Abstract
Chronic myeloid leukemia (CML) is characterized by a specific translocation of the c-abl oncogene on chromosome 9 to the break point cluster region (bcr) on chromosome 22, t(9; 22) (q34; q11). This translocation results in the expression of a 210-kD bcr-abl protein fusion gene product. The juxtaposition of the bcr and abl genes produces a novel junctional amino acid sequence, which may be presented by antigen-presenting cells and recognized specifically by human T lymphocytes. We have generated a CD4+ T lymphocyte line (NG-1) which recognizes the peptide epitope (GFKQSSKALQR) in association with HLA-DRβ1*0101-02. A comparison of antigen-presenting cells showed that CMRF-44+ blood dendritic cell presented a 12mer b3a2 peptide effectively. The b3a2 peptide was able to generate specific primary T-lymphocyte responses in other HLA-DR1 donors. We also show that bcr-abl, b3a2 peptide-specific T-lymphocyte lines proliferate in response to bcr-abl b3a2 containing cell lysates (K562 or CML PBMC derived) but not control (including b2a2 CML PBMC) lysates.
TREATMENT of relapsed chronic myeloid leukemia (CML), after HLA-matched sibling bone marrow transplantation (BMT), with donor leukocyte infusions has proven antileukemic effects.1,2 Nonetheless, this therapy, which is thought to reflect a graft-versus-leukemia (GVL) response against minor histocompatibility antigens on the leukemic clone3 is associated with significant mortality and morbidity from graft-versus-host disease (GVHD). A more specific form of leukemia immunotherapy, which increases the GVL while avoiding GVHD would be highly desirable. An antigenic target, which is not only leukemia specific but is also essential for the maintenance of the malignant phenotype, would theoretically minimize problems of nonspecific tissue damage and selection of antigen negative mutants during immunotherapy.
CML is characterized by a specific translocation of the c-abl oncogene (9q34) to the bcr region on chromosome 22 (22q11).4-6 Alternative recombination sites involving either the second or third exon of the bcr gene splicing to exon 2 of the abl gene yield two potential fusion gene transcripts, b2a2 and b3a2, respectively.7 The translated 210-kD bcr-abl fusion protein,8 which has abnormal tyrosine kinase activity, includes a potentially antigenic novel sequence at the fusion site: a novel amino acid is generated at the junctional site by the fusion event; in the b2a2 fusion a glutamic acid (E) is encoded, whereas in the b3a2 recombination event a lysine (K) is generated.
A bcr-abl peptide from the b3a2 fusion region has been found to be immunogenic in mice.9 In humans, binding of b3a2 peptides to HLA class I alleles A3, A11, and B810,11 has been described. Furthermore, these b3a2 peptides have been shown to prime CD8+ cytotoxic T lymphocytes (CTL) in vitro, although the capacity of these peptide specific CTL to lyse CML cells has not been determined. CD4+ b3a2 peptide-specific T-lymphocyte responses restricted by the HLA class II alleles DRβ1*1501,12 0401,13 and responses in HLA- DR11 and DR3 individuals14 have been described. ten Bosch et al13 showed that their HLA-DRβ1*0401 restricted clone proliferated after stimulation with CML cells obtained at blast crisis; however, Pawelec et al14 reported that their b3a2 peptide-specific CD4+ T-cell lines did not proliferate in response to HLA-matched CML cells. Thus, the susceptibility of CML cells to b3a2 peptide-specific therapy remains in doubt.
The bcr-abl fusion protein is CML specific and critical to the leukemogenic process,15,16 but if CML cells prove to be susceptible targets it will become critical to optimize any vaccination schedule for effective presentation of bcr-abl peptide to the immune system. The dendritic cell (DC) has the capacity to migrate through tissues, process and present antigen, and stimulate strong primary T-lymphocyte responses. As “nature's adjuvant,” DC are a logical choice for immunotherapy.17 Encouraging progress has been made using DC to initiate an antitumor response in mouse models18 and DC primed anti-idiotype responses to B-cell lymphomas have been described recently.19
This study describes the generation and characterization of an HLA-DR β1* 0101-02–restricted, CD4+ T-lymphocyte line that responds to an 11mer epitope spanning the junctional region of the b3a2 fusion protein. We have shown that CMRF-44+ DC can present the b3a2 peptide to responding T lymphocytes. Furthermore, we show that b3a2 peptide specific T lymphocytes are able to proliferate specifically in response to b3a2, but not b2a2, CML cell lysates.
MATERIALS AND METHODS
Blood donors and peripheral blood mononuclear cell (PBMC) preparation.Blood was collected, from normal volunteer donors and CML patients, into preservative-free heparin, according to Ethical Committe guidelines and with appropriate informed consent. PBMC were isolated from whole blood by Ficoll/Hypaque (Pharmacia, Uppsala, Sweden) (density = 1.077) density gradient centrifugation.
Antigen and peptide preparation.All peptides (see Table 1) were synthesized by Chiron Mimotopes (Clayton, Victoria, Australia) and purified to at least 70%. Peptides were dissolved in 0.1% acetic acid/distilled water to give a stock solution of 1 mg/mL, filter sterilized, and stored at −80°C.
Synthetic Peptides Used in This Study
| Name/No. . | Peptide Sequence . | No. of Amino Acids . |
|---|---|---|
| b2a2 | LTINKEEALQRP | 12 |
| b3a2 | GFKQSSKALQRP | 12 |
| 035 | HSATGFKQSSKA | 12 |
| 036 | ATGFKQSSKALQ | 12 |
| 799 | GFKQSSKALQR | 11 |
| 800 | FKQSSKALQRP | 11 |
| 037 | KQSSKALQRPVA | 12 |
| 038 | SSKALQRPVASD | 12 |
| 039 | KALQRPVASDFE | 12 |
| 801 | GFKQSSAALQRP | 12 |
| 605 | HSIPLTINKEEALQRPVASDF | 21 |
| 606 | HSATGFKQSSKALQRPVASDF | 21 |
| Name/No. . | Peptide Sequence . | No. of Amino Acids . |
|---|---|---|
| b2a2 | LTINKEEALQRP | 12 |
| b3a2 | GFKQSSKALQRP | 12 |
| 035 | HSATGFKQSSKA | 12 |
| 036 | ATGFKQSSKALQ | 12 |
| 799 | GFKQSSKALQR | 11 |
| 800 | FKQSSKALQRP | 11 |
| 037 | KQSSKALQRPVA | 12 |
| 038 | SSKALQRPVASD | 12 |
| 039 | KALQRPVASDFE | 12 |
| 801 | GFKQSSAALQRP | 12 |
| 605 | HSIPLTINKEEALQRPVASDF | 21 |
| 606 | HSATGFKQSSKALQRPVASDF | 21 |
Boldface letters indicate novel amino acid generated by translocation event.
Cell lysates were prepared from PBMC obtained from CML patients by resuspending PBMC at 1 × 108 cells/mL in distilled water followed by sonication for 10 seconds. Particulate matter was removed by centrifugation for 20 minutes at 36,260g using a Beckman Type 70./Ti Rotor (Beckman Instruments, Palo Alto, CA). The high-molecular-weight fraction of the bcr-abl–containing lysates were enriched by centrifugation through a Centricon-100 filter (Amicon, Beverly, MA) and resuspended in phosphate-buffered saline (PBS) to yield the equivalent of 1.0 × 108 cells/mL. The resultant fractions were filter sterilized before use as antigen in T-lymphocyte proliferation assays. K562 and HEL lysates were prepared in an identical manner except that cells were pelleted from tissue culture media before water lysis.
Monoclonal antibodies (MoAbs).The MoAbs used for blocking studies were anti–HLA-DP (B7/21), provided by Dr Frances Brodsky (University of California, San Francisco); anti-HLA class I (W6/32), anti–HLA-DQ (L227), and anti–HLA-DR (L243) obtained from American Type Culture Collection (ATCC, Rockville, MD). Anti-CD4 (OKT4) and CD8 (OKT8) antibodies for phenotyping the NG-1 line were also obtained from the ATCC. CMRF-44 (this laboratory20 ) and anti–CD14-PE (Becton Dickinson, Mountain View, CA) were used for DC and monocyte purification and FITC-SAM was purchased from Silenus (Hawthorn, Australia).
DC and monocyte purification.Highly purified DC and monocyte populations were isolated by the method of McLellan et al.21 Briefly, peripheral blood non-T cells were cultured at 1 × 107/mL for 16 hours in 10% AB before separation over a Nycoprep gradient (density = 1.068) (Nycomed, Oslo, Norway). The low-density cells obtained from the interface were double labeled with CMRF-44 and FITC-SAM, followed by CD14-PE. CMRF-44 strongly positive, CD14− cells were sorted to give a high purity (>90%) DC population20 and the CD14+ cells sorted to obtain monocytes (>95% purity).
Cell lines and media.The NG-1 line was cultured in RPMI-1640 supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mol/L L-glutamine, and 10% heat-inactivated AB serum. All other cell lines were maintained in the above media, but with 10% fetal calf serum (FCS) instead of AB serum. The L-cell transfectants expressing either HLA-DR β1*0101 (L57/23) or HLA-DR β1*0701 (L12/2) were kindly provided by Dr Brian Tait (Melbourne, Australia). The K562 line which expresses the b3a2 translocation,22 the HEL cell line,23 and the B95/8 EBV secreting line are maintained as laboratory stocks.
Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell lines were generated by culturing PBMC in 2 mL B95/8 supernatant for 2 hours at 37°C, then adjusting to a concentration of at least 1 × 106/mL in 10% FCS/RPMI-1640 containing 2 μg/mL cyclosporin A (Sandoz Pharma, Auckland, New Zealand).
Generation of T-lymphocyte lines and clones.PBMC were cultured at 2 × 106 cells/mL in 2 mL of 10% AB/RPMI-1640 in 24-well plates, together with a mixture of b2a2 and b3a2 peptides at 5 μg/mL each (3.7 μmol/L and 3.5 μmol/L, respectively). The cultures were restimulated every 7 days with peptide and 1 × 106/mL mitomycin C (Sigma Chemical Co, St Louis, MO) treated autologous PBMC (Mc PBMC) as APC. Samples (50 μL each in triplicate) were removed before and after the addition of antigen and assayed for proliferation after 2 more days of culture in a separate 96-well round-bottom plate. At the fourth stimulation interleukin-2 (IL-2) was added to 20 U/mL (Hoffman La Roche, Basel, Switzerland). One culture displayed specific proliferation in response to bcr-abl peptides after the fourth stimulation and this line (NG-1) was maintained by replacement of media and IL-2 and restimulation with PBMC and peptide every 7 to 10 days. After initial expansion the media was supplemented with an extra 10% FCS. The NG-1 line was cloned after the fifth stimulation by limiting dilution in 96-well flat-bottom plates. The cloning mix consisted of fresh autologous mitomycin C–treated PBMC at 2 × 105/well, IL-2 to 20 U/mL, and b3a2 peptide to 5 μg/mL in a final vol of 200 μL. Growing clones were expanded from wells seeded with less than 0.8 cells/well and after 10 days were tested for specificity.
T-lymphocyte proliferation assays.Proliferation was assayed using a standard 3H-thymidine incorporation assay. Sensitized T lymphocytes (2 to 5 × 104 cells/well) were cultured with varying numbers (usually 1 × 105) of mitomycin C–treated antigen presenting cells (APC) in 96-well U-bottomed plates (Beckton Dickinson). These were either autologous PBMC, EBV-transformed B-lymphoblastoid cell lines, HLA-DR–transfected L cells, autologous or HLA-DR1 positive DC, or monocytes. Peptide, cell lysates, or control treatments were included as indicated. For cell-lysate experiments, Mc PBMC were incubated with lysate in serum-free RPMI-1640 (SF RPMI) for 2 hours at 37°C and washed once before adding to T lymphocytes in 96-well plates.
After 48 hours 0.5 μCi 3H-thymidine (Amersham International, Arlington Heights, IL) was added for a further 16 hours before harvesting onto glass fiber filter mats and counting on a β-scintillation counter. Results are expressed as mean ± SEM of triplicate wells.
For blocking experiments, APC were incubated with MoAbs to major histocompatability complex (MHC) class I (W6/32) or HLA-DP (B7/21), HLA-DQ (L227), HLA-DR (L243) for 1 hour before incubation with the T lymphocytes and peptides.
Split-well method for assessing primary T-lymphocyte–specific responses.We used a method based on the split-well method of Gambacorti-Passerini.24 APC were prepared by pulsing 107 Mc PBMC in SF RPMI 1640 with 50 μmol/L 20mer b3a2 peptide for 2 hours at 37°C and cultured with 2 × 107 PBMC responders in a 25-cm2 flask. The final peptide concentration was 2.5 μmol/L and the initial AB serum concentration of 5% was increased to 10% the following day. The cultures were stimulated with pulsed APC every 7 days and IL-2 was added at day 10 to 2.5 U/mL. At the third stimulation (day 14) viable cells were isolated over Ficoll/Hypaque added and plated at 6.0 × 104 cells/well with 1.0 × 105 peptide-pulsed Mc PBMC and IL-2 was added to 5 U/mL on day 17. At the fourth stimulation (day 21) each well was split into four. Each replicate well was stimulated with thawed autologous Mc PBMC, pulsed with b3a2 peptide, b2a2 peptide, HEL (or b2a2 CML) cell lysate, or K562 (or b3a2 CML) cell lysate. All wells were assayed for proliferation after 2 further days of culture.
RESULTS
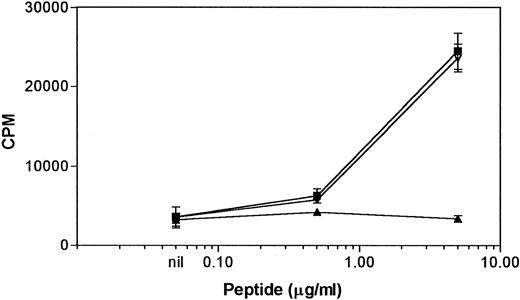
Generation of the bcr-abl b3a2 peptide-specific T-lymphocyte line NG-1.Fifteen PBMC cultures from different normal individuals were established and checked for bcr-abl peptide-specific proliferative responses. Although several initial promising results were obtained using PBMC stimulators, only one of these was maintained. A weak but antigen-specific response was detected in one individual (NG-1) after the fourth stimulation with b2a2/b3a2 peptide mixture. Further stimulation with either the b2a2/b3a2 mixture or each peptide alone showed a dose-dependent response to the b3a2 peptide, but not to the b2a2 peptide (Fig 1). The presence of the b2a2 peptide had no effect on the response to the b3a2 peptide. Flow cytometric analysis established the NG-1 line to be CD3+CD4+ CD8−. The HLA type of the donor (NG) was HLA-A2,3, B13,27, HLA-DRβ1*0101, 0701, HLA-DQβ1*02,0501.
Proliferative responses of the NG-1 line to the bcr-abl peptides. 5 × 104 NG-1 cells were incubated with 1 × 105 autologous mitomycin C–treated PBMC and indicated concentrations of b2a2/b3a2 peptide mix (▪), b3a2 peptide (▿), or b2a2 peptide (▴). Results are mean ± SEM of triplicate wells.
Proliferative responses of the NG-1 line to the bcr-abl peptides. 5 × 104 NG-1 cells were incubated with 1 × 105 autologous mitomycin C–treated PBMC and indicated concentrations of b2a2/b3a2 peptide mix (▪), b3a2 peptide (▿), or b2a2 peptide (▴). Results are mean ± SEM of triplicate wells.
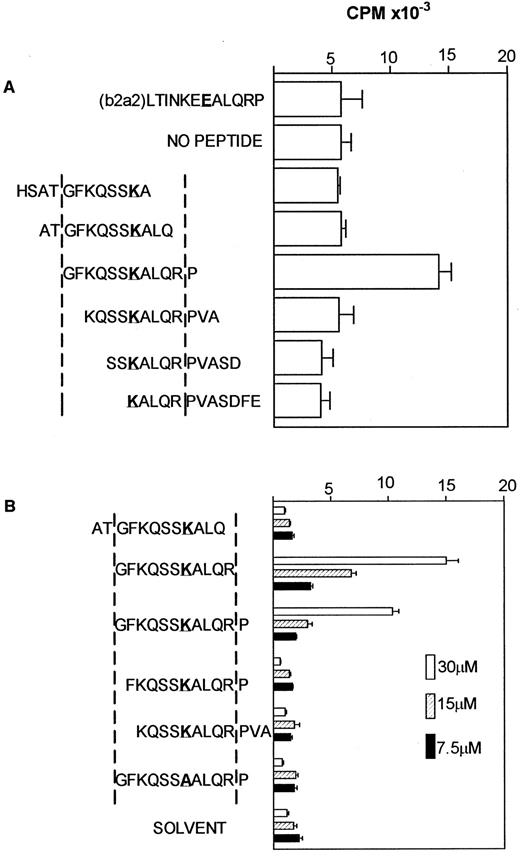
Definition of core peptide residues required for a response.To further characterize the specificity of the NG-1 line, a panel of peptides spanning the b3a2 fusion region was used to determine the minimum amino acid residues required for stimulation, using autologous PBMC as APC. Preliminary experiments with a panel of 12mer peptides spanning the bcr-abl fusion region (differing by two amino acids) showed that only the original b3a2 peptide used to generate the line stimulated a response (Fig 2A).
Definition of core peptide residues required for a response. (A) 5 × 104 NG-1 cells were cultured with 1.0 × 105 mitomycin C– treated autologous PBMC and indicated peptides at 5 μg/mL. Mean ± SEM of triplicate wells are shown. (K represents the amino acid generated by the fusion event). (B) 5 × 104 NG-1 cells were incubated with 1 × 105 autologous EBV-transformed B-lymphoblastoid cells (mitomycin C–treated) as APC and varying amounts of the truncated peptides as shown, or solvent as a negative control. Results are the mean ± SEM of triplicate wells.
Definition of core peptide residues required for a response. (A) 5 × 104 NG-1 cells were cultured with 1.0 × 105 mitomycin C– treated autologous PBMC and indicated peptides at 5 μg/mL. Mean ± SEM of triplicate wells are shown. (K represents the amino acid generated by the fusion event). (B) 5 × 104 NG-1 cells were incubated with 1 × 105 autologous EBV-transformed B-lymphoblastoid cells (mitomycin C–treated) as APC and varying amounts of the truncated peptides as shown, or solvent as a negative control. Results are the mean ± SEM of triplicate wells.
To define the minimum epitope further, b3a2 peptides with one deletion at each end were then tested. Only the original b3a2 (GFKQSSKALQRP) and a b3a2 peptide without the carboxyl terminal proline (GFKQSSKALQR) stimulated a proliferative response (Fig 2B). The peptide lacking the carboxyl terminal proline stimulated a greater proliferative response than the original 12mer b3a2 peptide in all three experiments defining this 11mer as the core sequence recognized. Substitution of the central lysine (K) with an alanine (A) (Fig 2B) ablated the stimulatory potential of the peptide, indicating that this amino acid generated by the recombination event is essential for recognition by NG-1.
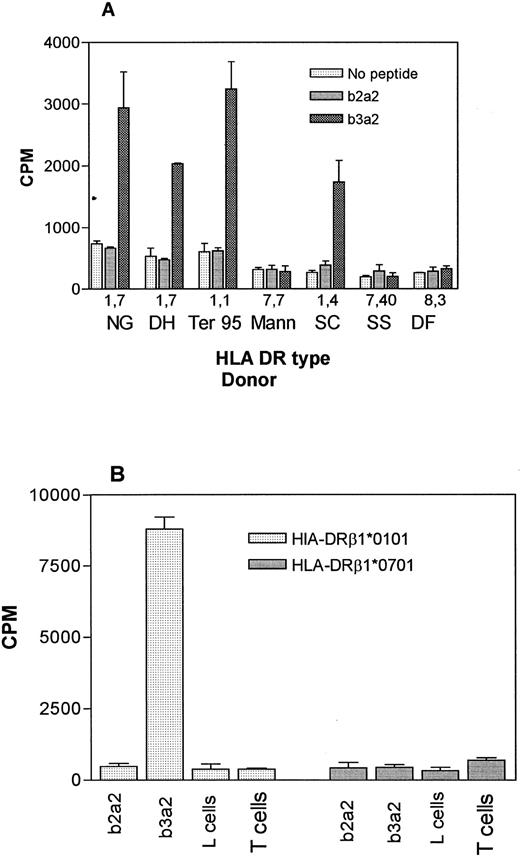
HLA restriction of the b3a2-specific response.Preliminary experiments showed that the response of NG-1 to autologous APC and b3a2 peptide was blocked by MoAb specific for HLA-DR but not MoAb to HLA-DP, HLA-DQ, or HLA-ABC (data not shown). To further define the HLA restriction, clones specific for the b3a2 peptide were generated by limiting dilution from the NG-1 line. One clone (NG-1, H11) was selected for further studies. Given the HLA type of donor, HLA-DR β1*0101 and/or HLA-DR β1*0701 might have been involved in the peptide presentation, so a panel of EBV-transformed B-lymphoblastoid cell lines of known HLA-DR type (Table 2) were used as APC to identify the restricting allele. Only cells that expressed HLA-DRβ1*010102 were capable of presenting b3a2 peptide to the clone NG-1, H11 (Fig 3A). No response was observed when a homozygous HLA-DRβ1*0701 line was used, thus excluding HLA-DRβ1*0701 as a restricting element. However, all of the HLA-DRβ1*0101 lines also expressed HLA-DQβ1*0501, so to confirm the HLA-DRβ1*0101-02 restriction, HLA-DRβ1*0101 and HLA-DRβ1*0701 transfected L cells were tested for their ability to present peptide. As shown in Fig 3B, the HLA-DRβ1*0101 transfected line was able to stimulate a b3a2 specific response, whereas no response was seen when the HLA-DRβ1*0701–transfected L cells were used, thus establishing the HLA-DRβ1*0101-02 restriction of the b3a2 response of NG-1. An HLA-DQβ1*0501–transfected cell line was not available and presentation by this allele cannot be excluded.
HLA Types of EBV-Transformed B-Lymphoblastoid Cell Lines Used as APC
| Name . | HLA-A . | HLA-B . | HLA-DRβ1* . | HLA-DQβ1* . |
|---|---|---|---|---|
| NG | 2, 30 | 13, 27 | 0101, 0701 | 02, 0501 |
| DH | 2, 3 | 7, 21 (50) | 0101, 0701 | 02, 0501 |
| Ter 95 | ND | ND | 0102, − | 0501, − |
| Mann | ND | ND | 0701, − | ND |
| SC | 2, − | 5, 12 | 0101, 04 | 0301, 0501 |
| SS | 2, − | 7, 40 | 02, 1302 | 0604/9, X |
| DF | 2, 3 | 7, 62 | 0301, 0801-04 | ND |
| Name . | HLA-A . | HLA-B . | HLA-DRβ1* . | HLA-DQβ1* . |
|---|---|---|---|---|
| NG | 2, 30 | 13, 27 | 0101, 0701 | 02, 0501 |
| DH | 2, 3 | 7, 21 (50) | 0101, 0701 | 02, 0501 |
| Ter 95 | ND | ND | 0102, − | 0501, − |
| Mann | ND | ND | 0701, − | ND |
| SC | 2, − | 5, 12 | 0101, 04 | 0301, 0501 |
| SS | 2, − | 7, 40 | 02, 1302 | 0604/9, X |
| DF | 2, 3 | 7, 62 | 0301, 0801-04 | ND |
HLA restriction of the b3a2 specific response. (A) Clone NG-1,Hll (4.0 × 104/well) T lymphocytes were incubated with 1 × 105 mitomycin C–treated EBV-transformed B-lymphoblastoid cells from individuals of known HLA-DR types (see Table 2) and b2a2 or b3a2 peptides at 20 μg/mL or equivalent volume of solvent. Results are the mean ± SEM of triplicate wells. (B) DRβ1*0101 transfected L cells present b3a2 peptide NG-1 cells (5.0 × .04/well) were incubated with 5 × 104 mitomycin C–treated L cells transfected with either HLA-DRβ1*0101 or HLA-DRβ1*0701 and 20 μg/mL of b2a2 or b3a2 peptide. L cells alone and NG-1 (T cells) alone were also included as negative controls. Mean ± SEM of triplicate wells are shown.
HLA restriction of the b3a2 specific response. (A) Clone NG-1,Hll (4.0 × 104/well) T lymphocytes were incubated with 1 × 105 mitomycin C–treated EBV-transformed B-lymphoblastoid cells from individuals of known HLA-DR types (see Table 2) and b2a2 or b3a2 peptides at 20 μg/mL or equivalent volume of solvent. Results are the mean ± SEM of triplicate wells. (B) DRβ1*0101 transfected L cells present b3a2 peptide NG-1 cells (5.0 × .04/well) were incubated with 5 × 104 mitomycin C–treated L cells transfected with either HLA-DRβ1*0101 or HLA-DRβ1*0701 and 20 μg/mL of b2a2 or b3a2 peptide. L cells alone and NG-1 (T cells) alone were also included as negative controls. Mean ± SEM of triplicate wells are shown.
The generality of the response in some other HLA-DR1 individuals was tested in a further four experiments using the split-well assay (Table 3). After initial priming and four stimulations, the donor NG again produced the highest number of specific wells (35 of 93), but specific responses were also noted in three other HLA-DR1 individuals.
Generation of bcr-abl Peptide-Specific T-Cell Lines From Normal DR1 Donors
| Donor . | Cells With SI* >2.0 . | SI Range . |
|---|---|---|
| 1 | 35/93 | 2.01-14.05 |
| 2 | 3/96 | 2.05-16.25 |
| 3 | 20/198 | 2.0-4.1 |
| 4 | 15/96 | 3.9-15.7 |
| Donor . | Cells With SI* >2.0 . | SI Range . |
|---|---|---|
| 1 | 35/93 | 2.01-14.05 |
| 2 | 3/96 | 2.05-16.25 |
| 3 | 20/198 | 2.0-4.1 |
| 4 | 15/96 | 3.9-15.7 |
Stimulation index.
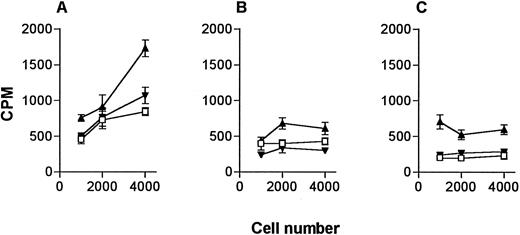
Ability of DC to present bcr-abl peptide.DC are likely to be the optimal cells for peptide vaccination given their particular ability to migrate to lymph nodes from subcutaneous sites.17 Therefore, we tested the ability of low numbers of purified peripheral blood DC to present peptide to the NG-1 line compared with equal cell numbers of other APC populations. As numbers of DC available for immunotherapy are likely to be limited, we compared the different APC at low cell numbers. DC (Fig 4A) and monocytes (Fig 4B) enriched and sorted from PBMC from an HLA-DR1+ donor were tested for their capacity to stimulate the proliferative response to the b3a2 peptide. These were compared to equal numbers of mitomycin C–treated HLA-DRβ1*0101–transfected L cells (Fig 4C). Some experiments included EBV-transformed B-lymphoblastoid cell lines; however, significant cross-reactivity was observed. These data show that DC were very effective in presenting this b3a2 peptide at low cell numbers.
Comparison of DC and monocytes for presentation of peptide to the NG-1 line. NG-1 cells (4.0 × 104) were incubated with indicated numbers of (A) sorted DC, (B) sorted monocytes, (C) HLA-DRβ1*0101 transfected L cells and 20 μg/mL of b3a2 (▴) or b2a2 (▾) peptide or equivalent volume of solvent (□). Results represent the mean of triplicate wells ± SEM.
Comparison of DC and monocytes for presentation of peptide to the NG-1 line. NG-1 cells (4.0 × 104) were incubated with indicated numbers of (A) sorted DC, (B) sorted monocytes, (C) HLA-DRβ1*0101 transfected L cells and 20 μg/mL of b3a2 (▴) or b2a2 (▾) peptide or equivalent volume of solvent (□). Results represent the mean of triplicate wells ± SEM.
Proliferative response of NG-1 to bcr-abl–containing lysates.The ability of the b3a2 peptide specific NG-1 line to proliferate in response to autologous or HLA-DR1 matched APC exposed to CML cell lysates which contained the fusion protein was investigated. PBMC from four CML patients (who had been typed for b2a2 or b3a2 translocation) were used to prepare lysates. These lysates were tested for mitogenicity and/or toxicity by assaying proliferation with HLA-DR mismatched mitomycin C–treated APC. Two samples (with high granulocyte counts) were toxic to NG-1 and were excluded; however, two samples (one b2a2 and one b3a2) which showed no evidence of mitogenic activity or toxicity were tested for their capacity to stimulate NG-1. Preliminary results showed a significant proliferative response to the lysate from the b3a2 patient but not the b2a2 patient (Student's t-test P < .001) (data not shown). Proliferation was also detected in response to the high-molecular-weight (>100 kD) fraction of a lysate prepared from the CML-derived cell line K562, which harbors the b3a2 translocation. This lysate did not stimulate NG1-H11, a b3a2 specific T-lymphocyte clone, to proliferate with HLA-DR mismatched APC (data not shown), thus demonstrating that this lysate is not mitogenic. Unfortunately, the original b3a2 peptide specific line could not be maintained for a sufficient length of time to confirm these results.
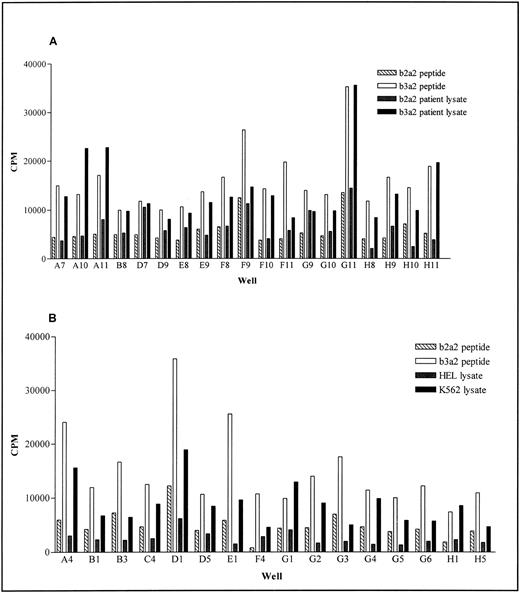
To verify these observations we generated additional b3a2 peptide specific lines from NG and tested their response to cell lysates from either K562 or CML patient PBMC using the split-well assay. When using cell lysates from CML patients, samples derived from patients with the b2a2 translocation make excellent negative controls. However, an appropriate negative control for K562 cell lysates was more difficult and we used the myeloid cell line HEL, as best equating with the K562 stage of differentiation. The latter had a minimal inhibitory effect in our proliferation assay but specific responses to K562 were likely, because in most cases the K562-induced proliferation is higher than that induced by b2a2 peptide pulsed PBMC. Of the 35 b3a2 peptide specific lines from the original donor (NG), 10 of 19 showed a specific response (SI > 2.0) to b3a2 patient cell lysate (Fig 5A) while 15 of 16 showed a specific response to K562 (Fig 5B).
Response of bcr-abl peptide specific lines to b3a2 containing cell lysates. PBMC from donor NG were stimulated four times with b3a2 peptide pulsed autologous mitomycin C–treated PBMC. Peptide specificity and response to cell lysate was determined after the fourth stimulation, using the split-well method. Results show the proliferation of peptide-specific wells, when stimulated with mitomycin C–treated PBMC pulsed with (A) b3a2 and b2a2 patient lysates and (B) K562 cell lysate or HEL cell lysate. In each case control b3a2 peptide and b2a2 peptide responses are shown.
Response of bcr-abl peptide specific lines to b3a2 containing cell lysates. PBMC from donor NG were stimulated four times with b3a2 peptide pulsed autologous mitomycin C–treated PBMC. Peptide specificity and response to cell lysate was determined after the fourth stimulation, using the split-well method. Results show the proliferation of peptide-specific wells, when stimulated with mitomycin C–treated PBMC pulsed with (A) b3a2 and b2a2 patient lysates and (B) K562 cell lysate or HEL cell lysate. In each case control b3a2 peptide and b2a2 peptide responses are shown.
DISCUSSION
We describe the characterization of a CD4+T-lymphocyte line that is specific for bcr-abl b3a2 peptide and restricted by HLA-DRβ1*0101-02. These cells proliferate in response to bcr-abl containing cell lysates, both from the erythroid leukemia line K562 and b3a2 but not b2a2 CML cell lysates. This critical result, demonstrated in humans for the first time, makes the point that at least some peptide-specific lines have the potential to recognize leukemic targets and is particularly relevant as recent evidence shows some free peptide/MHC complexes may be antigenically dissimilar to those resulting from intracellular processing of intact antigen.25 Specific responses to b3a2 peptide were also detected in other HLA-DR1 individuals. Furthermore, we have established that DC as the optimal APC also present the b3a2 peptide effectively to responding T lymphocytes.
There is mounting evidence that bcr-abl peptide specific T-lymphocyte lines are able to respond to peptides derived from whole protein, either in the form of cell lysate or as intact leukemic cells, suggesting that CML cells may be a target for a b3a2 immune response. Murine CD4+ T cells, specific for bcr-abl, b3a2 peptide have been shown to proliferate to the whole fusion protein, purified from cell extracts.9 Recently ten Bosch et al13 reported that a CD4+ T-lymphocyte line specific for bcr-abl b3a2 peptide proliferated in response to leukemic blasts obtained from a patient in CML blast crisis. These leukemic cells contained additional translocations and more differentiated forms of the same leukemia stimulated lesser proliferation. It is probable that these cells express additional costimulator molecules. Another recent report14 showed that bcr-abl specific CD4+ T-cell lines did not recognize the antigen presented by chronic-phase CML PBMC despite CD80 and HLA-DR expression. These investigators attributed this to insufficient expression of specific peptide/HLA-DR complexes on the CML cells. Likewise, work with pml/RAR-α peptides26 emphasizes the difficulties peptide-specific T-lymphocyte clones may have in recognizing leukemic cells expressing low levels of HLA class II and antigenic peptide. Our results give greater confidence that CD4+ proliferative responses are achievable to the CML specific b3a2 peptide and will, with perseverance, allow wider investigation of the ability of appropriately HLA-DR typed CML cells to stimulate specific CD4+ responses.
The capacity of a panel of bcr-abl peptides to bind HLA A and B molecules has been measured using in vitro HLA binding assays.10 No b2a2 peptides were able to bind with high affinities, although four b3a2 peptides were able to bind HLA A3, A11,B8 with high (<50 nmol/L) to moderate (≤500 nmol/L) affinities.10,11 These results suggest that a CD4+ response may be accompanied by a CD8+ T-lymphocyte response against b3a2 peptides in appropriate individuals. There have now been two reports of a human bcr-abl peptide specific cytotoxic T-lymphocyte responses11 27 using EBV-derived lymphoblastoid cells as targets but bcr-abl specific cytotoxic activity against CML cells have not been reported as yet.
Our experiments and the results discussed above confirm that it is possible to find, at least in some individuals, both CD4+ and CD8+ T-lymphocyte precursors with TCR rearrangements specific for the b3a2 fusion peptide in the context of autologous MHC molecules. One of our donors, NG, showed a greater capacity to respond to b3a2 peptide than other HLA-DR1 donors, although we did detect b3a2 peptide specific responses in other individuals. It is an intriguing possibility that this donor has another genetic characteristic, perhaps a polymorphism in their antigen processing pathway that results in better processing/presentation of the b3a2 epitope. Equally one might speculate that this individual has an unusually high responsive T-lymphocyte precursor frequency, perhaps reflecting a novel TCR repertoire.
Analysis of the TCR genes used in these receptors will be of considerable interest. There is now a growing list of HLA alleles that have been shown to present bcr-abl peptides to in vitro–generated T-lymphocyte lines. These include HLA-DR1 (this report), -4,13,-3, -11,14 -15,12 and the MHC class I allelles HLA-A3,11 -A11, and B8.10 Therefore, it is possible to contemplate a specific TCR as a recognition structure for bcr-abl peptides in most patients. Some re-engineering of T-lymphocyte responses in vivo or in vitro may be required to optimize this. It is noteworthy that CD4+ cytotoxic responses have been demonstrated using peptide loaded targets.26 Thus, it may be possible to engineer T-lymphocyte clones that are effective inhibitors of CML precursor growth, perhaps by monitoring their effects on in vitro colony growth assays. Simpler interventions such as the use of interferon to maximize HLA antigen/bcr-abl peptide expression on CML cells will also merit investigation.
Peripheral blood DC are well known as potent APC. Of course, it is possible, as DC in CML express the bcr-abl fusion transcript (manuscript in preparation), that DC collected during a leukemic phase could be used as the optimal APC source for generating both CD4+ and CD8+ T-lymphocyte responses. It is notable that DC were the most potent APC type for presenting the b3a2 peptide to the bcr-abl specific T-lymphocyte line in a secondary response. We are currently investigating the capacity of DC to stimulate a primary response to the optimal bcr-abl b3a2 peptide in association with DR1. Further refinement of DC peptide loading may also be possible, perhaps by using antibodies to the relevant protein or peptide. However, DC may also in certain circumstances generate tolerogenic responses, and further investigation of whether b3a2 T-lymphocyte responses are possible in appropriately typed CML patients is planned. Intriguing data showing abnormal TCR Vβ repertoires in CML patients28 suggest that this may be a possibility.
Taken together, our results encourage further investigation of the possible clinical application of bcr-abl peptide presented by autologous DC. We have shown that a peptide-specific line is able to respond to the whole protein in crude protein extract from CML cells. It seems reasonable to predict that the same response would result in vivo. Thus, if DC can be used to induce a strong antipeptide response (assuming tumor tolerance is not an issue), the T lymphocytes are likely to respond to the processed bcr-abl protein expressed as peptide-HLA complexes on CML cells. Given the apparently successful vaccination with DC and Ig idiotype in humans,19 29 there will be enthusiasm to vaccinate patients with autologous DC combined with bcr-abl proteins/peptides. This may be helpful, but further optimization of the in vitro vaccination process may ultimately provide better therapeutic agents, perhaps for infusion post autologous BMT.
ACKNOWLEDGMENT
We thank our blood donors for their generosity, Dr Frances Brodsky for providing the antibody to HLA-DP, Dr Brian Tait for the HLA-DR transfected L cell lines, and Sue Banks for helping prepare the manuscript.
Supported by the Cancer Society of New Zealand.
Address reprint requests to D.N.J. Hart, MD, Haematology/Immunology Research Group, Canterbury Health Laboratories, PO Box 151, Christchurch, 1, New Zealand.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal