Abstract
The Henshaw antigen (synonym: He or MNS6) is carried by an altered form of glycophorin B (GPB), but the molecular basis for its variable expression or quantitative polymorphism remains largely undefined. We report here the identification and analysis of a novel glycophorin He allele, GPHe(GL), which gives rise to the expression of two protein isoforms in the erythrocyte membrane. In addition to the nucleotide changes defining the epitopic sequence of He, a single C-to-G nucleotide transversion in exon V coding for the membrane domain was found to cause aberrant RNA splicings by creating a new acceptor splice site. In addition, a T-to-G transversion at −6 position of the acceptor splice site for exon IV was identified. Both full-length and truncated transcripts of GPHe(GL) were detected as the result of partial activation of the new acceptor splice site and partial inactivation of the normal splice sites. The full-length cDNA encoded He, S, and U antigens, whereas the three truncated ones lacked either the sequence for S and U antigens or a large portion of the membrane domain or both. The GPB gene on the other chromosome was apparently normal and its transcript encoded N, s, and U antigens. These results correlate alternative RNA splicing with the expression of two GPHe isoforms and thus delineate a new mechanism for the phenotypic diversity of membrane glycophorins.
THE SURFACE of human erythrocytes (red blood cells or RBCs) displays a large number of blood group antigens that are associated with integral membrane proteins. Among these, glycophorins A (GPA) and B (GPB) are major sialoglycoproteins that carry the M/N and S/s antigens.1 Both GPA and GPB are anchored in the RBC membrane via a hydrophobic domain that spans the lipid bilayer once.2 3 GPA carries the M or N antigen, which is determined by two amino acid substitutions at the amino terminus encoded by the second exon of the allelic genes. GPB also carries the N antigen at its amino terminus, but its associated S/s polymorphism results from an allelic variation, ATG versus ACG, in exon IV that specifies a Met-to-Thr change at position 29 of the protein.
Henshaw (synonym: He or MNS6) is a variant antigen of the MNS blood group system particularly prevalent in blacks4 from malaria endemic regions5 and its epitope has been localized to the amino terminus of an altered form of GPB.6 He differs from the antithetical N antigen at amino acid residues 1, 4, and 5: the former has the sequence Trp1-Ser-Thr-Ser-Gly5 , whereas the latter has the sequence Leu1-Ser-Thr-Thr-Glu5. The He antigen may travel with the Sta antigen on the same molecule,7 and it may also dissociate from the S, s and/or U antigens8 9 in at least 30% of S-s- individuals.10 In the latter situation, the strength of He expression on RBCs varies considerably, suggesting a heterogeneity of the underlying genetic background.
While the cotransmission of He and Sta has been attributed to a rare genetic recombination event,11 the linkage of He with S-s- and the resulting quantitative polymorphism have remained largely undefined. In one example, the molecular basis for He+(S-s-U-) has been associated with an aberrant RNA splicing event that results in a complete skipping of exon V in the GPHe(P2 ) gene.12 We report here studies on a He+ blood sample associated with different, and hitherto undescribed, aberrant RNA splicing events probably induced by two nucleotide transversions located in intron 4 and exon V, respectively. We show that aberrant splicing results in four He-bearing glycophorin transcripts two of which, the full-length form and the exon IV-deleted form, are most likely correlated with the expression of two GPHe protein isoforms in the erythrocyte membrane.
MATERIALS AND METHODS
Blood sample and hemagglutination testing.The He+ blood sample was obtained from a native South African (GL) who was found by screening with rabbit anti-He. Monoclonal anti-He (22G4) and anti-N (14E) were supplied by Gamma Biologicals, Inc (Houston, TX), and anti-S–like (monoclonal antibody [MoAb] 148) was supplied by Immucor (Norcross, GA). Hemagglutination was performed using standard serological testing in test tubes.
α-Chymotrypsin treatment of RBCs.α-Chymotrypsin (Type VII-TLCK treated) was purchased from Sigma Chemical Co (St Louis, MO). One volume of washed packed RBCs was mixed with four volumes of α-chymotrypsin (5 mg/mL in phosphate-buffered saline [PBS] pH 8.0), as described.13 This mixture was incubated at 37°C for 30 minutes with occasional agitation. The RBCs were then washed three times in PBS (pH 7.4) before use in hemagglutination studies or preparation of membranes for immunoblot analysis.
Immunoblotting analysis of membrane glycophorins.Immunoblotting was performed as previously described,10 and peroxidase-conjugated antimouse Ig was used as the secondary antibody.
Southern blot analysis of glycophorin genes.Genomic DNA was isolated from cells in the buffy coat of GL's blood sample. Both He− and He+ DNAs were used as controls. Southern blot analysis following restriction endonuclease digestion was performed as described.14
Transcript analysis by reverse-transcriptase polymerase chain reaction (RT-PCR) and cDNA sequencing.Total RNA was isolated from reticulocytes hemolysates15 and extracted with Trizol reagent using the supplier's protocol (BRL, Gaithersburg, MD). RT-PCR of glycophorin mRNAs was performed as previously described.12 Four primers were used for synthesis and amplification of GPB and GPHe cDNAs: P1, N-specific, 5′-GAAATTGTGAGCATATCAGCATT-3′ (sense, exon II); P2, He-specific, 5′-AAATTGTGAGCATATCAGCATGG-3′ (sense, exon II); P3, GPB-specific, 5′-GTTCTAG GCAAGATCAGGCAGCAT-3′ (antisense, exonVI); and P4, GPB-specific, 5′-AGAATACAGTAATAGTGAGGCAG-3′ (antisense, 3′-untranslated region or 3′-UTR). Two primers were used for synthesis and amplification of GPA cDNA: P5, 5′-GGAATTCCAGCTCATGATCTCAGGATG-3′ (sense strand attached with an EcoRI site GAATTC, exon I) and P6, 5′-TCCACATTTGGTTTGGTGAACAGATTC-3′ (antisense, exon VII or 3′-UTR).
For the analysis of GPB and GPHe transcripts, total RNAs were converted into single-stranded cDNAs using 20 ng of P4 in 10 μL of reaction volume (42°C for 1 hour). An aliquot (2.5 μL) of the cDNA products was then amplified using either P1+P3 or P2+P3 combination. This approach avoids coamplification of GPHe and GPB, thus producing specific DNA templates suitable for direct sequencing. PCR was run for 30 cycles as follows: 94°C for 60 seconds, 55°C for 45 seconds, and 72°C for 30 seconds; the last step was 55°C for 2 minutes and 72°C for 7 minutes. The resultant cDNA products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining. After purification by polyacrylamide gel electrophoresis and elution in TE buffer (10 mmol/L Tris-HCl, 0.1 mmol/L EDTA, pH 8.0), cDNA templates were recovered and directly cycle-sequenced on an automated DNA sequencer using chain terminators tagged with fluorescent dyes (Applied Biosystem, Foster City, CA).
Amplification of genomic sequences by allele-specific PCR.To confirm the results of cDNA sequence analysis and determine the exon-intron structures, allele-specific PCR16 of GPHe(GL) was performed using its 9.2- and 4.3-kb Msp I fragments as DNA templates. Four fragments, with each encompassing one exon, were amplified by the following combinations of primer pairs: for exon II, 5′-GTAAGATATATACTAAAAGCGCTTAGC-3′ (sense, intron 1) and 5′-TAACTCACAGTATTAT TTCTGTGAGAT-3′ (antisense, intron 2); for exon III, 5′-CATTCTTGTTCCCTTTCTCAACTTC-3′ (sense, intron 2) and 5′-AGAACTGTCATGAGTTACAGCTCGT-3′ (antisense, intron 3); for exon IV, 5′-CCCTCAGTTATGAGACAATTTGCT-3′ (sense, intron 3) and 5′-GCAATGGATAGTTTAAAAT GGAATGAC-3′(antisense, intron 4); and for exon V, 5′-TGGTCATTTATTTCAGACTTTCAT-3′ (sense, intron 4) and 5′-CTGTTTCTCTTTTGAGTTTAACTG-3′ (antisense, intron 5). The amplified genomic products were purified and then sequenced, as mentioned above. The exon-intron junction sequences were assessed to derive the score for the various splice sites, as described.17
RESULTS
Hemagglutination.RBCs of proband GL typed as M-N+S+s+U+ and had a strong expression of the He antigen as determined by reactions with mouse monoclonal, rabbit polyclonal, and human polyclonal anti-He antibodies. Other antigen typings were unremarkable. The direct antiglobulin test was negative. Unexpectedly, after α-chymotrypsin treatment, the proband's RBCs still reacted strongly with monoclonal anti-He, even though the S antigen was no longer detectable on these cells.
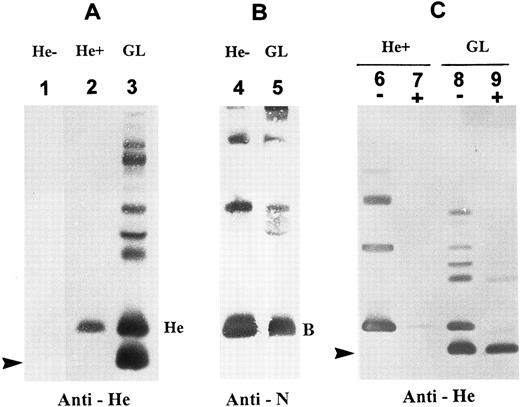
Detection of two membrane He+ protein isoforms by immunoblotting.Immunoblots with a monoclonal anti-He showed an expression in the GL's RBC membranes of two He+ glycophorin forms (designated GPHe-1 and GPHe-2). GPHe-1 was equivalent to GPB in molecular size (Mr 25,000), whereas GPHe-2 was a new fast-moving band with an Mr approximately 2,700 daltons less than GPB (Fig 1A, lane 3). The latter glycophorin species was absent in RBCs from both He− and He+ controls (Fig 1A, lanes 1 and 2). Immunoblotting with an anti-N MoAb detected GPB in the normal control and GL (Fig 1B), but the GPHe-2 species was nonreactive with the antibody (Fig 1B, lane 2). The GPHe-2 band was also detectable by anti-Me but not by anti-M (nonreactive with M−, He+ RBCs), anti-s or MoAb148 that is anti-S–like (data not shown). Together, these results indicated that the proband is heterozygous for a normal GPB gene and an altered GPHe gene producing two He+ membrane proteins.
Immunoblotting analysis of RBC membrane glycophorins. (A) Immunoblots probed with monoclonal anti-He. Lane designations are: 1, He−, He negative control; 2, He+, He positive control; and 3, proband GL. He monomer is seen in He+ but not He−, whereas two monomeric components of GPHe are present in GL (indicated by He and an arrow). Note that the upper bands seen in lane GL are due to overload and formation of homodimers and heterodimers with other glycophorins. (B) Immunoblots probed with monoclonal anti-N. Note that RBC membranes from proband GL contain a GPB form comparable to that from He− control (B denotes the monomeric form). (C) RBCs were treated with α-chymotrypsin and then membranes were prepared for immunoblotting with anti-He. A minus sign (−): untreated, and a plus sign (+): chymotrypsin-treated. This enzyme digestion almost completely removed the GPHe monomer with a size comparable to GPB but not at all the lower-molecular-weight GPHe species (arrow-indicated).
Immunoblotting analysis of RBC membrane glycophorins. (A) Immunoblots probed with monoclonal anti-He. Lane designations are: 1, He−, He negative control; 2, He+, He positive control; and 3, proband GL. He monomer is seen in He+ but not He−, whereas two monomeric components of GPHe are present in GL (indicated by He and an arrow). Note that the upper bands seen in lane GL are due to overload and formation of homodimers and heterodimers with other glycophorins. (B) Immunoblots probed with monoclonal anti-N. Note that RBC membranes from proband GL contain a GPB form comparable to that from He− control (B denotes the monomeric form). (C) RBCs were treated with α-chymotrypsin and then membranes were prepared for immunoblotting with anti-He. A minus sign (−): untreated, and a plus sign (+): chymotrypsin-treated. This enzyme digestion almost completely removed the GPHe monomer with a size comparable to GPB but not at all the lower-molecular-weight GPHe species (arrow-indicated).
To further explore the structural differences between the two He+ protein isoforms, α-chymotrypsin digestion of RBCs followed by immunoblotting analysis with anti-He was performed. The results showed that GPHe-1 from both He+ control and GL was α-chymotrypsin–cleavable (Fig 1C, lanes 7 and 9), whereas GPHe-2 from GL was not (Fig 1C, lane 9). This finding indicated that the enzyme cleavage sites in the latter were either absent or conformationally perturbed due to a truncation of the corresponding amino acid sequence.
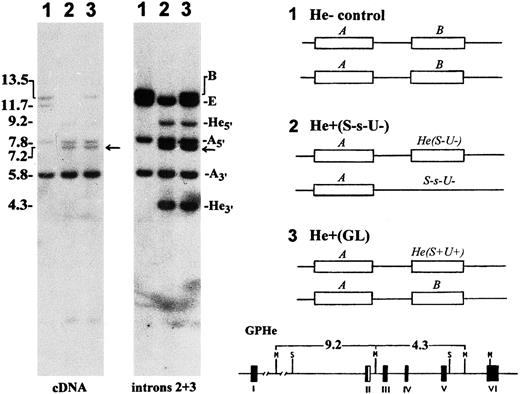
Genetic polymorphism of He detected by Southern blotting.In proband GL, Msp I blots hybridized with glycophorin cDNA and genomic DNA probes showed a gross alteration in the GPB gene but not the GPA gene (Fig 2, autoradiograms at left). In addition to a 13.5-kb band for the normal GPB gene, there were two new bands of 9.2-kb and 4.3-kb in size that originated from the GPHe gene on the homologous chromosome. These two bands were previously shown to arise by the introduction of a unique Msp I cleavage site into the second intron of GPHe gene, perhaps through a gene conversion-like event.12 Analysis of unrelated He− and He+ genomic DNAs showed that only the He+ samples display this polymorphism (gels not shown), suggesting its tight linkage with He. A genetic marker, the 7.2-kb Msp I band, known to be tightly associated with S-s-,14 was also seen in GL. These data confirmed that the diploid genome of GL carries a normal GPB gene and an altered GPHe gene (designated GPHe(GL), Fig 2, right).
Southern blot analysis of glycophorin genes. Left, genomic DNAs were digested with Msp I and hybridized with GPA cDNA and intron 2 and 3 probes, as indicated. Lanes 1, He− control (M+N+He-S+s-U+); 2, He+ control (M-N+He+S-s-U-); and 3, proband GL (M-N+He+S+s+U+). The size (kb) and gene origin of various bands are indicated at left and right margins, respectively. Arrow points to the 7.2-kb marker band known to be tightly linked with S-s-. Right, the probable genotypes for the GYPA locus in the three subjects are schematically shown in which the GPE gene is omitted for the sake of simplicity. He(S-U-) denotes nondeleted haplotype and S-s-U- GPB-deleted haplotype. GL is a heterozygote for the He(S+U+) haplotype. The GPHe gene-specific 9.2- and 4.3-kb bands originating from the introduction of a unique Msp I site into intron 2 is indicated at bottom.
Southern blot analysis of glycophorin genes. Left, genomic DNAs were digested with Msp I and hybridized with GPA cDNA and intron 2 and 3 probes, as indicated. Lanes 1, He− control (M+N+He-S+s-U+); 2, He+ control (M-N+He+S-s-U-); and 3, proband GL (M-N+He+S+s+U+). The size (kb) and gene origin of various bands are indicated at left and right margins, respectively. Arrow points to the 7.2-kb marker band known to be tightly linked with S-s-. Right, the probable genotypes for the GYPA locus in the three subjects are schematically shown in which the GPE gene is omitted for the sake of simplicity. He(S-U-) denotes nondeleted haplotype and S-s-U- GPB-deleted haplotype. GL is a heterozygote for the He(S+U+) haplotype. The GPHe gene-specific 9.2- and 4.3-kb bands originating from the introduction of a unique Msp I site into intron 2 is indicated at bottom.
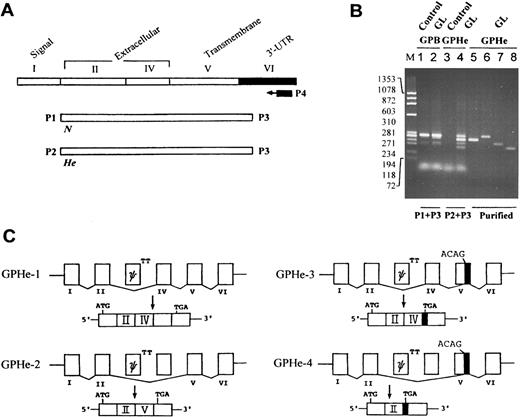
Splicing pattern of GPHe(GL) transcripts.To establish whether the two GPHe protein isoforms are derived from the same gene or two different genes, RT-PCR and cDNA sequencing were used to investigate the composition and structure of glycophorin transcripts (Fig 3A). Compared to normal controls, no abnormality was found in GL with regard to GPA (data not shown) and GPB transcripts (Fig 3B, lanes 1 and 2). However, GPHe(GL) cDNAs amplified with P2 and P3 consisted of four species, one comparable to, while others smaller than, GPB in size (Fig 3B, lane 4). Nucleotide sequencing identified the pattern of various exon-exon junctions in the four He-carrying transcripts (Fig 3C). GPHe-1 was a normally spliced form in which exon II and exon IV were connected; this skipping event is expected, since the donor splice site for GPB exon III is known to be defective.18 GPHe-2 was an aberrantly spliced form, lacking exon IV and thus having an exon II-exon V in-frame connection. In GPHe-3, exon IV was retained, but it was joined to the 3′ end of exon V, thereby resulting in a premature stop codon. GPHe-4 lacked both exon IV and a large portion of exon V, causing a further truncation of the coding sequence but a same premature termination as seen in GPHe-3 (see below).
RT-PCR analysis and splicing pattern of GPHe(GL) transcripts. (A) Strategy for gene-specific RT-PCR analysis. The correspondence of exons to different protein domains and the location of primers for cDNA synthesis and amplification are shown. (B) Agarose gel electrophoresis of GPB and GPHe(GL) cDNAs. M is the HaeIII-cleaved size marker of φX174 DNA. Lanes 1 and 3, He− control; and lanes 2 and 4, GL. When primers P2 and P3 were used, multiple cDNA forms are seen in GL, while no product is found in control. Lanes 5 through 8 show reanalysis of the PAGE-purified cDNAs of GPHe-2, 1, 3, and 4 observed in lane 4. (C) The pattern of exon-exon connections in GPHe-1 to 4 isoforms. Like GPB, exon III of GPHe(GL) is also a pseudoexon (denoted ψ) attached with a defective donor site, TT. GPHe-1 and GPHe-2 are products of in-frame splicing, whereas GPHe-3 and GPHe-4 are products of out-of-frame splicing with the same frameshift and premature termination (see Fig 4A). The newly created acceptor site (ACAG) and termination codon TGA in exon V are indicated. The residual exon V sequence spliced into GPHe-3 and GPHe-4 is blackened.
RT-PCR analysis and splicing pattern of GPHe(GL) transcripts. (A) Strategy for gene-specific RT-PCR analysis. The correspondence of exons to different protein domains and the location of primers for cDNA synthesis and amplification are shown. (B) Agarose gel electrophoresis of GPB and GPHe(GL) cDNAs. M is the HaeIII-cleaved size marker of φX174 DNA. Lanes 1 and 3, He− control; and lanes 2 and 4, GL. When primers P2 and P3 were used, multiple cDNA forms are seen in GL, while no product is found in control. Lanes 5 through 8 show reanalysis of the PAGE-purified cDNAs of GPHe-2, 1, 3, and 4 observed in lane 4. (C) The pattern of exon-exon connections in GPHe-1 to 4 isoforms. Like GPB, exon III of GPHe(GL) is also a pseudoexon (denoted ψ) attached with a defective donor site, TT. GPHe-1 and GPHe-2 are products of in-frame splicing, whereas GPHe-3 and GPHe-4 are products of out-of-frame splicing with the same frameshift and premature termination (see Fig 4A). The newly created acceptor site (ACAG) and termination codon TGA in exon V are indicated. The residual exon V sequence spliced into GPHe-3 and GPHe-4 is blackened.
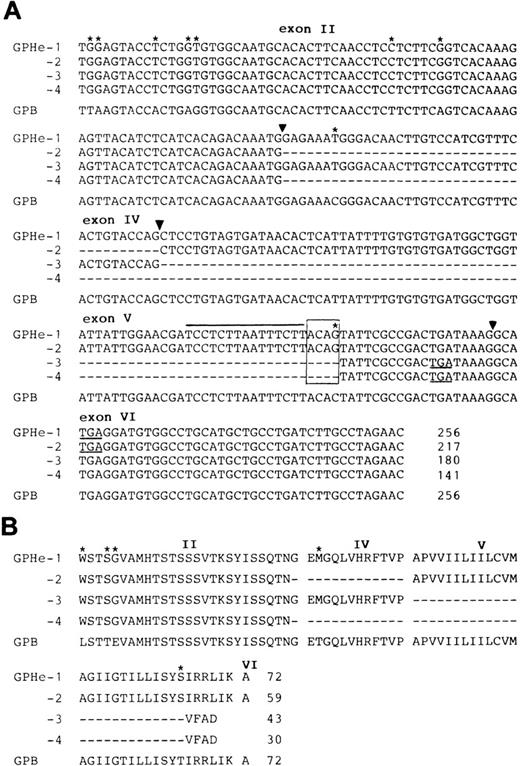
Nucleotide and deduced amino acid sequences of GPHe isoforms.Figure 4 shows the nucleotide and deduced amino acid sequences of GPB and the four GPHe transcripts obtained from GL's erythroid cells. The GPB sequence should encode the N and s antigens since it contains Leu, Glu, and Thr at positions 1, 5, and 29, respectively. All GPHe(GL) forms were predicted to carry Trp, Ser, and Gly residues at positions 1, 4, and 5, which define the He antigen.6 It is also apparent from comparison with GPB that all GPHe transcripts carry the same two silent nucleotide substitutions in exon II, nt 39T-to-C, and nt 45A-to-G (Fig 4A). These results confirmed that all GPHe(GL) cDNAs originated from the same precursor messenger RNA (Pre-mRNA) by alternative splicing (Fig 3C).
Nucleotide and deduced amino acid sequences of the four GPHe(GL) isoforms. (A) Nucleotide sequences of the four GPHe(GL) cDNA species. The GPB sequence from GL is listed for comparison. Nucleotide differences are marked by stars and deleted sequences in the cDNA products by dashes. The new acceptor splice site ACAG is boxed and its upstream pyrimidine-rich sequence indicated by a straight line. Exons are numbered and their boundaries denoted by triangles. Termination codons (TGA) are underlined. (B) Alignment of the deduced amino acid sequences for GPHe(GL) isoforms. Amino acid variations are denoted by stars and deleted sequences by dashes. Gaps interrupting the amino acid sequence pertain to the exon-exon junctions. The total amino acid number of each putative polypeptide is indicated. Note that GPHe-3 and 4 are premature chain termination products because of a shift in the open reading frame.
Nucleotide and deduced amino acid sequences of the four GPHe(GL) isoforms. (A) Nucleotide sequences of the four GPHe(GL) cDNA species. The GPB sequence from GL is listed for comparison. Nucleotide differences are marked by stars and deleted sequences in the cDNA products by dashes. The new acceptor splice site ACAG is boxed and its upstream pyrimidine-rich sequence indicated by a straight line. Exons are numbered and their boundaries denoted by triangles. Termination codons (TGA) are underlined. (B) Alignment of the deduced amino acid sequences for GPHe(GL) isoforms. Amino acid variations are denoted by stars and deleted sequences by dashes. Gaps interrupting the amino acid sequence pertain to the exon-exon junctions. The total amino acid number of each putative polypeptide is indicated. Note that GPHe-3 and 4 are premature chain termination products because of a shift in the open reading frame.
Comparison of GPB and GPHe-1 further showed that there was a single C-to-G base change in exon V that resulted in a Thr-to-Ser substitution at position 65 (Fig 4B). In conjunction with the upstream pyrimidine-rich sequence, this guanyl nucleotide created a new cryptic acceptor splice site, ACAG (Fig 4B, overlined and boxed). As shown, the sequence of exon IV was completely excluded from both GPHe-2 and 4, whereas the sequence of exon V was partially excluded from GPHe-3 and 4, exactly at the position of AG dinucleotide. These results showed a partial activation of the new acceptor splice site and partial inactivation of the normal splice sites.
While GPHe-1 should contain 72 amino acids with a Met residue at position 29 for the S antigen, the truncated GPHe forms lack either the Met residue or the membrane domain or both because of the different skipping events involving exon IV and/or exon V (Fig 4A). GPHe-2 maintained a correct open reading frame and thus was predicted to encode a polypeptide with 59 amino acids; however, exclusion of the exon IV sequence ought to abolish the expression of S and U antigens on its protein. With regard to GPHe-3 and GPHe-4, both carried the same frameshift and thus should encode two putative proteins with 43 and 30 amino acids, respectively (Fig 4B). In these products, only a short stretch of amino acids at the carboxyl terminus are hydrophobic due to a large truncation of the transmembrane segment as well as a premature chain termination. No evidence was obtained yet to indicate their surface expression, even though GPHe-3 was expected to retain a linear sequence of exon IV encoding the S and U antigens.
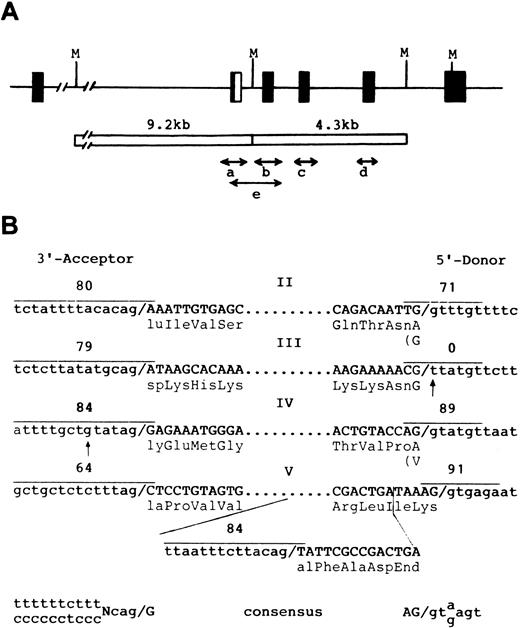
Genomic sequences and exon-intron structures of the GPHe(GL) gene.To establish whether any splice mutations occur, five genomic segments covering exons II through V were obtained by allele-specific PCR (Fig 5A) and their nucleotide sequences were determined (Fig 5B). This analysis confirmed the presence of a defective 5′ donor site attached to exon III of the GPHe(GL) gene (Fig 3C, TT instead of GT) and of 9 nucleotide differences identified by cDNA sequencing (Fig 4A). Significantly, a point mutation (t→g) in the pyrimidine stretch of the acceptor site of exon IV was also identified. Although it would only reduce the splice site score from 90 to 84, the aberrant splicing events associated with GPHe(GL) pre-mRNA could be the combined effects of this mutation and the creation of a new acceptor site within exon V. Figure 5B shows the exon-intron junction sequences of the GPHe (GL) gene and comparison of scores for the new and original splice sites. This assessment indicates that the new acceptor site has a score comparable to the donor site in intron 4 (84 v 89), but much higher than that of the adjacent acceptor site that is only 76-base pairs apart (84 v 64).
Genomic structure of the GPHe(GL) allele and nucleotide sequences of exon-intron junctions. (A) Strategy for genomic amplification of exons and their flanking sequences. Four segments with each covering a unique exon were amplified from either the 9.2- or 4.3-kb Msp I fragment. Segment e, which overlaps a and b, was amplified from total genomic DNA using the He-specific primer P2 and the reverse primer for the b segment (see Materials and Methods). As shown, the GPHe gene from GL also is a GPB-A-B hybrid in the exon II-intron 2 junction region.12 (B) Exon-intron junction structures and splice site scores for the determined acceptor and donor splice sites (overlined). Exon sequences are in capital letters and their encoded amino acid residues are shown. Dots denote omission. Two point mutations, a g→t transversion in the 5′ donor gt element of exon III and a t→g transversion at −6 position near the exon IV acceptor site, are indicated by arrows. The newly created acceptor splice site in exon V and its score are also shown. The consensus sequences of the 3′ acceptor and 5′ donor splice sites are listed at the bottom.
Genomic structure of the GPHe(GL) allele and nucleotide sequences of exon-intron junctions. (A) Strategy for genomic amplification of exons and their flanking sequences. Four segments with each covering a unique exon were amplified from either the 9.2- or 4.3-kb Msp I fragment. Segment e, which overlaps a and b, was amplified from total genomic DNA using the He-specific primer P2 and the reverse primer for the b segment (see Materials and Methods). As shown, the GPHe gene from GL also is a GPB-A-B hybrid in the exon II-intron 2 junction region.12 (B) Exon-intron junction structures and splice site scores for the determined acceptor and donor splice sites (overlined). Exon sequences are in capital letters and their encoded amino acid residues are shown. Dots denote omission. Two point mutations, a g→t transversion in the 5′ donor gt element of exon III and a t→g transversion at −6 position near the exon IV acceptor site, are indicated by arrows. The newly created acceptor splice site in exon V and its score are also shown. The consensus sequences of the 3′ acceptor and 5′ donor splice sites are listed at the bottom.
DISCUSSION
In this study, we describe the identification and molecular analysis of a novel glycophorin allele, GPHe(GL), which produces two He+ protein isoforms in the erythrocyte membrane. We have shown that this gene codes for He, S, and U antigens but contains two nucleotide transversions. The T-to-G mutation is located at −6 position of the acceptor splice site for exon IV, whereas the C-to-G substitution resides in exon V encoding the membrane-spanning segment. Evidence is presented that the latter point mutation creates a new acceptor splice site whose partial activation causes aberrant RNA splicings. We have also shown that the proband carries a normal GPB gene on the homologous chromosome that encodes N, s, and U antigens. These results correlate alternative splicing with the expression of two GPHe isoforms and delineate a new mechanism underlying the polymorphism of He antigen.
The coexistence of both He and S coding sequences in the GPHe-1 transcript conforms to the expression of corresponding antigens on the variant erythrocytes and to the occurrence of a He+ species that comigrated with normal GPB on immunoblots. GPHe-2, rather than GPHe-3 or GPHe-4, is more likely to represent the lower Mr He+ protein species expressed in the RBC membrane, because GPHe-2 is a major transcript and encodes a polypeptide with an intact transmembrane segment. In GPHe-2, the absence of an exon IV sequence also is consistent with the lack of reactivity of its protein with anti-S–like MoAb. Moreover, in view of the potential cleavage sites of α-chymotrypsin in GPB,19 such a deletion readily explains the abolished enzyme digestion of the GPHe-2 protein. With regard to GPHe-3 and GPHe-4, the low level of transcript expression, the deletion of a large internal sequence, and the truncation of a major portion of the membrane domain would impede the disposition of their putative protein products in the plasma membrane. Detailed transfection studies using the GPHe(GL) cDNAs are required to provide a definitive proof for the gene-phenotype correlation.
With the GPHe gene analyzed here, three different forms of the He antigen have now been characterized at the molecular level. In coexpression of He and Sta,7 genetic recombination via the third introns of GPHe and GPA genes leads to a hybrid gene GPHe(Sta) encoding both antigens.11 As to the linkage of He with S-s-U-, the responsible GPHe(P2 ) gene contains two splicing mutations, one being identical with that described here and the other being a G-to-T change at +5 position of the consensus donor splice site.12 In a concerted fashion, the two mutations cause a complete skipping of exon V, resulting in a frameshift and an elongated new hydrophobic sequence for membrane anchoring. Interestingly, the protein product only displays the He reactivity, although it contains a linear sequence for both He and S (and perhaps U) antigens. In contrast, the existing example shows a different splicing pattern in which the joining of exon IV and/or exon V is affected due to the partial activation of the new acceptor splice site and the partial inactivation of the normal splice sites. Accordingly, the exon IV-deleted transcript GPHe-2 lacks the coding sequence for the S and U antigens and thus expresses only the He antigen. These findings support the proposal that diverse mechanisms may underlie the phenotypic variation of He, whether qualitatively or quantitatively.10
Pre-mRNA splicing is a hierarchical cellular process by which splice sites at the exon-intron boundaries can be selected precisely.20,21 The information essential for the selection includes the nearly invariant GT and AG elements at the 5′ and 3′ ends of introns and a branch point close to the 3′ acceptor splice site.17 A survey of human disease genes has shown that point substitutions in GT and AG account for the majority of splicing mutations, constantly causing aberrant RNA splicing.22 However, the effects on pre-mRNA splicing of those mutations not occurring in the predetermined GT-AG elements at exon-intron junctions are not readily predictable. The multiple exon skipping events described here apparently occurred as the result of the combined effects of the T-to-G change close to the exon IV acceptor site and the C-to-G mutation in exon V leading to a new acceptor site. With regard to exon IV and/or exon V partial skipping, it remains to be investigated whether the two altered nucleotides exert their effects concertedly or independently during pre-mRNA splicing. Nevertheless, the selection of the new acceptor site in exon V appears to be related to its sequence context, spatial distribution, and balanced competition with other splice sites. First, the AG element is coincident with the occurrence of a stretch of pyrimidine-rich sequence and thus shows a high similarity to the consensus acceptor site.17 Second, due to a proximity or position effect,23-26 the new splice site might compete with the upstream acceptor and downstream donor sites and thus result in alternative splicing. Third, the selective usage of the new acceptor site could be related to the inevitable emergence of two premature stop codons in exon V, as nonsense mutations are known determinants of splice site selection.27 Along with our further understanding of the molecular basis for He variation, it should be possible to determine how different combinations of mutations affect the processing of GPHe-related primary transcripts by direct experimentation involving splicing assays performed on model pre-mRNA constructs.
ACKNOWLEDGMENT
We thank John Moulds from Gamma Biologicals for rabbit anti-He and monoclonal anti-He and anti-N, and Susan Rolih from Immucor for human anti-He and monoclonal anti-S–like. We are grateful to Dr Colvin Redman for reading the manuscript and helpful discussion. Technical assistance from Rita Batts, Gregory Halverson, and Frances Green is highly appreciated. Thanks are also due to Tellervo Huima-Byron and Yelena Oskov for making photoprints.
Supported by Grants No. HL54459 and GM16389 from the National Institutes of Health, Bethesda, MD and NBF94-10 from the National Blood Foundation (Bethesda, MD).
Address reprint request to Cheng-Han Huang, MD, PhD, Lindsley F. Kimball Research Institute, New York Blood Center, 310 E 67th St, New York, NY 10021.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal