Abstract
Because in humans mast cells and basophils tend to possess nonsegmented and segmented/multi-lobular nuclei, respectively, nuclear morphology has been a major criterion for assessing the lineage of metachromatic cells of hematopoietic origin. Immature metachromatic cells with mono- and multi-lobular nuclei were both obtained when bone marrow cells from BALB/c mice were cultured for 3 weeks in the presence of interleukin-3. Analogous to the indigenous mature mast cells that reside in the peritoneal cavity and skin, both populations of in vitro–derived cells expressed the surface receptor c-kit, the chymase mouse mast cell protease (mMCP) 5, the tryptase mMCP-6, and the exopeptidase carboxypeptidase A (mMC-CPA). Immunogold electron microscopy confirmed the granule location of mMC-CPA and mMCP-6 in both populations of cells, and cytochemical analysis confirmed the presence of chymotryptic enzymes in the granules. Because mature mast cells possessing multi-lobular nuclei also were occasionally found in the skeletal muscle and jejunum of the BALB/c mouse, the V3 mouse mast cell line was used to investigate the developmental relationship of mast cells that have very different nuclear structures. After the adoptive transfer of V3 mast cells into BALB/c mice, v-abl–immortalized mast cells with mono- and multi-lobular nuclei were detected in the lymph nodes and other tissues of the mastocytosis mice that expressed c-kit, mMCP-5, mMCP-6, and mMC-CPA. These studies indicate that mouse mast cells can exhibit varied nuclear profiles. Moreover, the nuclear morphology of this cell type gives no insight as to its protease phenotype or stage of development.
MAST CELLS, like basophils, possess high-affinity receptors for IgE (FcεRI) on their cell surfaces and contain numerous histamine-rich secretory granules that become metachromatic when stained with cationic dyes. Because antibodies to basophil-specific proteins are not available and because antibodies to mast cell–specific proteins became available only recently, mouse mast cells and basophils have been distinguished primarily by ultrastructural criteria.1-4 Attempts have been made to distinguish basophils from mast cells by the number and morphology of their granules. However, the observation that the granules in a single rat5 or mouse6 7 intraepithelial mast cell can differ dramatically in their size, maturation, and ultrastructure indicates that granule ultrastructure, by itself, cannot be used to definitively phenotype a metachromatic cell. Thus, the nuclear profile of a cell thought to be in the basophil or mast cell lineage is presently one of the major structural features used to distinguish in vivo–differentiated mouse basophils and mast cells.
Immature populations of FcεRI+/metachromatic cells are obtained when progenitor cells from the bone marrow (mBMMC) and other organs are cultured for 3 to 4 weeks in the presence of purified interleukin-3 (IL-3), recombinant IL-3, or IL-3–enriched cell-conditioned media.8-11 Most of the cells in these different cultures are mononuclear but some possess multi-lobular nuclei. While Galli et al12-14 concluded that this latter population of cells in the cultures probably are mast cells, Rottem et al15 concluded that they probably are basophils.
Because mature human mast cells expresses substantially more c-kit on their surfaces than mature human basophils, the relative abundance of this plasma membrane receptor is regularly used to distinguish the two populations of cells in humans.16 Mouse mast cells also express c-kit on their surfaces.17 Moreover, they store in their secretory granules various combinations of an exopeptidase (termed mouse mast cell carboxypeptidase A [mMC-CPA]) and at least nine different serine proteases (termed mouse mast cell protease [mMCP] 1 to mMCP-9).18-29 cDNAs and genes that encode all of these granule proteases except mMCP-3 have been cloned and sequenced, and protease-specific antibodies have been obtained in rabbits that recognize mMCP-1, mMCP-2, mMCP-5, mMCP-6, and mMCP-7.7,30-33 Along with the cDNAs, the protease-specific antibodies have been useful for phenotyping in vivo–differentiated mouse mast cells and for monitoring the regulated differentiation and maturation of hematopoietic progenitor cells into mast cells in vitro and in vivo. As assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)/immunoblot and N-terminal amino acid analysis of resolved granular protein, RNA blot analysis, and/or immunohistochemical analysis, mast cells have been detected at different tissue sites in the BALB/c mouse that express various panels of granule proteases. For example, the mast cells that increase in number in the jejunal lamina propria of V3 mastocytosis mice and in the jejunal epithelium of Trichinella spiralis–infected mice at the height of the infection preferentially express mMCP-1 and mMCP-2, whereas the mast cells in the jejunal muscle of these mice preferentially express mMCP-4, mMCP-5, mMCP-6, and mMC-CPA.7,19,21,30,34,35 Immunohistochemical analysis of the mastocytosis that develops in the jejunum during helminth infection7 or in different tissues of BALB/c mice after the adoptive transfer of the v-abl–immortalized V3 mast cell line34 indicate that the protease phenotype of a mast cell can be modulated and is not predetermined in the BM.
As assessed by RNA blot analysis, mBMMC cultures contain high levels of the transcripts that encode FcεRI, c-kit, mMCP-5, mMCP-6, mMCP-7, and mMC-CPA.17,18,23,24 36-38 Most of these transcripts are readily detected 1 to 2 weeks after BM cells are exposed to IL-3–enriched medium. Because mature, cutaneous mast cells of the BALB/c mouse express FcεRI, c-kit, mMCP-5, mMCP-6, mMCP-7, and mMC-CPA, we and others have concluded that most, if not all, of the cells in the 3- to 4-week-old cultures belong to the mast cell lineage. In the present study, we show that the metachromatic cells in the 3- to 4-week-old BALB/c mBMMC cultures that contain multi-lobular nuclei express c-kit, mMCP-5, mMCP-6, and mMC-CPA, as do the predominant mononuclear mBMMC. We also show that mast cells with multi-lobular nuclei can be found occasionally in the skeletal muscle of normal mice, the jejunum of helminth-infected mice, and in various tissues of V3 mastocytosis mice. Thus, in the mouse, mast cells can exhibit very different nuclear profiles.
MATERIALS AND METHODS
In vitro– and in vivo–derived mast cells.mBMMC were obtained by culturing BALB/c mouse BM cells for 3 to 4 weeks in enriched medium (RPMI-1640 supplemented with 10% fetal calf serum [FCS] [GIBCO, Grand Island, NY], 2 mmol/L L-glutamine, 0.1 mmol/L nonessential amino acids, 100 U/mL penicillin, and 100 μg/mL streptomycin) containing either 50% WEHI-3 cell (line TIB-68; American Type Culture Collection [ATCC], Rockville, MD) conditioned medium or recombinant IL-3 in a humidified atmosphere at 37°C and 5% CO2 .10 V3 mastocytosis mice were generated by injecting ≈106 cultured V3 mast cells intravenously into the tail veins of each 6-week-old BALB/c mouse (The Jackson Laboratory, Bar Harbor, ME).34 Previous DNA blot analysis revealed that the V3 mast cell line contains a single copy of the pGDv-abl provirus in its genome. Because the oncogene was inserted at a site distinct from that in the V7 mast cell line and the V8 lymphoblastic cell line, the V3 mast cell line is of clonal origin. V3 mastocytosis mice were used 2 to 4 weeks after their adoptive transfer. Mouse Kirsten sarcoma virus–immortalized mast cells (KiSV-MC) were maintained in 100% enriched medium as previously described.39 Serosal mast cells were obtained under sterile conditions from Sprague-Dawley rats (The Jackson Laboratory) by peritoneal lavage with Tyrode's buffer containing 5% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 20 μg/mL gentamicin, and then were enriched to 90% to 99% purity by density-gradient centrifugation in 25% metrizamide.40 Swiss albino mouse 3T3 fibroblasts (line CCL-92; ATCC) were grown in enriched medium. BALB/c mice were infected with T spiralis as described to evaluate the mast cells in the jejunal epithelium at the height of the infection.7,30 35
Production of a mMC-CPA-specific antibody.The deduced amino acid sequences of mMC-CPA18 and rat MC-CPA41 were compared with that of other metallocarboxypeptidases42-46 to define potential antigenic regions. As assessed by the Protein Identification Resource database of the National Biomedical Foundation (Bethesda, MD), residues 146 to 157 are not present in any other carboxypeptidase or protein. Because of its novelty, hydrophilicity, and potential antigenicity, we speculated that antibodies directed against the peptide Asp-Val-Ser-Trp-Ser-Ser-Pro-Asn-Thr-Asp-Lys would be specific for mMC-CPA and rat MC-CPA. Thus, a synthetic peptide (3 mg) corresponding to this sequence was suspended in 5 mL of 10 mmol/L phosphate-buffered saline (PBS) containing 5 mg keyhole limpet hemocyanin (Sigma Chemical Co, St Louis, MO), and coupling was performed in the presence of 0.25% glutaraldehyde (Sigma) at 4°C. Noncoupled peptide was removed by dialyzing the reaction mixture against PBS using a membrane with a 12-kD cutoff. Polyclonal antibodies to the coupled peptide were raised in New Zealand White rabbits according to standard immunization protocols in which each rabbit was injected intramuscularly (IM) with an emulsion consisting of 0.5 mL of the coupled peptide (≈500 μg) mixed with an equal volume of TiterMax synthetic adjuvant (CyRx Crop, Norcross, CA). The immunized animals received booster injections IM monthly, and their sera were collected at 2-week intervals over a number of months. An enzyme-linked immunosorbent assay (ELISA) was used to evaluate the presence of specific antibodies in the sera, and antibodies that exhibited strong reactivities against the immunizing peptide were purified with a peptide-affinity column, as previously described for anti–mMCP-2 Ig and anti–mMCP-5 Ig.30 31
The first 10 residues of the immunizing peptide also are found in the corresponding region of rat MC-CPA.41 Because considerably more MC-CPA containing mast cells can be purified from a normal rat than from a normal mouse, the native exopeptidase was purified from rat serosal mast cells on a potato carboxypeptidase-inhibitor Sepharose column18 41 to evaluate whether or not mMC-CPA and rat MC-CPA were both recognized by the antipeptide antibody. When the wells of the microtiter plates were coated with purified rat MC-CPA and incubated with the purified antibody, the antibody avidly bound to purified rat MC-CPA in the ELISA at a greater than 1,000-fold dilution (data not shown). As assessed by SDS-PAGE/immunoblot analysis, anti–mMC-CPA Ig identified an ≈36-kD protein in whole cell lysates and granule-enriched preparations from mBMMC, mouse KiSV-MC, and purified rat serosal mast cells. In contrast, it failed to recognize any protein in the lysates of mouse fibroblasts. The ≈36-kD protein was not recognized by preimmune sera, and the reactivity of anti–mMC-CPA Ig was abolished by preincubation with the immunizing peptide but not with the synthetic peptide that corresponds to residues 276 to 298 of mMC-CPA.
Histochemistry, enzyme cytochemistry, and immunohistochemistry.For histological examination, cytocentrifuge preparations of BALB/c mBMMC were air dried and stained for 20 seconds in a 5% ethanolic solution of methylene blue.47 Serial 1.5-μm thick glycolmethacrylate sections of lymph node tissue from V3 mastocytosis mice, tongue skeletal muscle from normal BALB/c mice, and jejunum from T spiralis–infected BALB/c mice were air dried and incubated sequentially with double-strength hematoxylin for 2 minutes, 1% aqueous eosin Y for 15 minutes, azure II for 1 minute, and then with ethylene glycol monomethyl ether for 5 seconds.48 Individual mast cells containing chymotryptic activities were identified in tissue sections by the method of Leder.49 mBMMC and fixed tissue sections were incubated at 30°C for 1 hour with a solution containing naphthol AS-D chloroacetate; the resulting preparations were rinsed and counterstained with hematoxylin. Mast cells containing tryptic activities were identified in tissue sections by the cytochemistry method of Osman et al,50 with Z-Ala-Ala-Lys-4-methoxy-2-naphthylamide (AAK) used as the substrate.
Light and electron microscopic immunohistochemistry were performed as described.7,30-34,51 For light microscopy, collected tissues were fixed for 4 hours at room temperature in 4% paraformaldehyde in 0.1 mol/L sodium phosphate, pH 7.6. Alternatively, selected samples were fixed in Methacarn or Carnoy's solution. Preparations were washed twice with PBS containing 2% dimethyl sulfoxide and were suspended in 50 mmol/L NH4Cl overnight at 4°C. The specimens were dehydrated and embedded in accordance with the JB-4 embedding kit instructions from Polysciences Inc (Warrington, PA). For electron microscopy, tissues were fixed in 0.25% glutaraldehyde and 4% paraformaldehyde and then frozen in liquid nitrogen. Tissue sections and/or cells were stained with either rat monoclonal antimouse c-kit Ig (PharMingen, San Diego, CA) followed by goat-antirat IgG (PharMingen) or with affinity-purified rabbit anti–mMCP-2 Ig, anti–mMCP-5 Ig, anti–mMCP-6 Ig, or anti–mMC-CPA Ig followed by goat-antirabbit IgG.30-32
RESULTS
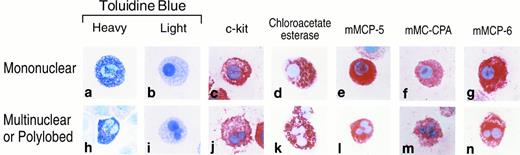
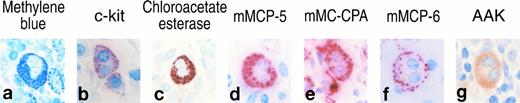
Histochemistry, enzyme cytochemistry, and immunohistochemistry of mBMMC that have different nuclear profiles.Metachromatic mBMMC were obtained that possessed different nuclear morphologies when BALB/c mouse BM cells were cultured for greater than 3 weeks in the presence of IL-3 (Fig 1). When >300 BALB/c mBMMC were analyzed in each of three experiments, 8%, 14%, and 15% of the cells in the culture had multi-lobular nuclei. Regardless of whether these mBMMC were heavily or lightly granulated in terms of their metachromasia when stained with toluidine blue, they phenotypically resembled the mononuclear cells in the culture. Although these cells contained low levels of tryptase activity (data not shown), they contained substantial amounts of chloroacetate esterase (chymase) activity (Fig 1). As assessed immunohistochemically at low magnification, both populations of mBMMC expressed c-kit, mMCP-5, mMCP-6, and mMC-CPA. When the mBMMC with mono- and multi-lobular nuclei were examined with transmission electron microscopy and immunogold cytochemistry, most of the secretory granules in both populations of cells were relatively immature (Figs 2 and 3). Nevertheless, some cells in both cultures possessed electron dense granules and these granules were stained by anti–mMC-CPA Ig and anti–mMCP-6 Ig (Fig 2). The chromatin was condensed to the same extent on the nuclear membrane in both populations of mBMMC. Analysis of four different levels through a single mBMMC showed that the apparent segmented nature of the nucleus was not a consequence of the section analyzed (Fig 3). Further ultrastructural analysis revealed that the varied lobes of the nucleus can be joined by a thin connection.
Histochemistry, enzyme cytochemistry, and immunohistochemistry of cells in 4-week cultures of BALB/c mBMMC that possess mono- (a through g) and multi-lobular (h through n) nuclei. Cytocentrifuge preparations of cells were evaluated for toluidine blue reactive granules (a, b, h, i) that contained chloroacetate esterase enzymatic activity (d, k), mMCP-5 protein (e, l), mMC-CPA protein (f, m), or mMCP-6 protein (g, n). Cells also were evaluated immunohistochemically for their surface expression of c-kit (c, j).
Histochemistry, enzyme cytochemistry, and immunohistochemistry of cells in 4-week cultures of BALB/c mBMMC that possess mono- (a through g) and multi-lobular (h through n) nuclei. Cytocentrifuge preparations of cells were evaluated for toluidine blue reactive granules (a, b, h, i) that contained chloroacetate esterase enzymatic activity (d, k), mMCP-5 protein (e, l), mMC-CPA protein (f, m), or mMCP-6 protein (g, n). Cells also were evaluated immunohistochemically for their surface expression of c-kit (c, j).
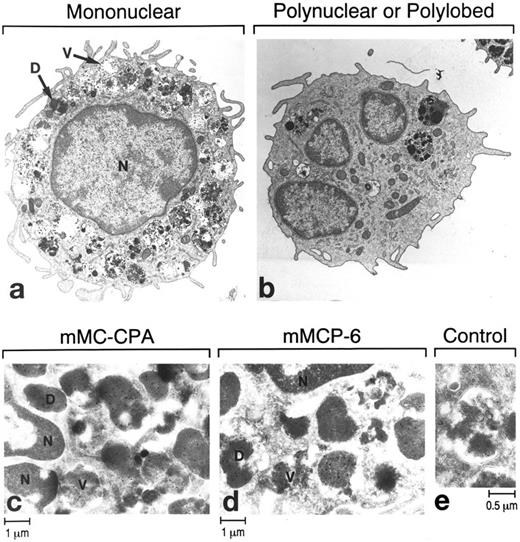
Immunoelectron microscopy of mBMMC with mono- (a) and multi-lobular (b through e) nuclei. Most cells in the culture have numerous multi-vesicular structures in their granules (V). However, in terms of their electron density, some of the granules (D) in these cultured cells are nearly as mature as those in tissue mast cells. Frozen thin sections of the mBMMC were stained with immunogold anti–mMC-CPA Ig (c), anti–mMCP-6 Ig (d), or a control rabbit Ig (e). Ten-nanometer gold particles were used with the antibodies. N, nucleus.
Immunoelectron microscopy of mBMMC with mono- (a) and multi-lobular (b through e) nuclei. Most cells in the culture have numerous multi-vesicular structures in their granules (V). However, in terms of their electron density, some of the granules (D) in these cultured cells are nearly as mature as those in tissue mast cells. Frozen thin sections of the mBMMC were stained with immunogold anti–mMC-CPA Ig (c), anti–mMCP-6 Ig (d), or a control rabbit Ig (e). Ten-nanometer gold particles were used with the antibodies. N, nucleus.
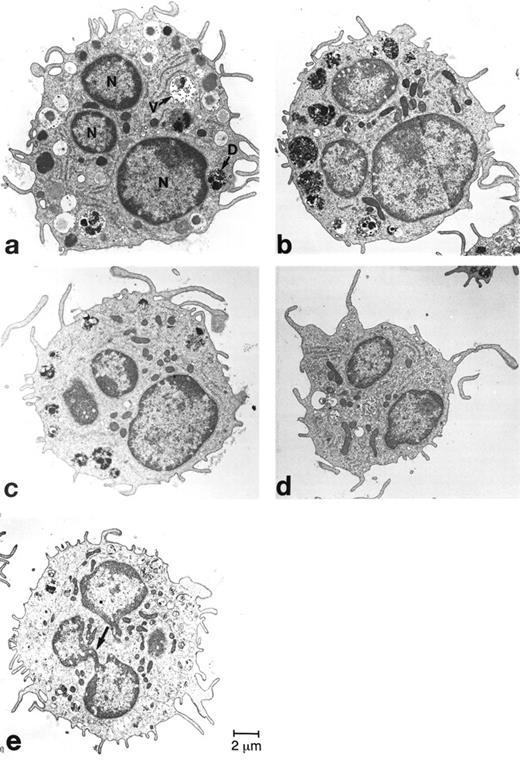
Ultrastructural analysis of mBMMC. Four levels through a single mBMMC are analyzed in panels (a) through (d). The electron micrograph depicted in panel (e) shows a thin connection joining the two lobes of the nucleus of another mBMMC. N, lobes of the nucleus; D, relatively dense granules; V, relatively immature granules containing predominantly multi-vesicular structures.
Ultrastructural analysis of mBMMC. Four levels through a single mBMMC are analyzed in panels (a) through (d). The electron micrograph depicted in panel (e) shows a thin connection joining the two lobes of the nucleus of another mBMMC. N, lobes of the nucleus; D, relatively dense granules; V, relatively immature granules containing predominantly multi-vesicular structures.
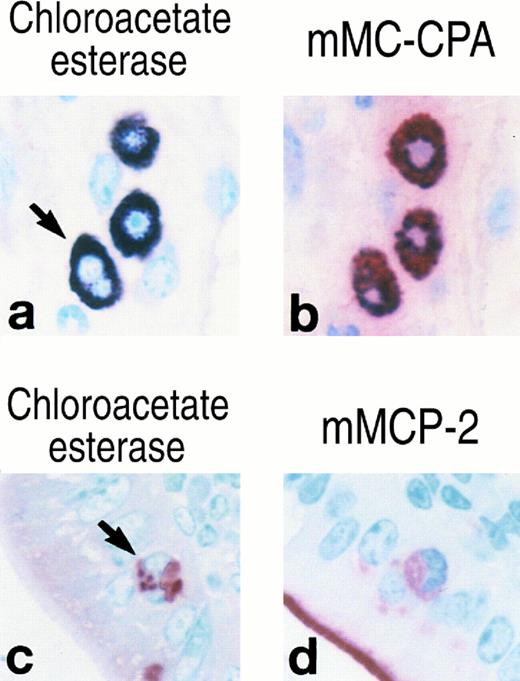
Histochemistry, enzyme cytochemistry, and immunohistochemistry of mast cells in normal BALB/c mice and in V3 mastocytosis mice that possess multi-lobular nuclei.Because of the thinness of the sections, it is not always possible to determine the nuclear configuration of a tissue-localized mast cell precisely. Nevertheless, metachromatic+/chloroacetate esterase+ cells that appear to possess multi-lobular nuclei were occasionally seen in the skeletal muscle of the tongue of normal BALB/c mice and the jejunal epithelium of T spiralis–infected BALB/c mice (Fig 4, see page 385). The observation that the individual nuclear lobes in these cells are smaller in size than the intact nucleus of a mononuclear mast cell in the same section suggests that the nuclear lobes represent portions of the same nucleus rather than distinct nuclei. Analogous to the mononuclear mast cells at these two tissue sites, the cells with multi-lobular nuclei in the muscle could be stained by anti–mMC-CPA Ig but not anti–mMCP-2 Ig, whereas those in the jejunum could be stained by anti–mMCP-2 Ig but not anti–mMC-CPA Ig (Fig 4).
Cytochemistry and immunohistochemistry of mast cells in the skeletal muscle of a normal BALB/c mouse (a, b) and the jejunal epithelium of a T spiralis–infected BALB/c mouse (c, d). Serial sections were evaluated for chloroacetate esterase enzymatic activity (a, c), immunoreactive mMC-CPA (b), and immunoreactive mMCP-2 (d). The arrows indicate mast cells that appear to possess multi-lobular nuclei. Panel (a) was heavily counterstained with methyl green. Thus, the three chloroacetate esterase+ mast cells depicted in this panel exhibit a blue color. The red reaction product on the brush border of the villi (d) is due to endogenous intestinal alkaline phosphatase. The presence of this product indicates that the color substrate was active in the immunohistochemical reaction.
Cytochemistry and immunohistochemistry of mast cells in the skeletal muscle of a normal BALB/c mouse (a, b) and the jejunal epithelium of a T spiralis–infected BALB/c mouse (c, d). Serial sections were evaluated for chloroacetate esterase enzymatic activity (a, c), immunoreactive mMC-CPA (b), and immunoreactive mMCP-2 (d). The arrows indicate mast cells that appear to possess multi-lobular nuclei. Panel (a) was heavily counterstained with methyl green. Thus, the three chloroacetate esterase+ mast cells depicted in this panel exhibit a blue color. The red reaction product on the brush border of the villi (d) is due to endogenous intestinal alkaline phosphatase. The presence of this product indicates that the color substrate was active in the immunohistochemical reaction.
V3 mastocytosis mice were used to determine if an immature mast cell–committed line could give rise to mast cells in vivo with very different nuclear profiles. When greater than 100 V3 mast cells were analyzed in each of four separate experiments, 1%, 2%, 3%, and 4% of the cells in the culture contained multi-lobular nuclei. As obtained previously,34 a prominent mastocytosis developed in a number of tissues of the BALB/c mouse after the adoptive transfer of the v-abl–immortalized V3 mast cell line into this animal. Most of the methylene blue+/toluidine blue+ V3 mast cells found in the mesenteric lymph nodes of these animals appeared to be mononuclear. However, occasionally a methylene blue+ V3 mast cell could be detected that appeared to contain a multi-lobular nucleus (Fig 5, see page 385). Although V3 mast cells with multi-lobular nuclei were most readily found in the mesenteric lymph nodes, similar cells were observed in liver, spleen, and intestine of V3 mastocytosis mice (data not shown). Analysis of tissue sections of varied organs of three different animals showed that 0.7% of the 1,400 V3-MC examined contained multi-lobular nuclei. Because sections are analyzed rather than isolated whole cells, this percentage of V3 mast cells in tissues that have multi-lobular nuclei is an underestimation. Nevertheless, like mononuclear V3 mast cells, these cells contained chloroacetate esterase and AAK (tryptase) activities. As assessed immunohistochemically, both populations of V3 mast cells in lymph nodes expressed c-kit, mMCP-5, mMCP-6, and mMC-CPA.
Histochemistry, cytochemistry, and immunohistochemistry of V3 mast cells in the lymph nodes of mastocytosis mice. The V3 mast cells in the various tissue sections that have multi-lobular nuclei were evaluated for the expression of c-kit (b) and for methylene blue reactive granules (a) that contain chloroacetate esterase enzymatic activity (c), mMCP-5 protein (d), mMC-CPA protein (e), mMCP-6 protein (f ), and AAK enzymatic activity (g).
Histochemistry, cytochemistry, and immunohistochemistry of V3 mast cells in the lymph nodes of mastocytosis mice. The V3 mast cells in the various tissue sections that have multi-lobular nuclei were evaluated for the expression of c-kit (b) and for methylene blue reactive granules (a) that contain chloroacetate esterase enzymatic activity (c), mMCP-5 protein (d), mMC-CPA protein (e), mMCP-6 protein (f ), and AAK enzymatic activity (g).
DISCUSSION
Although in vitro–differentiated mast cells possessing multi-lobular nuclei have been found occasionally in culture-derived mouse mast cells,12-15 the consensus opinion is that in vivo–differentiated mast cells in this species are primarily mononuclear. Thus, nuclear morphology is one of the criteria routinely used to distinguish mast cells from basophils in the mouse. We now show that it is possible to obtain mouse mast cells in vitro and in vivo that possess segmented/multi-lobular nuclei, and that an immature mouse mast cell-committed line can give rise to mast cells in vivo with very different nuclear morphologies.
Mast cells and basophils in humans are granule-containing hematopoietic cells that become metachromatic when stained with cationic dyes. mBMMC and all tissue-localized mouse mast cells that have been analyzed so far contain large amounts of at least one chymase in their granules, and most of these mast cells also express at least one tryptase. Thus, even when tissue sections are fixed, dehydrated, and embedded, mast cells are readily recognized in mouse tissues because of their pronounced tryptic and chymotryptic activities.7,49,50 With enzyme cytochemistry and immunohistochemistry, it was discovered that the toluidine blue+/methylene blue+ cells with multi-lobular nuclei in mBMMC cultures (Fig 1) and in the mesenteric lymph nodes of V3 mastocytosis mice (Fig 5) all contain abundant levels of mMCP-5 and mMCP-6 in their granules. Although mMCP-5 and mMCP-6 are both serine proteases, they are members of two distinct families whose genes reside on chromosomes 14 and 17, respectively. Thus, these immunohistochemical and cytochemical findings suggested that the metachromatic cells in the analyzed cultures with multi-lobular nuclei are mast cells, as Galli et al12-14 concluded in their ultrastructural analysis of comparable cultured cells. Dvorak et al52 noted the transient appearance of metachromatic cells very early in mBMMC cultures which morphologically resemble basophils. Because these in vitro–derived cells were not analyzed in the present investigation, we cannot support or dispute the concept that basophils exist in the mouse as a developmentally distinct cell type. However, the finding that in vitro– and in vivo–differentiated mouse mast cells can have different nuclear profiles now indicates that the lineage of a metachromatic mouse cell cannot be determined conclusively by the shape of the cell's nucleus alone.
In contrast to their granule serine proteases, only one carboxypeptidase has been identified in the granules of mouse, rat, and human mast cells.18,41,53,54 In humans, MC-CPA protein is preferentially found in the tryptase+/chymase+/cathepsin G+ subset of mast cells that reside in the skin and certain other tissue sites.55 Basophils in the peripheral blood of humans do not contain detectable amounts of this exopeptidase in their granules.55 In mice and rats,18,20,41 MC-CPA is a major granule constituent of the serosal mast cells isolated from the peritoneal cavity. The ear and skin of BALB/c mice also contain high steady-state levels of the mMC-CPA transcript,33 and in situ hybridization analysis56 has shown that most, if not all, of the mast cells in these connective tissue sites express mMC-CPA. Because high levels of the mMC-CPA transcript are present in the BALB/c mBMMC cultures,18 it has been presumed that the prominent mononuclear cells in these cultures store the exopeptidase in their granules. Therefore, whether the mMCP-5+ cells in V3 mastocytosis mice and in the mBMMC cultures that contain multi-lobular nuclei also store mMC-CPA protein in their granules was of interest. To this end, an mMC-CPA–specific antibody was derived with an antipeptide approach. When immunohistochemistry was performed with anti–MC-CPA Ig, the granules of both populations of mBMMC in the culture were strongly reactive (Figs 1 and 2), as were the granules of mast cells in the skeletal muscle of the normal BALB/c mouse (Fig 4) and the V3 mast cells in the lymph node (Fig 5) that possessed multi-lobular nuclei. Analogous to mature mast cells,17 these V3 mast cells (Fig 5) and mBMMC (Fig 1) also expressed c-kit.
Because a higher percentage of the cells in cultures of mBMMC15 and human cord blood/BM57-61 have multi-lobular nuclei when hematopoietic progenitor cells are cultured in the presence of IL-3 rather than in c-kit ligand, IL-3 seems to promote nuclear segmentation. What other factors are involved and the functional significance of the nuclear changes remain to be ascertained. Nuclear segmentation of neutrophils and eosinophils is a late event in the maturation processes of these granulocytes. Nevertheless, the nuclear segmentation process of mouse mast cells does not appear to depend on the cell being at its final stages of granule maturation because these mast cells in the mBMMC cultures and in the varied tissues of the V3 mastocytosis mouse are only of modest maturity in terms of their granulation.
In a number of ways (eg, presence of metachromatic secretory granules that contain histamine, expression of membrane-bound FcεRI, FcεRI-mediated metabolism of arachidonic acid to leukotrienes, and FcεRI-mediated expression of IL-4 and IL-13) human mast cells are more similar to basophils than are any other cells in the body. The discovery of an “intermediate” cell in the BM and peripheral blood of patients with chronic myelogenous leukemia possessing certain ultrastructural features of normal peripheral blood basophils and certain ultrastructural features of normal tissue mast cells62 suggested that the two populations of metachromatic cells were developmentally related. However, because of nuclear morphology and certain biochemical differences,16,63-67 it is generally accepted that basophils and mast cells in humans are derived from distinct progenitors. For example, in terms of granule constituents, chymase and tryptase are relatively mast cell–specific markers at protein and mRNA levels.64,67 Nevertheless, Li et al68 recently found that a metachromatic/FcεRI+/tryptase+/chymase+ population of cells that resembled basophils in terms of nuclear morphology developed when human BM cells from normal donors were cultured for 6 weeks in the presence of c-kit ligand and conditioned medium derived from the HBM-M cell line.
In vivo and in vitro studies have shown that granule protease expression in a mouse mast cell is neither fixed nor predetermined in the BM.7,30,34,35,37,69 The ability of a BALB/c mouse mast cell to rapidly change its chymase phenotype is regulated, in part, by a cytokine-dependent, posttranscriptional mechanism.70 The posttranscriptional findings in the mouse, coupled with the in vitro findings of Li et al,68 now raise the possibility that human basophils are more closely related developmentally to human mast cells than previously recognized. If the ultimate phenotype of a basophil is not fixed but is instead regulated by its microenvironment (as are tissue mast cells in the mouse), the failure of a peripheral blood human basophil to express high levels of c-kit, tryptase, chymase, and/or MC-CPA protein may simply be a consequence of the cell failing to localize in the relevant tissue for an extended period of time.
Whether or not mast cells and basophils in the human are more closely related than previously thought, it is now clear that mouse mast cells can possess very different nuclear profiles. Moreover, the detection in tissues of immature and mature mouse mast cells with multi-lobular nuclei that express different panels of granule proteases indicates that the nuclear morphology of a mouse mast cell gives no insight as to its protease phenotype or its stage of development.
ACKNOWLEDGMENT
The technical assistance of Xuzhen Hu, Kathy M. Grattan, and Maria Ericsson are gratefully acknowledged.
Supported by Grants No. AI-22531, AI-23483, AI-31599, AR-36308, HL-36110, and HL-48598 from the National Institutes of Health.
Address reprint requests to Richard L. Stevens, PhD, Harvard Medical School, Seeley G. Mudd Bldg, Room 617, 250 Longwood Ave, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal