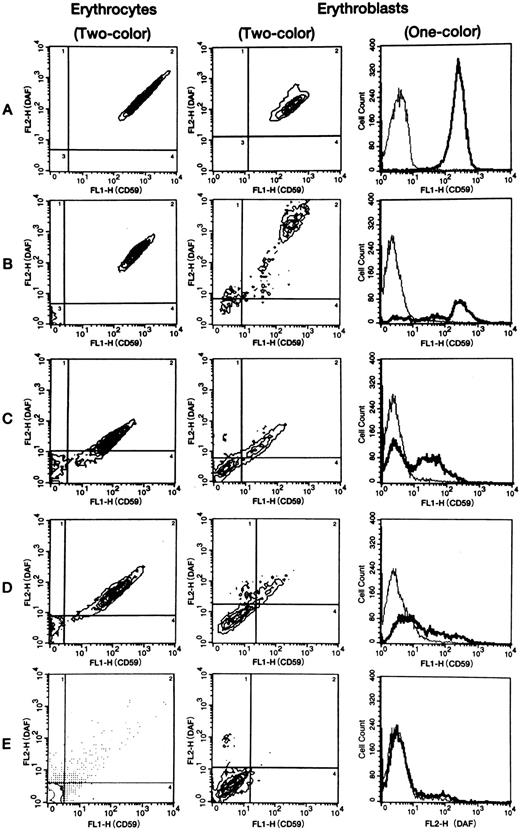
DAF and CD59/MACIF expression by erythrocytes.Two-color flow cytometric analysis was performed to assess DAF and CD59/MACIF expression by erythrocytes from all nine PNH patients and 31 healthy volunteers. Erythrocytes of all healthy controls consisted of a single population with double-positive fluorescence (Fig 1A). In contrast, erythrocytes from PNH cases 1 to 6 consisted of separate positive and negative populations (Table 1 and Fig 1B). Erythrocytes of cases 7, 8, and 9, respectively, consisted of intermediate and negative populations, positive, intermediate, and negative populations, and a single double-negative population (Table 1 and Fig 1C to E). The erythrocyte populations found in all PNH patients are summarized in Table 1. The double-negative population varied from 1.6% to 95%. In case 4, the size of the negative population of erythrocytes was probably affected by recent blood transfusion.
Representative flow cytometric patterns of membrane CD59/MACIF and/or DAF expression by normal or PNH erythrocytes and erythroblasts. In 2-color analysis, 4 regions (shown by the lines) were set using the flow cytometric data of negative controls examined at the same time. Expression of CD59/MACIF and DAF by PNH erythrocytes and erythroblasts was defined as negative, intermediate, or positive based on the intensity of fluorescein isothiocyanate fluorescence (FL1) and phycoerythrin fluorescence (FL2). Erythrocytes of all 31 volunteers and erythroblasts of four volunteers consisted of a single population showing high-level expression of both proteins. In 1-color analysis of CD59/MACIF or DAF expression by PNH erythrocytes and erythroblasts, 3 fractions (negative, intermediate, and positive) were also detected. In contrast, normal erythrocytes and erythroblasts showed a single population with high-level expression. Thin lines show negative controls, thick lines show tested subjects, and vertical axes represent cell counts. A, normal volunteer; B, case 3; C, case 7; D, case 8; E, case 9.
Representative flow cytometric patterns of membrane CD59/MACIF and/or DAF expression by normal or PNH erythrocytes and erythroblasts. In 2-color analysis, 4 regions (shown by the lines) were set using the flow cytometric data of negative controls examined at the same time. Expression of CD59/MACIF and DAF by PNH erythrocytes and erythroblasts was defined as negative, intermediate, or positive based on the intensity of fluorescein isothiocyanate fluorescence (FL1) and phycoerythrin fluorescence (FL2). Erythrocytes of all 31 volunteers and erythroblasts of four volunteers consisted of a single population showing high-level expression of both proteins. In 1-color analysis of CD59/MACIF or DAF expression by PNH erythrocytes and erythroblasts, 3 fractions (negative, intermediate, and positive) were also detected. In contrast, normal erythrocytes and erythroblasts showed a single population with high-level expression. Thin lines show negative controls, thick lines show tested subjects, and vertical axes represent cell counts. A, normal volunteer; B, case 3; C, case 7; D, case 8; E, case 9.
DAF and CD59/MACIF expression by erythroblasts.One- and two-color flow cytometric analysis was performed to assess DAF and/or CD59/MACIF expression by erythroblasts from all nine PNH patients and four healthy controls. Erythroblasts of the healthy controls consisted of a single population with double-positive fluorescence (Fig 1A). In contrast, erythroblasts from PNH cases 1 to 6 consisted of positive, intermediate, and negative populations on both one- and two-color flow cytometric analysis (Fig 1B and Table 2). In cases 1, 4, and 5, discordant expression of DAF and CD59/MACIF was detected by one-color analysis (Table 2). In cases 7 and 9, erythroblasts consisted of intermediate and negative populations (Fig 1C and E, and Table 2), while case 8 had positive, intermediate, and negative populations (Fig 1D and Table 2). In case 9, the intermediate population was not so clear on two-color analysis (Fig 1E), but we found a distinct intermediate population of erythroblasts derived from BFU-E colonies from the peripheral blood (data not shown) in an examination performed at almost the same time. The populations shown in each subject by one- and two-color analysis are summarized in Table 2. The double-negative population detected by two-color analysis ranged from 16.3% to 80.8% of all erythroblasts in the PNH patients. The intermediate population detected by two-color analysis was less than 10% in cases 1 and 2, whereas it was above 10% in the other patients. Although the intermediate population was higher than average in cases 7 and 8, who also had an intermediate population of erythrocytes, the intermediate population of erythroblasts was also increased in cases 3 and 6, who had no intermediate erythrocytes. One-color analysis of DAF expression showed that the intermediate population in cases 1 and 3 to 6 was almost the same size as in cases 7 and 8.
Two-Color and One-Color Flow Cytometric Analysis of CD59/MACIF and/or DAF Expression by Erythroblasts
| . | . | . | . | One-Color Analysis (%) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . |
| . | Two-Color Analysis (%) . | CD59/MACIF . | DAF . | ||||||
| . | . | . | . | . | . | . | . | . | . |
| Case No. . | Positive . | Intermediate . | Negative . | Positive . | Intermediate . | Negative . | Positive . | Intermediate . | Negative . |
| PNH patients | |||||||||
| 1 | 59.5 | 4.9 | 35.6 | 55.9 | 5.7 | 38.4 | 19.3 | 44.7 | 35.9 |
| 2 | 12.6 | 6.7 | 80.8 | 11.3 | 4.8 | 83.8 | 4.3 | 8.7 | 87.0 |
| 3 | 60.0 | 23.7 | 16.3 | 59.9 | 21.5 | 18.6 | 55.1 | 27.5 | 17.4 |
| 4 | 44.1 | 16.9 | 39.0 | 37.0 | 18.0 | 45.0 | 47.3 | 36.1 | 16.6 |
| 5 | 37.6 | 12.6 | 49.8 | 36.4 | 19.7 | 43.9 | 16.8 | 34.7 | 48.5 |
| 6 | 15.1 | 49.1 | 35.8 | 10.7 | 42.4 | 46.9 | 10.6 | 42.9 | 46.5 |
| 7 | — | 62.4 | 37.6 | — | 48.0 | 52.0 | — | 52.1 | 47.9 |
| 8 | 16.4 | 30.0 | 53.6 | 8.4 | 24.9 | 66.7 | 14.7 | 32.5 | 52.8 |
| 9 | — | 19.5 | 80.4 | — | 8.5 | 91.5 | — | 13.2 | 86.8 |
| Normal individuals | |||||||||
| 1 | 98.9 | — | — | 99.0 | — | — | 98.4 | — | — |
| 2 | 98.7 | — | — | 98.9 | — | — | 98.4 | — | — |
| 3 | 98.1 | — | — | 98.3 | — | — | 97.5 | — | — |
| 4 | 99.0 | — | — | 99.1 | — | — | 98.9 | — | — |
| . | . | . | . | One-Color Analysis (%) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . |
| . | Two-Color Analysis (%) . | CD59/MACIF . | DAF . | ||||||
| . | . | . | . | . | . | . | . | . | . |
| Case No. . | Positive . | Intermediate . | Negative . | Positive . | Intermediate . | Negative . | Positive . | Intermediate . | Negative . |
| PNH patients | |||||||||
| 1 | 59.5 | 4.9 | 35.6 | 55.9 | 5.7 | 38.4 | 19.3 | 44.7 | 35.9 |
| 2 | 12.6 | 6.7 | 80.8 | 11.3 | 4.8 | 83.8 | 4.3 | 8.7 | 87.0 |
| 3 | 60.0 | 23.7 | 16.3 | 59.9 | 21.5 | 18.6 | 55.1 | 27.5 | 17.4 |
| 4 | 44.1 | 16.9 | 39.0 | 37.0 | 18.0 | 45.0 | 47.3 | 36.1 | 16.6 |
| 5 | 37.6 | 12.6 | 49.8 | 36.4 | 19.7 | 43.9 | 16.8 | 34.7 | 48.5 |
| 6 | 15.1 | 49.1 | 35.8 | 10.7 | 42.4 | 46.9 | 10.6 | 42.9 | 46.5 |
| 7 | — | 62.4 | 37.6 | — | 48.0 | 52.0 | — | 52.1 | 47.9 |
| 8 | 16.4 | 30.0 | 53.6 | 8.4 | 24.9 | 66.7 | 14.7 | 32.5 | 52.8 |
| 9 | — | 19.5 | 80.4 | — | 8.5 | 91.5 | — | 13.2 | 86.8 |
| Normal individuals | |||||||||
| 1 | 98.9 | — | — | 99.0 | — | — | 98.4 | — | — |
| 2 | 98.7 | — | — | 98.9 | — | — | 98.4 | — | — |
| 3 | 98.1 | — | — | 98.3 | — | — | 97.5 | — | — |
| 4 | 99.0 | — | — | 99.1 | — | — | 98.9 | — | — |
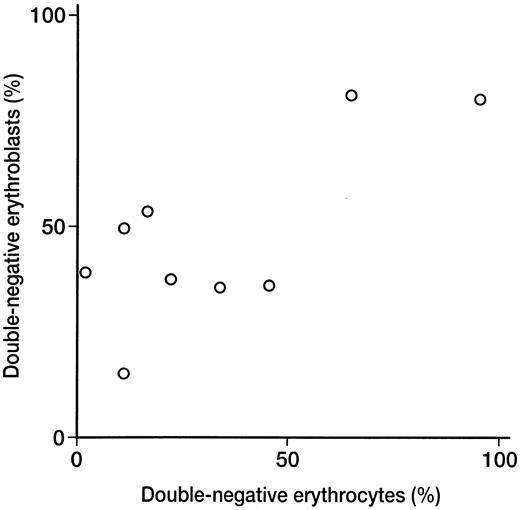
Relationship between double-negative erythrocytes and erythroblasts.When the percentage of double-negative erythrocytes was compared with that of double-negative erythroblasts detected by two-color analysis in nine PNH patients, a significant correlation was found (r = .741, P < .05; Fig 2). In seven of nine patients, the percentage of erythroblasts negative for DAF and CD59/MACIF was higher than the percentage of negative erythrocytes (Tables 1 and 2).
Relationship between double-negative erythroblast and erythrocyte populations detected by 2-color flow cytometric analysis in 9 PNH patients. There was a significant correlation between the 2 populations (r = .741, P < .05).
Relationship between double-negative erythroblast and erythrocyte populations detected by 2-color flow cytometric analysis in 9 PNH patients. There was a significant correlation between the 2 populations (r = .741, P < .05).
Erythropoietic cell culture.The number of BFU-E colonies and the morphologic features of erythroblasts cultured from all PNH patients and healthy controls are summarized in Table 3. Six of nine PNH patients showed a decrease of bone marrow BFU-E colonies when compared with healthy controls (51 ∼ 123, n = 5). Microscopic examination of the erythropoietic cell cultures indicated that cell purity was almost 100% in all subjects (data not shown), with the majority of erythroblasts being polychromatophilic or orthochromatophilic (Table 3).
Number of BFU-E Colonies Obtained by Erythropoietic Cell Culture, Morphologic Features of Cultured Erythroblasts, and Percentage of Apoptotic Erythroblasts on TUNEL Analysis
| Case No. . | No. of BFU-E Colonies (/1.0 × 105 cells)3-150 . | Morphologic Findings (%) . | Apoptotic Erythroblasts (%) . | ||
|---|---|---|---|---|---|
| . | . | BAS . | POLY . | ORTH . | . |
| PNH patients | |||||
| 1 | 51, 48, 52 | 3.0 | 33.0 | 64.0 | 2.6, 3.1 |
| 2 | 73, 69, 76 | 1.0 | 42.5 | 56.5 | 3.2, 3.53-151 |
| 3 | 9, 7, 12 | 0.0 | 18.5 | 81.5 | 1.8, 2.0 |
| 4 | 4, 2, 3 | 0.0 | 52.0 | 48.0 | NT |
| 5 | 62, 58, 59 | 1.0 | 21.5 | 77.5 | NT |
| 6 | 14, 13, 10 | 4.0 | 28.0 | 68.0 | 3.3, 3.63-151 |
| 7 | 61, 70, 63 | 0.0 | 40.5 | 59.5 | 1.4, 1.8 |
| 8 | 22, 19, 20 | 2.0 | 10.0 | 88.0 | 1.1, 1.5 |
| 9 | 23, 16, 18 | 0.0 | 39.5 | 60.5 | NT |
| Normal individuals | |||||
| 1 | 48, 62, 53 | 3.6 | 58.2 | 38.2 | 0.6, 0.6 |
| 2 | 54, 56, 52 | 2.0 | 50.0 | 48.0 | 1.6, 1.8 |
| 3 | 55, 63, 50 | 0.0 | 72.0 | 28.0 | 1.9, 1.5 |
| 4 | 60, 48, 54 | 1.4 | 48.0 | 50.6 | 1.3, 1.2 |
| Case No. . | No. of BFU-E Colonies (/1.0 × 105 cells)3-150 . | Morphologic Findings (%) . | Apoptotic Erythroblasts (%) . | ||
|---|---|---|---|---|---|
| . | . | BAS . | POLY . | ORTH . | . |
| PNH patients | |||||
| 1 | 51, 48, 52 | 3.0 | 33.0 | 64.0 | 2.6, 3.1 |
| 2 | 73, 69, 76 | 1.0 | 42.5 | 56.5 | 3.2, 3.53-151 |
| 3 | 9, 7, 12 | 0.0 | 18.5 | 81.5 | 1.8, 2.0 |
| 4 | 4, 2, 3 | 0.0 | 52.0 | 48.0 | NT |
| 5 | 62, 58, 59 | 1.0 | 21.5 | 77.5 | NT |
| 6 | 14, 13, 10 | 4.0 | 28.0 | 68.0 | 3.3, 3.63-151 |
| 7 | 61, 70, 63 | 0.0 | 40.5 | 59.5 | 1.4, 1.8 |
| 8 | 22, 19, 20 | 2.0 | 10.0 | 88.0 | 1.1, 1.5 |
| 9 | 23, 16, 18 | 0.0 | 39.5 | 60.5 | NT |
| Normal individuals | |||||
| 1 | 48, 62, 53 | 3.6 | 58.2 | 38.2 | 0.6, 0.6 |
| 2 | 54, 56, 52 | 2.0 | 50.0 | 48.0 | 1.6, 1.8 |
| 3 | 55, 63, 50 | 0.0 | 72.0 | 28.0 | 1.9, 1.5 |
| 4 | 60, 48, 54 | 1.4 | 48.0 | 50.6 | 1.3, 1.2 |
Abbreviations: BAS, basochromatophilic erythroblasts; POLY, polychromatophilic erythroblasts; ORTH, orthochromatophilic erythroblasts; NT, not tested.
In the normal individuals, 3 × 105 peripheral blood mononuclear cells were used.
P < .05 v normal individuals by Student's t test.
Apoptosis of erythroblasts.TUNEL analysis was performed to assess apoptosis of erythroblasts obtained from erythropoietic cell cultures in six of nine PNH patients and four of 31 healthy controls. The results are shown in Table 3. Although the percentage of apoptotic cells in cases 2 and 6 showed a significant difference (P < .05) versus that in the erythroblasts of healthy controls, the percentage of apoptotic cells showed no overall significant difference between control and PNH groups.
DISCUSSION
To investigate the process of erythropoiesis in PNH, we studied the expression of DAF and CD59/MACIF by circulating erythrocytes and cultured erythroblasts from nine PNH patients by flow cytometry. In six out of nine PNH patients, erythrocytes consisted of positive and negative populations, whereas erythrocytes of the other three patients consisted of intermediate and negative populations, positive, intermediate, and negative populations, and a single double-negative population, respectively (Fig 1 and Table 1). Although the proportion of erythrocytes negative for DAF and CD59/MACIF was higher in cases 2 and 9 than in the other patients, both patients also had a high hemoglobin level (Table 1). Case 2 had received androgen therapy for 95 months before the study, and the hemoglobin was increased as a result. During this period, episodes of hemoglobinuria occurred every 1 to 3 months, although these were not severe. Case 9 had been treated with prednisolone and was clinically stable. It is probable that progression of anemia and episodes of hemoglobinuria were largely prevented in this patient by administration of prednisolone.40,41 Recently, Nakakuma et al.42 reported that autologous serum selectively hemolyzed Th+ PNH erythrocytes but not Th− PNH erythrocytes or Th+ control erythrocytes in vitro. This may also help to explain why cases 2 and 9 had a high hemoglobin and did not suffer from severe hemoglobinuria. The serum concentration of C9 was within the normal range in both patients (case 2, 5.8 mg/dl; case 9, 7.2 mg/dl). We previously reported that two-color flow cytometric analysis of erythrocytes is superior to the traditional tests for diagnosis of PNH, after comparing two-color analysis with the CLS test in 59 patients.27 Two-color analysis can be used both for diagnosis and for determining the phenotype of PNH erythrocytes.43 In this study, the percentage of patients with PNH II erythrocytes (the intermediate population on two-color analysis) and PNH III erythrocytes (the negative population) was lower than reported by Rosse44 or Shichishima et al,45 and the percentage of patients with PNH I (the positive population), II, and III erythrocytes was higher.27 Unfortunately, none of our patients had PNH I and II erythrocytes or PNH II erythrocytes alone in the present study. However, it is well known that most patients with this disease have PNH I and III erythrocytes. It has been reported that the complement sensitivity of PNH erythroblasts in vivo and in vitro has almost the same characteristics, as shown by the relationship between the complement concentration and the percentage of cells undergoing lysis in the CLS test.8,11 24 In the present study, we indicated that the percentage of double-negative erythrocytes showed a significant correlation with that of double-negative erythroblasts in nine PNH patients (Fig 2). These findings suggest that the phenotype of PNH erythroblasts in vitro is largely related to that of bone marrow erythroblasts.
It has been reported that hypersensitivity of erythroid precursors in vitro and erythroblasts in vitro or in vivo to complement can be detected by the sugar-water test,46 Ham's test,34 or the CLS test24 in patients with PNH.7,8,10,11 This finding may imply some complement-mediated changes of the characteristics of erythroid precursor cells in the bone marrow, with a resulting decrease of erythroid progenitor cells. In the present study, six of nine PNH patients showed a decrease of BFU-E colonies in the bone marrow, and the patient who underwent transformation to aplastic anemia showed a marked decrease (Table 3). The expression of DAF and CD59/MACIF on erythroid precursors in vitro or in vivo has been studied in PNH patients by three groups. Moore et al47 reported that DAF-deficient cultured erythroblasts are derived from BFU-E expressing DAF, and that DAF-negative erythroid progenitors do not form BFU-E colonies. They also reported that cultured erythroblasts derived from individual BFU-E colonies included both DAF-positive and DAF-negative cells. On the other hand, Kanamaru et al48 reported that DAF-negative cultured erythroblasts were derived from both DAF-negative and -positive erythroid precursor cells. Both of these reports could suggest that the expression of GPI-anchored membrane proteins by PNH cultured erythroblasts is determined during differentiation of erythroid progenitor cells. Moreover, Terstappen et al49 separated early and late erythroid progenitors in vivo by flow cytometry using CD71 and CD45 monoclonal antibodies in two patients with PNH, and found that these cells consisted of CD59− and CD59+ populations. This suggests that the expression of GPI-anchored proteins by PNH erythroblasts may already be determined at the BFU-E level. Unfortunately, they did not compare the phenotypes of circulating erythrocytes and erythroid precursors in detail and did not assess the intermediate population of erythroid precursors. In the present study, we compared the expression of DAF and CD59/MACIF on circulating erythrocytes versus cultured erythroblasts. We found both PNH II and PNH III erythroblasts in all patients examined irrespective of the presence or absence of PNH II erythrocytes in the peripheral blood (Fig 1 and Tables 1 and 2). This finding suggests that the phenotype of PNH erythrocytes is not determined at the erythroblast level, and that PNH II erythroblasts may disappear during maturation to mature erythrocytes. However, it is not clear from our data whether the phenotype of erythroid precursors changes during erythroid differentiation, or whether erythroid precursors with an intermediate phenotype show impaired erythroid differentiation in vivo. In the present study, one-color analysis of DAF or CD59/MACIF expression by cultured erythroblasts showed discordant expression in some of our PNH patients (cases 1, 4, and 5; Table 2). However, this discordant expression was seen in two or three of the erythroblast populations determined by two-color flow cytometric analysis, probably due to overlapping of the fluorescence distribution curves, suggesting that discordant expression on one-color analysis may not have any specific meaning.
Ware et al30 recently reported that type II reticulocytes in peripheral blood accounted for greater than 5% of cells in only three of 25 PNH patients, although type II mature erythrocytes accounted for greater than 5% of cells in 10 patients. This finding suggests that PNH II erythroblasts may disappear at the reticulocyte level during maturation. However, it may also suggest that the phenotype of PNH erythrocytes is determined in the second stage of maturation from erythroblasts to mature erythrocytes. Our results indicate that if PNH II erythroblasts disappear during maturation, the erythrocytes consist of negative and positive populations (cases 1 to 6) or a double-negative population only (case 9). In contrast, if PNH II erythroblasts survive during maturation, the erythrocytes consist of negative, intermediate, and positive populations (case 8) or negative and intermediate populations (case 7). Therefore, it appears that the final phenotype of mature erythrocytes is determined during maturation from erythroblasts to erythrocytes by the disappearance or persistence of PNH II erythroblasts. Iwamoto et al29 and Ware et al30 both reported that the population of reticulocytes negative for DAF and CD59 in the peripheral blood was markedly higher than the population of negative erythrocytes. In the present study, we found that the percentage of cultured erythroblasts negative for DAF and CD59/MACIF was also higher than that of negative circulating erythrocytes in seven of nine patients, and whereas the percentages were similar in the other two patients (cases 3 and 6). However, cases 7 and 8 with an intermediate population of erythrocytes did not necessarily have a higher percentage of PNH II erythroblasts in comparison to the other patients who had no intermediate population on flow cytometry. This may indicate that the disappearance of PNH II erythroblasts during maturation to reticulocytes is related to other mechanisms in addition to hypersensitivity to complement.
Interestingly, Jones and Morgan50 reported that the loss of DAF and CD59 was closely related to apoptosis in human polymorphonuclear neutrophils, and they observed a marked reduction in the expression of GPI-anchored CD16 by apoptotic polymorphonuclear neutrophils. It is known that apoptosis is a major factor in the maintenance of normal erythropoiesis, and it has been shown that erythropoietin controls erythrocyte production by retarding DNA breakdown and preventing apoptosis in progenitor cells isolated from the spleens of mice infected with the anemia-inducing strain of Friend leukemia virus.51 In the present study, we investigated whether cultured erythroblasts underwent apoptosis in six of nine PNH patients using TUNEL analysis. Apoptosis was observed at a low level, but the percentage of apoptotic PNH erythroblasts was not significantly different from that of healthy erythroblasts (Table 3). Although the percentage of apoptotic cells in cases 2 and 6 was significantly different from that in healthy controls, we could not investigate the phenotype of apoptotic erythroblasts in these patients. Thus, it is possible that some loss of PNH erythroblasts occurs by apoptosis, as well as loss related to complement-mediated damage in the bone marrow.8,11 However, these findings also suggest that apoptosis does not play an important role in determining GPI-linked protein expression, because of the low frequency of apoptotic PNH erythroblasts. Therefore, we could not exclude the possibility that erythroid precursors with an intermediate phenotype in vivo were expanded by erythropoietic cell culture in some of our PNH patients. Some reports have indicated that mutation of the phosphatidylinositol glycan-class A gene,52 which is associated with the first step in GPI core synthesis, may be responsible for the intermediate defect of GPI proteins in PNH cells.53-55 Recently, Endo et al56 found that missense mutations of the phosphatidylinositol glycan-class A gene that cause partial loss of function produced the PNH II phenotype, whereas mutations that cause complete loss of function (eg, deletions, insertions, or nonsense mutations) produced the PNH III phenotype in T-cell lines with various phenotypes derived from a PNH patient. There have been similar reports on PNH patients with two distinct somatic mutations of the phosphatidylinositol glycan-class A gene.55,57,58 However, it is thought at present that the percentage of PNH patients having above two abnormalities of phosphatidylinositol glycan-class A gene is lower than that having PNH II erythrocytes, which was about 40% in our previous study of 59 PNH patients.27 59 Therefore, we think that the discrepancy between the high frequency of PNH patients with intermediate erythroblasts in vitro and the commonly accepted percentage of missense mutations may be resolved by improved methods of gene analysis and/or by analyzing the phosphatidylinositol glycan-class A gene in cultured erythroblasts from PNH patients. Accordingly, a defect of the phosphatidylinositol glycan-class A may sometimes underlie the appearance of an intermediate population, since these defects usually result in a gene than can be transcribed with an altered amino acid sequence. However, it is certainly not clear that this is the only way such cells arise, and our present findings suggest that some cells may lose GPI protein expression during maturation or that PNH erythroblasts may sometimes be lost by apoptosis.
In conclusion, our results suggest that the final phenotype of mature erythrocytes in PNH is determined during maturation from erythroblasts to erythrocytes by the disappearance or persistence of PNH II erythroblasts. In addition, some PNH erythroblasts in vitro may be lost by apoptosis, but apoptosis does not play an important role in determining GPI-linked protein expression.
ACKNOWLEDGMENT
We thank Dr Taroh Kinoshita (Osaka University, Japan) and Dr Yuuji Sugita (Showa University, Japan), who provided the monoclonal antibodies for DAF and CD59/MACIF, respectively.
Supported in part by a Grant for General Scientific Research (06671104) from the Ministry of Education, Science, and Culture of Japan.
Address reprint requests to Yukio Maruyama, MD, First Department of Internal Medicine, Fukushima Medical College, 1 Hikariga-oka, Fukushima, Fukushima 960-12, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal