Abstract
An experimental animal model of meningeal leukemia was developed in the nude rat, rnu/rnu, using the human-derived acute lymphoblastic leukemia cell line HPB-ALL. Anesthetized rats were placed in a modified stereotaxic frame and then injected intrathecally, at the level of the cisterna magna, with human leukemic cells. Cerebrospinal fluid and tissue samples from brain, spinal cord, heart, liver, kidney, spleen, bone marrow, and cervical lymph nodes were subjected to histopathologic examination and molecular genetic screening by clonotype primer-directed polymerase chain reaction (CPD-PCR). Ninety-three percent of animals (n = 14) developed signs of meningeal irritation leading to death 30 to 63 days postinjection (median, 36.0 days, mean, 38.7); death occurred between 30 and 39 days in 77% of all animals. Leukemic cells progressively infiltrated the pericerebellar and pericerebral subarachnoid space and infiltrated the Virchow-Robin (perivascular) space. The infiltrating meningeal leukemia closely resembled the pathologic presentation in the human condition. By CPD-PCR, leukemic cells were first detected in cerebrospinal fluid (CSF ) on day 4 postinjection, were variably present over the ensuing 17 days, and were consistently detected after day 21. At terminal stages, CPD-PCR tissue surveys showed leukemic DNA in all brains and spinal cords and rarely in cervical lymph nodes, but leukemic DNA was not detected in any other tissue screened. Leukemic meningitis was reliably produced with a predictable survival time. Intrathecal administration of leukemic cells was an efficient means of transmitting leukemic meningitis and it compartmentalized the disease to the central nervous system (CNS), eliminating potential complications of systemic illness. The use of human-derived cell lines may render this model more relevant to the development of future therapeutic strategies to treat leukemia and lymphoma that invade the CNS.
LEUKEMIA and lymphoma are the leading causes of cancer-related deaths for men and women combined under 35 years of age, with an estimated 28,600 new cases of leukemia in 1995.1 Substantial improvement in chemotherapy, bone marrow transplantation, and radiotherapy has resulted in a greater number of patients achieving clinical remission. However, prolonged survival has been accompanied by an increase in the frequency of central nervous system (CNS) involvement.2 Even with CNS prophylaxis, more than 10% of children and adults with leukemia develop leukemic meningitis, which is often fatal.2-4 Cranial irradiation and intrathecal chemotherapy, which are used both prophylactically and as standard therapy, have significant toxicity: seizures, myelosuppression, and motor, cognitive, and neuropsychological deficits.5-7 Meningeal involvement is a frequent and often lethal complication of leukemia, commonly refractory to our current, toxic treatments.
The development of new therapies requires the use of animal models of leukemic meningitis. Older animal models employ systemic or intracerebral administration of syngeneic murine leukemia lines.8,9 Recently, human leukemia and lymphoma cell lines have been used to develop models in severe combined immunodeficiency (SCID) and nude mice.10 11 Unfortunately, these models produce cancer, which is not confined to the CNS, and the systemic disease may confound interpretation of therapeutic efficacy for drugs being tested to control meningeal leukemia.
We developed an experimental model of leukemic meningitis in the nude rat, rnu/rnu, by intrathecal injection of the human derived T-cell line, HPB-ALL. The evolution of meningeal leukemia is documented by histopathologic analysis and by use of gene amplification by polymerase chain reaction (PCR) to detect minute amounts of the unique human T-cell clonotype against rat background.12 Leukemic meningitis is reliably produced, with a predictable survival time and virtually no evidence of systemic disease. We believe the model will be a useful tool in the development of new therapeutic strategies for leukemia and lymphoma that invade the CNS.
MATERIALS AND METHODS
Animal care.The animal research protocol was reviewed and approved by the Animal Subcommittee, Veterans Administration Medical Center, Buffalo, NY. All aspects of animal care were in accordance with National Institutes of Health (NIH) guidelines. Nude rats, rnu/rnu, originally obtained from Harlan Sprague Dawley (Indianapolis, IN) were bred and maintained at the VAMC Animal Facility (Buffalo, NY) in specific pathogen-free conditions. All animal procedures were performed in a laminar flow hood.
Intrathecal implantation of leukemic cells.HPB-ALL cells, maintained in RPMI 1640 media supplemented with 7% fetal calf serum, 50 U/mL penicillin and 50 μg/mL streptomycin, were diluted to between 4 × 106 and 1.5 × 107 cells/mL in RPMI 1640 before injection. Male nude rats weighing at least 270 g were anesthetized with 1 mL/kg ketamine/xylazine (80 mg/kg and 4.0 mg/kg, respectively) and placed in a stereotaxic frame (David Kopf Instruments, Model 900, Tujunga, CA) modified from Frankman's frame for repeated sampling of cerebrospinal fluid (CSF ).13 The back of the head and neck were shaved, sterily prepared, and draped, and a 250-μL Hamilton syringe with a 26s gauge needle mounted in a micromanipulator was advanced horizontally through a 2-mm incision, made in the skin at the level of the cisterna magna, until the dura was punctured (usually between 7.0 and 9.5 mm deep from skin level). Slight negative pressure was applied to the syringe until CSF was obtained. Ten microliters of the HPB-ALL cell suspension was injected over 3 minutes, and the needle was left in place for 5 minutes before being withdrawn over 5 minutes. The incision was closed with one 4-0 silk suture. Animals received tetracycline hydrochloride [1 teaspoon powder (The Butler Co, Columbus, OH) dissolved in a 16-oz capacity water bottle] for 7 days following surgery. Forty-two rats were implanted with HPB-ALL cells and separated into two groups, a survival study group (n = 15) and a timed histologic study group (n = 27).
CSF sampling.At multiple intervals postintrathecal injection, CSF was sampled from anesthetized rats in a manner similar to the process of intrathecal cell injection described above. Approximately 100 μL CSF per cisternal tap was aspirated with a 250-μL Hamilton syringe and 26s gauge needle, snap frozen, and stored before DNA extraction and subsequent PCR-based molecular genetic analyses. Sampling was performed at 1, 2, 4, 7, 11, 14, 19, 20, 21, and 28 days postleukemic cell implantation. In animals killed for the timed histologic study, a CSF sample was taken at the time of death. For the survival study, CSF was sampled at time points during the observation period, and a small sample of leukemic cells was removed from the subarachnoid space of the posterior fossa at the time of death.
Cytopathologic examination of CSF.Eight animals were separately injected with HPB-ALL cells, and two animals each at days 1, 7, 14, and 21 were sampled for cytologic examination of CSF. CSF cell counts were determined by Coulter Counter, and cytospin preparations were designed to contain a minimum of 500 cells per slide. Fixed cells were stained with Wright/Giemsa. Examinations of CSF preparations were performed by an independent pathologist experienced in cytologic assessment of CSF.
DNA and oligonucleotide preparation.High-molecular-weight DNA was isolated from the transformed T-cell line HPB-ALL as previously described.14 CSF was first subjected to a 10-minute high-speed centrifugation to pellet cells before DNA extraction, while rat tissues were minced in phosphate-buffered saline (PBS) and washed by low-speed centrifugation before extraction.
Fluorescent oligonucleotide primers were synthesized on an automated 391 DNA synthesizer (Applied Biosystems, Foster City, CA). The HPB-ALL T-cell receptor beta chain specific primers were 5′-labeled with fluorescein amidate (FAM) and constant region primers were labeled with 6-hexachlorofluorescein (HEX). All primers were purified using oligonucleotide purification cartridges (OPC) (ABI, Foster City, CA) according to manufacturer's directions.
Molecular characterization and detection of human leukemic cells.HPB-ALL cells contain unique DNA sequences formed by the juxtaposition of the variable/diversity/joining (VDJ) regions of the T-cell receptor beta chain during T-cell maturation.15 Using FAM-labeled primers specific solely for this unique DNA sequence, we used clonotype primer-directed PCR (CPD-PCR, described in detail in Beers12 ) to detect human HPB-ALL DNA in CSF and a variety of rat tissues. To control for the quality of DNA and the integrity of the PCR reactions, we screened all DNA samples using HEX-labeled primers, which reacted equally well with the constant region of the T-cell receptor in either rat or human DNA. The primers have the following 5′ → 3′ sequences: HPB-ALL (+) primer (TCR-βV5 specific): FAM-TCTCAGCTCGCCAGTTCCCTAACTATAGCTCTGA; HPB-ALL(−) primer (clonotype specific): FAM-AGTACTGGGTTTTCCGCGAGCTG; TCR-βC (+) primer: HEX-GTGTTCCCACCCGAGGTCGCTGTGTTTGAGCC; TCR-βC (−) primer: HEX-GTGCTGACCCCACTGTGCACCTCCTTCCCATT. All PCRs were performed as previously described.12
Molecular genetic detection of leukemic HPB-ALL TCR-βVDJ DNA was performed by a gel electrophoresis/laser detection system using Genescanner analysis (Genescanner 377; Perkin Elmer, ABI Division, Foster City, CA). The electrophoresed, fluorescently labeled amplicons were analyzed using Genescanner software, V1.2.
Tissue preparation and histologic examination.Animals were killed in a CO2 chamber and the following organs removed: brain, spinal cord (lumbar region), heart, liver, kidney, spleen, bone marrow (femoral), and cervical lymph nodes. Specimens were flash frozen in isopentane on dry ice and stored at −70°C before analysis of DNA. The most rostral 6-mm coronal section of the frontal lobes was removed for DNA analysis. The remaining brain tissue was then flash frozen, as above, before preparation for histologic examination.
Human meningeal leukemia in the nude rat: Survival curve. Fourteen nude rats were injected intrathecally with an HPB-ALL cell suspension and died at a median of 36.0 days and mean of 38.7 days postinjection (SEM, 8.6 days). Note that death occurred between 30 and 39 days postinjection (ie, within a range of 10 days) in 77% of the animals (arrows).
Human meningeal leukemia in the nude rat: Survival curve. Fourteen nude rats were injected intrathecally with an HPB-ALL cell suspension and died at a median of 36.0 days and mean of 38.7 days postinjection (SEM, 8.6 days). Note that death occurred between 30 and 39 days postinjection (ie, within a range of 10 days) in 77% of the animals (arrows).
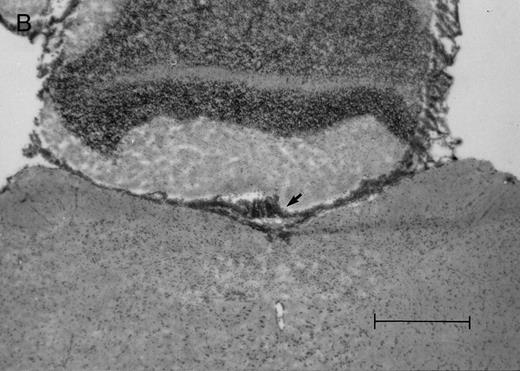
Histologic progression of leukemic meningitis. All sections are 12-μm coronal sections. (A) Cisterna magna at 14 days postinjection showing a small cluster of HPB-ALL cells (arrow) in the subarachnoid space, H&E, bar = 0.64 mm. (B) Cisterna magna at 21 days postinjection showing large groups of HPB-ALL cells in the subarachnoid space (arrow), H&E, bar = 0.64 mm. Compare the large groups of cells shown here with the small cluster at 14 days postinjection shown in (A).
(C and D) Cerebral level of the animal shown in (B), showing a very small cluster of HPB-ALL cells in the subarachnoid space (arrow), H&E (C), and leukocyte common antigen (LCA) (D), bar = 0.09 mm. Compare the sparse leukemic cell density here with the posterior fossa section in (B).
(E) Cerebral level of an animal that died during the natural progression of the disease. Note the extensive replacement of the subarachnoid space of the interhemispheric fissure by HPB-ALL cells and the invasion of Virchow-Robin spaces, H&E, bar = 0.56 mm.
Histologic progression of leukemic meningitis. All sections are 12-μm coronal sections. (A) Cisterna magna at 14 days postinjection showing a small cluster of HPB-ALL cells (arrow) in the subarachnoid space, H&E, bar = 0.64 mm. (B) Cisterna magna at 21 days postinjection showing large groups of HPB-ALL cells in the subarachnoid space (arrow), H&E, bar = 0.64 mm. Compare the large groups of cells shown here with the small cluster at 14 days postinjection shown in (A).
(C and D) Cerebral level of the animal shown in (B), showing a very small cluster of HPB-ALL cells in the subarachnoid space (arrow), H&E (C), and leukocyte common antigen (LCA) (D), bar = 0.09 mm. Compare the sparse leukemic cell density here with the posterior fossa section in (B).
(E) Cerebral level of an animal that died during the natural progression of the disease. Note the extensive replacement of the subarachnoid space of the interhemispheric fissure by HPB-ALL cells and the invasion of Virchow-Robin spaces, H&E, bar = 0.56 mm.
Cerebral and cerebellar 12 μm-thick coronal sections were cryosectioned (Reichert-Jung Cryocut 1800, Nussloch bei Heidelberg, Germany), thaw-mounted on gelatin-coated slides, fixed in acetone for 10 minutes, and stored frozen at −70°C. Immunohistochemical staining was performed by incubation in leukocyte common antigen, 2B11, and PD7/26 antibody (Dako Corp, Carpinteria, CA) at a concentration of 1:50 in 3% bovine serum albumin/PBS according to standard procedures. Sections were then treated with biotinylated horse antimouse IgG and Vectastain ABC reagent as per manufacturer's recommendations (Vector Laboratories, Burlingame, CA). Color development was done in PBS containing 0.05% diaminobenzidine (Sigma, St Louis, MO), 0.01% H2O2 and 0.04% NiCl. Separately, adjacent sections were stained with hematoxylin and eosin.
RESULTS
The intrathecal administration of human leukemic HPB-ALL cells into the nude rat resulted in the near universal development of leukemic meningitis. In the survival study, 15 animals were injected intrathecally and followed for signs of leukemic meningitis. One animal died due to the anesthetic and is not counted in any of the results. Symptoms attributed to the development of meningeal leukemia included lethargy, loss of righting ability, head-tilt, occasional intraorbital hemorrhage, labored breathing, and weight loss. Two animals died within 24 hours of demonstrating these symptoms, so for humane purposes, the remainder of the animals were killed at the onset of symptoms. Thirteen animals (93%) developed signs of meningeal irritation at a median of 36 days and a range of 30 and 63 days postinjection (mean, 38.7 days, standard error of the mean [SEM] 8.6) and time to death fell between 30 and 39 days, ie, within a range of 10 days, in 77% of all animals that succumbed (Fig 1). A single animal showed no clinical signs of disease and was killed on day 70 with no histologic evidence of disease.
In the timed histologic study, animals were killed sequentially postintrathecal HPB-ALL cell injection. Three animals each at 1, 2, 4, 7, 11, 14, and 19 days, two animals at 20 days, and seven animals at 21 days postinjection underwent CSF sampling and were immediately killed. At the 19 day time point, only CSF was sampled, as the same group of animals was also sampled and then killed as part of the 21 day time point. On histologic examination, brain sections from animals killed between 1 and 11 days postinjection showed rare, dispersed leukemic cells in the subarachnoid space at both the cerebral and cerebellar levels. At day 14, very small clusters of HPB-ALL cells could be observed in the subarachnoid space at the cerebellar level in two of three animals (Fig 2A). By 21 days postinjection, large groups of cells could be seen in the subarachnoid space of all animals in the cerebellar sections (Fig 2B). Collections of leukemic cells were concentrated in the most posterior cerebellar sections, in the meninges of the cisterna magna. Small clusters of leukemic cells or scattered leukemic cells were observed in the subarachnoid space of cerebral sections (Fig 2C and D). In animals that developed symptoms during the course of the natural progression of leukemic meningitis, there was extensive infiltration of the subarachnoid space by leukemic cells throughout the brain, with the most dense involvement in the basal region. This was accompanied by permeation of the Virchow-Robin spaces (Fig 2E). The pattern was strikingly similar to leukemic meningitis in humans.
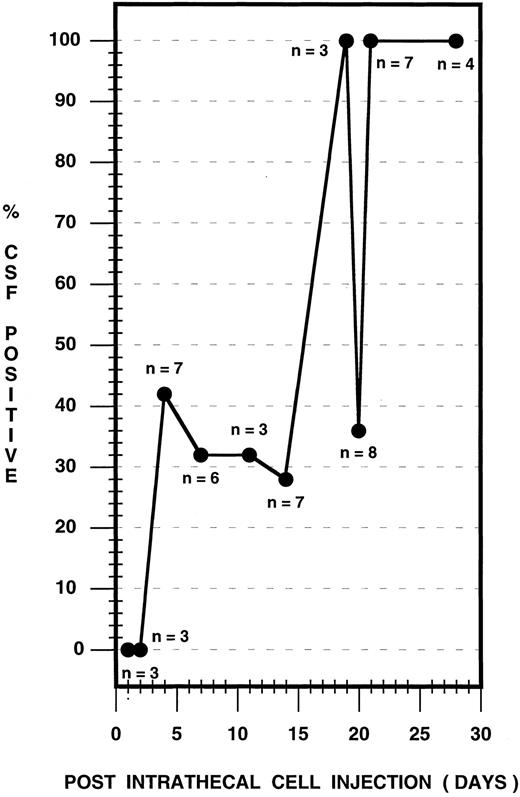
Using CPD-PCR, the earliest detection of leukemic cells in the CSF occurred on day 4 postinjection (Fig 3). The presence of leukemic cells in the CSF was variable over the ensuing 17-day period, with between 28% and 100% of rats sampled expressing HPB-ALL DNA in the CSF (Fig 3). Before day 21, in 10 cases from the survival study and in two cases from the timed histologic study, CSF was negative for HPB-ALL DNA, although animals subsequently developed symptoms of the disease or brain tissue was positive. By day 21 postinjection and subsequently, 100% of rats consistently contained leukemic cellular DNA in the CSF. Cytological analysis of CSF samples showed no atypical cells before day 21 and a small population of atypical cells, compatible with the presence of leukemia, at 21 days (data not shown).
CSF leukemic pleocytosis in the nude rat. CSF samples from both survival and timed histologic studies were used and sampling was performed as follows: Survival study (n = 14): Animals were selected at random for CSF sampling during the observation period and were sampled in groups of three to six animals at 4, 7, 14, 20, and 28 days postinjection. Timed histologic study (n = 27): Three animals each at 1, 2, 4, 7, 11, 14, and 19 days, two animals at 20 days, and seven animals at 21 days postinjection were sampled, followed immediately by death. The total number of CSF samples analyzed is depicted for each time point.
CSF leukemic pleocytosis in the nude rat. CSF samples from both survival and timed histologic studies were used and sampling was performed as follows: Survival study (n = 14): Animals were selected at random for CSF sampling during the observation period and were sampled in groups of three to six animals at 4, 7, 14, 20, and 28 days postinjection. Timed histologic study (n = 27): Three animals each at 1, 2, 4, 7, 11, 14, and 19 days, two animals at 20 days, and seven animals at 21 days postinjection were sampled, followed immediately by death. The total number of CSF samples analyzed is depicted for each time point.
A tissue survey by CPD-PCR showed that leukemic cells were rarely detected in the brain or spinal cords before 7 days following injection. At 7, 11, and 14 days postinjection, one third of rat brains or spinal cords were positive for leukemic DNA, and by 20 days postinjection, 100% of animals were consistently leukemic DNA positive in the frontal brain and/or spinal cord. Signals of amplified HPB-ALL DNA were found in all brains and spinal cords of animals exhibiting symptoms of leukemic meningitis and in four rats a positive signal for leukemic DNA was also detected in the cervical lymph nodes. It must be noted, however, that the positive signal in the lymph nodes was less than 1% of the amplitude of the signal for the brains of those animals (Fig 4). For all other tissues and organs screened, including femoral bone marrow, CPD-PCR failed to show the presence of leukemic involvement in either study group (Table 1).
Detection of HPB-ALL DNA in brain and cervical lymph node. One microgram of DNA from either the brain or cervical lymph node of the same animal was subjected to amplification by PCR using the fluorescent primers described. One microliter of the resulting reaction mixture was subjected to fractionation on a 6% denaturant polyacrylamide gel containing 8 mol/L urea and run for 3 hours in the Model 373 Genetic Analyzer (Applied Biosystems) and the results analyzed by Genescanner software (Applied Biosystems) as described. Signals from the brain (open graph) and lymph node (darkened graph, arrow) have been superimposed to be on the same scale. The expected fragment size of the HPB-ALL T-cell receptor VDJ region being amplified is 112 bases. After correcting for the recovery of standards, the relative areas of the brain and lymph node peaks are in the approximate ratio of 100:1.
Detection of HPB-ALL DNA in brain and cervical lymph node. One microgram of DNA from either the brain or cervical lymph node of the same animal was subjected to amplification by PCR using the fluorescent primers described. One microliter of the resulting reaction mixture was subjected to fractionation on a 6% denaturant polyacrylamide gel containing 8 mol/L urea and run for 3 hours in the Model 373 Genetic Analyzer (Applied Biosystems) and the results analyzed by Genescanner software (Applied Biosystems) as described. Signals from the brain (open graph) and lymph node (darkened graph, arrow) have been superimposed to be on the same scale. The expected fragment size of the HPB-ALL T-cell receptor VDJ region being amplified is 112 bases. After correcting for the recovery of standards, the relative areas of the brain and lymph node peaks are in the approximate ratio of 100:1.
Molecular Genetic Detection of Leukemic DNA: Organ Survey
| Organ . | Survival Study* . | Time Course Study . |
|---|---|---|
| . | Percentage Positive . | Percentage Positive . |
| Heart | 0 (n = 12) | 0 (n = 27), all time points |
| Liver | 0 (n = 12) | 0 (n = 27), all time points |
| Kidney | 0 (n = 12) | 0 (n = 27), all time points |
| Spleen | 0 (n = 12) | 0 (n = 27), all time points |
| Bone marrow | 0 (n = 2) | 0 (n = 27), all time points |
| Lymph node cervical | 25 (n = 12) | 0 (n = 27), all time points |
| Spinal cord lumbar | 100 (n = 11) | 0 (n = 15), at 1, 2, 4, 11, and 14 d |
| 33 (n = 3), at 7 d | ||
| 100 (n = 2), at 20 d | ||
| 100 (n = 7), at 21 d | ||
| Brain | ||
| frontal | 93 (n = 14) | 0 (n = 9), at 1, 4, and 7 d |
| 33 (n = 3), at 2 d | ||
| 33 (n = 3), at 11 d | ||
| 33 (n = 3), at 14 d | ||
| 100 (n = 2), at 20 d | ||
| 86 (n = 7), at 21 d |
| Organ . | Survival Study* . | Time Course Study . |
|---|---|---|
| . | Percentage Positive . | Percentage Positive . |
| Heart | 0 (n = 12) | 0 (n = 27), all time points |
| Liver | 0 (n = 12) | 0 (n = 27), all time points |
| Kidney | 0 (n = 12) | 0 (n = 27), all time points |
| Spleen | 0 (n = 12) | 0 (n = 27), all time points |
| Bone marrow | 0 (n = 2) | 0 (n = 27), all time points |
| Lymph node cervical | 25 (n = 12) | 0 (n = 27), all time points |
| Spinal cord lumbar | 100 (n = 11) | 0 (n = 15), at 1, 2, 4, 11, and 14 d |
| 33 (n = 3), at 7 d | ||
| 100 (n = 2), at 20 d | ||
| 100 (n = 7), at 21 d | ||
| Brain | ||
| frontal | 93 (n = 14) | 0 (n = 9), at 1, 4, and 7 d |
| 33 (n = 3), at 2 d | ||
| 33 (n = 3), at 11 d | ||
| 33 (n = 3), at 14 d | ||
| 100 (n = 2), at 20 d | ||
| 86 (n = 7), at 21 d |
In 2 of 14 animals, only brain was removed and examined.
DISCUSSION
Immunodeficient animals readily accept xenogeneic tissue and are ideal hosts to develop various experimental models of human cancer. SCID and nude mouse models of meningeal leukemia have recently been described.10 11 Our study uses the nude rat to develop a new model of meningeal leukemia.
We injected human leukemic cells into the CSF via the cisterna magna of nude rats producing leukemic meningitis in 93% of rats. The one failure to develop meningeal leukemia may have been caused by cell clumping in the needle, causing inadequate cell delivery. Alternatively, leukemic cells may have been cleared by a residually intact cellular immune system.16 In a SCID model of disseminated leukemia, mice with high IgM levels developed disease in an unreliable fashion or not at all.17 IgM levels in nude rats are as high as their euthymic counterparts and their natural killer cell levels have been reported to be increased.18
Our model of human leukemic meningitis in the nude rat shows a predictable survival time; in the majority of cases, death occurred 30 to 39 days postinjection. Survival times in other CNS leukemia models have ranged widely from 10 to 50 days.8-11 The 39-day mean survival in our model was comparable to that found in a SCID mouse model established by Gunther et al.10 Most models report systemic disease in addition to CNS disease. In our model, leukemic DNA was detected in cervical lymph nodes in four rats at the terminal stage of disease. This may reflect instances of leukemic metastasis to adjacent extra CNS sites or, more likely, lymphatic drainage of dead leukemic cell debris through normal lymph channels from CNS sites or from extra CNS sites via cells escaping through the dural puncture following cisternal injection. We did not find leukemic DNA in any other organ, including femoral bone marrow, avoiding possible complications from systemic leukemia. However, we did not analyze skull marrow and cannot assume that there was no infiltration in this area.
We observed similar clinical symptoms to those seen in other models, but differences were noted as well. Most models describe severe paralysis and seizures, which we did not observe.8,10,11 The lethargy we noted may have been followed by paralysis had we allowed the disease to progress another 12 to 24 hours. Similar to our observations though, lethargy and weight loss are noted.8,10,11 Histologically, all models show a similar sheet of cells in the subarachnoid space and many mention invasion of the Virchow-Robin spaces similar to our observations in advanced disease.8-11
The CPD-PCR methodology employed here has a demonstrated sensitivity of detecting 1 pg leukemic DNA in 1 μg polyclonal DNA input or the equivalent of detecting 1 leukemic cell in 106 cells.12,19 20 Serial CSF analyses performed by PCR were unable to detect leukemic DNA at 24- and 48-hour time points postinjection. It is possible that animals killed at early time points received ineffectual injections, but the high rate of disease development in the survival study makes this unlikely. It is probable that many leukemic cells were unable to survive the in vitro to in vivo transition. In addition, leukemic cells may have been cleared by natural killer cell activity shortly after intrathecal administration. Viable cells probably adhered to the meninges. This is supported by the cases in the timed histologic study where leukemic DNA was detected in brain tissue, but was not detected in CSF at time points earlier than 21 days. Leukemic DNA was first detected in the CSF on day 4 postinjection and in a variable number of animals up to 3 weeks. By day 21, CSF was consistently positive for the presence of leukemic DNA. These observations were in agreement with the cytopathologic data; CSF was never positive for atypical lymphocytes before 21 days. Clonotype primer-directed PCR was more sensitive than cytology in detecting leukemic cells, as the PCR method could occasionally detect cells before 21 days postinjection. As the meningeal leukemia progressed, cells may have begun sloughing off into the CSF. The data suggest that between 2 and 3 weeks postinjection, a massive increase in cell number occurred. The profile of advancing CSF leukemic DNA positivity paralleled disease progression, and this was supported by serial analyses of brain and spinal cord tissue by PCR and brain histopathology obtained at corresponding time points postinjection. Both methods showed a variable pattern of leukemic cell detection before 21 days and consistent detection of disease thereafter.
At day 14 postinjection, there was histopathologic evidence that the cells were concentrated in the meninges surrounding the cisterna magna and caudal cerebellum. The paucity of cells seen in cerebral sections at day 21 suggests that massive expansion occurred at the cisternal site before cells spread in the CSF. However, HPB-ALL DNA was detected in the frontal brain and/or lumbar spinal cord at 21 days in all animals, confirming a limited leukemic spread from the injection site. This observation suggests that screening by PCR is more sensitive than histologic examination for detecting very small numbers of leukemic cells. The histologic appearance at the time of death was dramatic, with extensive replacement of the subarachnoid space by leukemic cells and infiltration of the Virchow-Robin spaces deep into the brain parenchyma. This picture is nearly identical to the histopathology observed in patients with leukemic meningitis and supports the conclusion that the experimental animal model presented here is a close representation of the human disease.
The nude rat model of human meningeal leukemia should be useful in developing novel, less toxic treatments for CNS leukemia including gene therapy, immunotherapy, and alternative drug therapies. Studies are currently under way to evaluate new treatment modalities using this experimental animal model.
Supported in part by grants from the Endowment for the Neurosciences, North Bellmore, NY; and The Buffalo General Hospital Margaret Duffy and Robert Cameron Troup Memorial Fund for Cancer Research, Buffalo General Hospital, Buffalo, NY.
Address reprint requests to Robert J. Plunkett, MD, Department of Neurosurgery, Buffalo General Hospital, E-2, 100 High St, Buffalo, NY 14203.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal