Abstract
The mitogen-dependent induction of cyclin D–dependent kinase activity is required for cells to enter the DNA synthetic (S) phase of their division cycle. Immature 32Dcl3 myeloid cells (32D) proliferating in the presence of interleukin-3 (IL-3) normally express cyclins D2 and D3, which assemble into binary holoenzyme complexes with their catalytic subunits, CDK4 and CDK6. When 32D cells are switched to medium containing granulocyte colony-stimulating factor (G-CSF ) instead of IL-3, D-type cyclins are degraded and, in the absence of their associated kinase activity, the cells arrest in the first gap phase (G1 ) of the cell cycle and differentiate to neutrophils. We derived 32D cells in which the expression of p19INK4d, a specific polypeptide inhibitor of CDK4 and CDK6, is regulated by the heavy metal-inducible sheep metallothionein promoter. Induction of p19INK4d in response to zinc prolonged cell survival in the absence of growth factor treatment. When maintained in medium containing both IL-3 and zinc, these cells lost cyclin D–dependent kinase activity, underwent G1 phase arrest, and acquired certain morphologic, antigenic, and functional properties of mononuclear phagocytes. Cells induced to express p19INK4d did not synthesize receptors for macrophage colony-stimulating factor (M-CSF/CSF-1) and reverted to an immature myeloid phenotype when shifted back into medium containing IL-3 alone. These cells exhibited accelerated differentiation to neutrophils in response to G-CSF but also gave rise to macrophage-like cells when maintained in medium containing both G-CSF and zinc. Therefore, the acquisition of macrophage properties in response to zinc treatment neither depended upon IL-3 nor upon G1 phase arrest per se and instead reflects some ability of p19INK4d, and presumably cyclin D–dependent kinases, to affect myeloid differentiation.
MAINTENANCE OF CELLS in a proliferative state requires the activities of cyclin-dependent kinases (CDKs) that regulate progression through distinct cell cycle transitions. Best characterized is the oscillatory activation of the holoenzyme composed of cyclin B (the regulatory subunit) and CDK1 (the catalytic subunit, also known as CDC2 in the yeast Schizosaccharomyces pombe and CDC28 in Saccharomyces cerevisiae ), whose abrupt activation and subsequent degradation are universally required for mitotic entry and exit, respectively, in all eukaryotes.1 In mammalian cells, progression through the first gap phase (G1 ) into the DNA synthetic (S) phase requires the activity of at least two distinct classes of G1 cyclins (types D and E) and their associated catalytic partners. As quiescent cells enter the cycle, different D-type cyclins (D1, D2, and D3) are induced by various mitogens in a lineage-dependent manner and assemble under growth factor control with their catalytic subunits, CDK4 and CDK6.2 The activity of cyclin D–dependent kinases increases throughout G1 phase as long as mitogen stimulation continues, and in mid to late G1 , these enzymes catalyze the phosphorylation of the retinoblastoma protein (Rb), thereby helping to reverse its growth suppressive function.3,4 One consequence of Rb phosphorylation is the activation of a class of transcription factors (the E2Fs) that coordinately regulate genes whose expression is necessary for DNA synthesis.5,6 Among these is cyclin E, which, when complexed to CDK2, completes the inactivation of Rb and also triggers the onset of S phase by phosphorylating additional substrates.7-9 The timing of Rb phosphorylation in late G1 phase occurs as cells pass a restriction point where they no longer require mitogens for subsequent progression through the cell cycle and, conversely, can no longer be inhibited by certain antiproliferative cytokines.10 11 The irreversibility of the restriction point transition is in part caused by the shift in the control of Rb phosphorylation from a mitogen (D-type)-dependent kinase to a mitogen-independent (E-type) one.
Inhibition of cyclin D–dependent kinases prevents cells from passing the restriction point, so although cells can progress through S, G2 , and M phases without the activity of these enzymes, they accumulate in G1 phase and may then exit the cycle into a quiescent (G0 ) state.12-14 The simplest example of this process occurs upon mitogen withdrawal.15 Because the D-type cyclins are intrinsically unstable proteins, cells deprived of growth factors quickly lose cyclin D–dependent kinase activity and arrest in G1 phase, usually within a single cycle. But even in the face of persistent mitogen stimulation, G1 arrest can be induced by specific polypeptide inhibitors of CDK4 and CDK6, the so-called INK4 proteins of which four have now been identified.16-20 Importantly, cells that lack Rb function, either as a result of Rb mutation or loss, are refractory to the effects of INK4 overexpression,18,21-24 thereby underscoring the existence of a biochemical pathway in which INK4 proteins act “upstream” of cyclin D–dependent kinases to negatively regulate Rb phosphorylation. In contradistinction to the effects of mitogens on the D-type cyclins, we might well imagine that INK4 proteins would be induced by antiproliferative signals, and this is the case for p15INK4b, which is synthesized in response to stimulation by transforming growth factor-β.17 25
Although the activity of D-type CDKs is nonessential for G1 progression in cells lacking Rb, this does not preclude the possibility that these enzymes phosphorylate other substrates. For example, the activation of D-type CDKs as cells enter the cycle may be important in canceling the activity of inhibitors that hold cells in a quiescent state. Alternatively, cyclin D–dependent kinases could act during G1 phase to interfere with differentiation-specific programs that might otherwise be executed in noncycling cells. In agreement with this concept, it was previously shown that the enforced overexpression of cyclins D2 or D3 in immature IL-3–dependent 32Dcl3 myeloid cells shortened their G1 phase26 and prevented their ability to differentiate to neutrophils in response to G-CSF.27
To further explore the possibility that D-type CDKs might affect differentiation-specific or apoptotic programs, we have now studied the effects of inducible INK4 protein expression on interleukin-3 (IL-3)-dependent proliferation and granulocyte colony-stimulating factor (G-CSF )-induced differentiation in 32Dcl3 cells. These cells, and others of the myeloid lineage, normally express two INK4 family members, p18INK4c and p19INK4d.18,19 Their synthesis oscillates throughout the cell cycle with the highest levels observed in the S and G2 phases and the lowest in G1 phase, the latter being the only interval where elevated INK4 protein expression can inhibit cell proliferation.19 We now show that enforced overexpression of p19INK4d and concomitant inhibition of cyclin D–dependent kinase activity in 32D cells not only blocks G1 phase progression but also prolongs cell survival in the absence of supportive growth factors and, surprisingly, induces certain features of macrophage differentiation in the presence of IL-3. The p19 overexpressors exhibited accelerated differentiation to neutrophils in response to G-CSF, but even under these conditions, some cells acquired macrophage-like properties. Therefore, apart from their role in facilitating G1 progression, the cyclin D–dependent kinases may negatively influence the execution of differentiation programs.
MATERIALS AND METHODS
Cell lines and culture conditions.IL-3–dependent 32Dcl3 cells (32D cells)28 29 were provided by Dr J.N. Ihle (St Jude Children's Research Hospital, Memphis, TN) and were maintained in RPMI 1640 medium (Mediatech, Inc, Herndon, VA) supplemented with murine IL-3 (25 U/mL), and 10% fetal bovine serum (FBS; BioWhittaker, Walkersville, MD). Cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C. Growth rates of parental 32D cells and their derivatives (discussed later) were determined by seeding cells at 1 × 105 cells per mL in 5 mL of complete media in 60-mm diameter tissue culture dishes and enumerating them daily after suspension in medium containing trypan blue dye. Unless otherwise stated in the figure legends, p19 expression was induced by adding 75 μmol/L ZnCl2 to the culture medium. The time courses for zinc induction and G-CSF treatment are indicated in the figures and their legends.
DNA constructs and electroporation.A Not I-BamHI fragment containing mouse cDNA encoding p19INK4d 19 was inserted unidirectionally into Not I-BamHI cloning sites downstream of the sheep metallothionein promoter in the eukaryotic expression vector, pMT-CB6+ (a gift from F. Rauscher, Wistar Institute, Philadelphia, PA) as used by others.30 The resulting pMT-CB6+p19 vector is bicistronic and contains a neomycin resistance gene (neo ) regulated independently by the Simian Virus 40 promoter. Circular vector plasmid DNA purified through cesium chloride gradients was linearized by restriction with Pvu I, and 50 μg was electroporated27 31 into 5 × 106 32D cells using a gene pulsar apparatus (Bio-Rad, Richmond, CA) at 960 μF and 290 V. Electroporated cells were cultured for 24 hours in complete medium and then selected for 10 days in medium containing 750 μg/mL G418 (GIBCO-BRL, Gaithersburg, MD). Clonal transformants were selected from single cells by limiting dilution, and six p19-inducible clones were chosen for further study. Control 32D cell lines were generated in parallel by introducing the naked pMT-CB6+ vector and selecting the cells in G418.
Protein analyses.Direct immunoblotting was performed using total cell lysates from 3 × 105 cells per gel lane. After disruption of the cells in cell lysis buffer (50 mmol/L Tris HCl, pH 8.0, 120 mmol/L NaCl, 0.5% NP-40), boiled lysates were resolved by electrophoresis on denaturing polyacrylamide gels containing sodium dodecyl sulfate (SDS) and transferred to nitrocellulose. Immunoblotting was performed32 using a goat anti-mouse p19 polyclonal antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) followed by incubation with a secondary donkey antibody directed to goat immunoglobulin G (IgG) conjugated to horseradish peroxidase (Santa Cruz Biotechnology Inc). Proteins were detected by enhanced chemiluminescence (ECL, Amersham, Arlington Heights, IL) according to the manufacturer's instructions. The levels of D cyclins and CDK proteins in total cell lysates from equivalent numbers of 32D cells were estimated by comparing the intensity of blot signals obtained using antibodies with the individual components versus those generated with defined amounts of the recombinant proteins run in adjacent lanes of the same gel. Recombinant cyclin D2, cyclin D3, CDK4, and CDK6 were prepared using a baculovirus expression system as described33 and were detected as above using monoclonal antibodies to the cyclins34,35 or antisera to the CDK4 (serum RZ ) and CDK6 (serum RJJ ) carboxyltermini.35 36
In experiments designed to measure p19-CDK complexes formed in vivo, p19INK4d was recovered by immunoprecipitation after detergent lysis of 5 × 106 cells in buffer containing protease inhibitors.37 Washed immune precipitates generated with polyclonal rabbit antisera to p19 and recovered on protein A-Sepharose19 were separated by electrophoresis on 15% denaturing polyacrylamide gels, transferred to nitrocellulose, and immunoblotted as above using the same antisera to p19, or antisera directed to the CDK4 or CDK6 carboxyltermini. In analogous experiments designed to detect complexes containing cyclin D3, washed precipitates generated with monoclonal antibody to mouse cyclin D334 were separated on gels and blotted with the same antibody or with antisera directed to p19 or CDK4. Recombinant proteins produced under baculoviral vector control in insect Sf9 cells were used as positive controls as indicated in the legends of Figs 1-3.
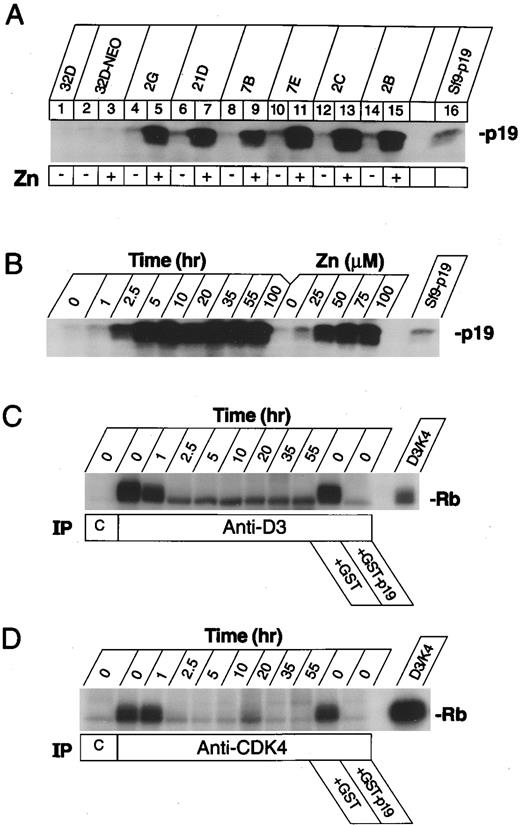
Induction of p19INK4d and inhibition of cyclin D–dependent Rb kinase activity in zinc-induced 32D clones. (A) Immunoblot analysis of parental 32D cells (lane 1), a polyclonal population of control cells receiving the empty pMT-CB6+ vector (lanes 2 and 3), and six single cell-derived subclones electroporated with the pMT-CB6+p19 vector (lanes 4 to 15) before (even numbered lanes) or after (odd numbered lanes) treatment with 75 μmol/L zinc for 4 hours. An Sf9 cell lysate containing recombinant p19 was used as an internal control to mark the mobility of the protein in the gel (lane 16). (B) Time course after zinc-addition, and dose-dependence of p19 expression. A p19-inducible clone was grown in IL-3 and 75 μmol/L zinc for the indicated times in hours, or treated with the indicated concentrations (μmol/L) of zinc for 5 hours. Sf9 cell lysates containing p19 were used to mark the position of p19 in the gel (right lane, Sf9-p19). (C) Induced p19 inhibits cyclin D3–dependent kinase activity. Cell lysates were prepared from clone 2C at the indicated times in hours after zinc-addition and were precipitated with an irrelevant control monoclonal antibody (lane C) or with a monoclonal antibody to mouse cyclin D3 (D3-19D5-13). Washed immune complexes were assayed for kinase activity in vitro using bacterial GST-Rb fusion protein as substrate. Lysates from untreated cells (time = 0) were treated as indicated for 5 minutes at room temperature with 1 μg recombinant GST-p19 or GST alone to verify that p19 inhibits the Rb kinase activity precipitated from these cells. Recombinant cyclin D3-CDK4 complexes were prepared in Sf9 cells and used as a positive control for enzyme activity (right lane). (D) The same lysates as in (C) were immunoprecipitated with nonimmune rabbit serum (lane C) or with rabbit antiserum (RZ ) directed to the CDK4 C-terminus, and washed precipitates were assayed for Rb kinase activity as in (C).
Induction of p19INK4d and inhibition of cyclin D–dependent Rb kinase activity in zinc-induced 32D clones. (A) Immunoblot analysis of parental 32D cells (lane 1), a polyclonal population of control cells receiving the empty pMT-CB6+ vector (lanes 2 and 3), and six single cell-derived subclones electroporated with the pMT-CB6+p19 vector (lanes 4 to 15) before (even numbered lanes) or after (odd numbered lanes) treatment with 75 μmol/L zinc for 4 hours. An Sf9 cell lysate containing recombinant p19 was used as an internal control to mark the mobility of the protein in the gel (lane 16). (B) Time course after zinc-addition, and dose-dependence of p19 expression. A p19-inducible clone was grown in IL-3 and 75 μmol/L zinc for the indicated times in hours, or treated with the indicated concentrations (μmol/L) of zinc for 5 hours. Sf9 cell lysates containing p19 were used to mark the position of p19 in the gel (right lane, Sf9-p19). (C) Induced p19 inhibits cyclin D3–dependent kinase activity. Cell lysates were prepared from clone 2C at the indicated times in hours after zinc-addition and were precipitated with an irrelevant control monoclonal antibody (lane C) or with a monoclonal antibody to mouse cyclin D3 (D3-19D5-13). Washed immune complexes were assayed for kinase activity in vitro using bacterial GST-Rb fusion protein as substrate. Lysates from untreated cells (time = 0) were treated as indicated for 5 minutes at room temperature with 1 μg recombinant GST-p19 or GST alone to verify that p19 inhibits the Rb kinase activity precipitated from these cells. Recombinant cyclin D3-CDK4 complexes were prepared in Sf9 cells and used as a positive control for enzyme activity (right lane). (D) The same lysates as in (C) were immunoprecipitated with nonimmune rabbit serum (lane C) or with rabbit antiserum (RZ ) directed to the CDK4 C-terminus, and washed precipitates were assayed for Rb kinase activity as in (C).
Cyclin D–dependent protein kinase activity in immune complexes was measured as described35 after precipitation of lysates from 2 × 107 cells with rat monoclonal antibodies to cyclins D2 or D334 or with rabbit antisera to the CDK4 or CDK6 carboxyltermini.35,36 Bacterially produced GST-Rb substrate was prepared as described33 38 and, after phosphorylation in vitro, was resolved by electrophoresis on denaturing 7.5% polyacrylamide gels which were dried and subjected to autoradiography.
To detect receptors for colony-stimulating factor-1 (CSF-1R), 32D cells and control mouse BAC1.2F5 macrophages39 were metabolically labeled for 1.5 hours with 200 μCi/mL L-[35S]methionine (specific activity, 1,000 Ci/mmol; ICN, Irvine, CA) in methionine-free medium containing dialyzed FBS. 32D clones were labeled in the presence of IL-3. Lysates were precipitated with antisera to the recombinant feline v-fms40 41 or human c-fms31 42 gene products, both of which crossreact strongly with murine CSF-1R. Precipitated proteins were resolved on denaturing polyacrylamide gels and detected by autoradiography as described.43
Coupled reverse transcription and polymerase chain reaction (RT-PCR) assay.Polyadenylated RNA was isolated using a mRNA isolation kit from Pharmacia (Piscataway, NJ), and cDNA was synthesized from 1 μg of mRNA templates according to the manufacturer's instructions (Strata-Script RT-PCR kit; Stratagene, La Jolla, CA). After amplification with primers specific for mouse CSF-1R44 (sense primer, 5′-CCAGAACTGGTTGTAGAGCC-3′; antisense, 5′-CAGCTTGCTAGGCTCC-AATT-3′) with 35 cycles of denaturation (94°C, 1 minute), annealing (55°C, 2 minutes) and extension (72°C, 3 minutes), products were electrophoresed on 1.5% agarose gels and visualized by ethidium bromide staining.
Cell morphology and immunohistochemistry.Cells collected with a Cytospin-3 cytocentrifuge (Shandon, Pittsburgh, PA) were processed by May-Grünwald-Giemsa staining (Sigma Chemicals, St Louis, MO) or for immunocytochemistry and enzyme cytochemistry. Cytocentrifuge preparations were air-dried and fixed for 10 minutes at 4°C, washed with phosphate-buffered saline (PBS), and incubated for 50 minutes at room temperature with antibodies diluted in PBS and containing 1% bovine serum albumin (PBS+). Cells were reacted either with monoclonal antibodies directed to murine CSF-1R (Upstate Biotechnology Inc, Lake Placid NY) or to Mac-3 (clone M3/84.6.34; American Type Culture Collection, Rockville, MD). After washing twice in PBS+, the preparations were incubated for 45 minutes with a mouse anti-rat horse radish peroxidase-conjugated immunoglobulin (Jackson ImmunoResearch Laboratories Inc, West Grove, PA) diluted in PBS+ with 10% vol/vol normal mouse serum. Peroxidase activity was shown with 3,3′-diaminobenzidine tetrahydrochloride as substrate (Sigma). Isotype-matched control immunoglobulins (PharMingen, San Diego, CA) were used in place of anti–CSF-1R or anti–Mac-3 as negative controls. Acid phosphatase activity was shown45 with naphthol AS-BI phosphate (Sigma) as substrate and hexazotized pararosaniline as diazonium salt (90 minutes at 37°C).
Fc-receptors and phagocytosis.Fc-receptor (FcR)-positive cells were detected by rosette formation with IgG-coated sheep red blood cells (SRBC).46 A 1.6% vol/vol suspension of washed SRBC was made in PBS+, and mouse anti-SRBC IgG was added at a subhemagglutinating concentration. After a 30-minute incubation at 37°C, the SRBC were washed twice in PBS+ and resuspended in PBS+ to a 1.6% vol/vol suspension (SRBC solution). 32D cells (2 × 105 cells) were incubated for 1 hour at 4°C with 200 μL SRBC solution, and the percentage of cells binding three or more erythrocytes per cell was determined by examination of the suspension under a light microscope. Immunophagocytosis was assayed by incubating 2 × 105 cells in 200 μL SRBC solution for 1 hour at 37°C. After osmotic lysis of extracellular SRBC using 0.83 mol/L NH4Cl2 solution,46 32D cells were cytocentrifuged and processed for May-Grünwald-Giemsa staining. The percentage of phagocytosing cells was microscopically determined. In both assays, uncoated SRBC were used as negative controls.
Flow cytometric analyses.Cell suspensions screened for expression of specific cell surface antigens were incubated for 20 minutes at 4°C with PBS+ containing 10% vol/vol normal mouse serum, followed by a 30-minute incubation with optimal concentrations of the indicated monoclonal antibodies diluted in the same solution. Monoclonal antibodies used for staining included phycoerythrin (PE)-conjugated anti–Mac-1 (PharMingen), anti–Mac-3 (PharMingen), and anti–F4/80 antibody (Caltag Lab, San Francisco, CA), and fluorescein isothiocyanate (FITC)-conjugated anti-CD14 (PharMingen), together with isotype-matched control antibodies (PharMingen). Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) as described.47 Where indicated, the DNA content of cells was measured by flow-cytometric analysis of propidium iodide-stained nuclei, as previously described.15
RESULTS
Inducible overexpression of p19INK4d in 32Dcl3 myeloid cells.32Dcl3 cells were transfected with an expression vector encoding p19INK4d under the control of the sheep metallothionein promoter, and stable clones were generated that overexpressed the p19 protein in response to zinc-treatment. Immunoblotting (Fig 1A) revealed that asynchronously proliferating, parental 32D cells (lane 1) and neomycin-resistant derivatives transformed with the naked expression vector (lane 2) expressed low levels of endogenous p19 when propagated in medium containing IL-3 (see also Fig 3A, lanes 2 and 3). Because of “leakiness” of the expression vector, six representative clones (lanes 4 to 15) expressed somewhat higher levels of p19 when grown under noninducing conditions (even-numbered lanes). Despite this constitutive increase in basal p19 synthesis, these levels of the inhibitor were insufficient to block cells in G1 phase, as observed in previous studies with rodent fibroblasts.19 Addition of 75 μmol/L zinc to the media resulted in marked increases in p19 accumulation within 4 hours in each clone (odd-numbered lanes). Induction of p19 in response to heavy metals was dose-dependent and rapid, being maximal by 5 hours after treatment of cells with 75 μmol/L zinc, and high levels of the protein persisted as long as cells were maintained in medium containing IL-3 and zinc (Fig 1B). Despite braking effects on their proliferative rate, and changes in their differentiation status (discussed later), cells maintained in both IL-3 and zinc remained fully viable. Induction of p19 was reversible, and basal levels of p19 expression were again achieved within 3 to 4 days after removal of zinc from the medium (data not shown).
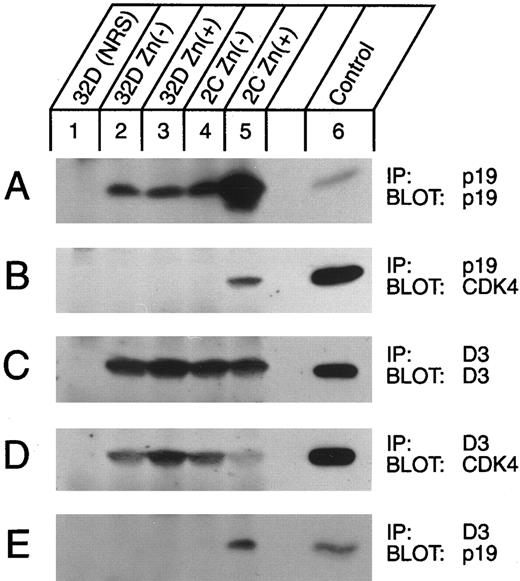
Detection of p19-CDK complexes in vivo. Lysates of parental 32D cells transfected with the naked vector (lanes 2 and 3) or of clone 2C cells (lanes 4 and 5) either untreated or treated for 5 hours with 75 μmol/L zinc (as indicated above the lanes) were immunoprecipitated with antisera to p19 or to cyclin D3 (designated by IP, right margin) and then blotted with antibodies to p19, cyclin D3, or CDK4 (as indicated by Blot, right margin). Lysates from control cells were precipitated with nonimmune serum (NRS, lanes 1). Recombinant proteins produced in Sf9 cells were loaded as positive controls (lane 6) for the immunoblots.
Detection of p19-CDK complexes in vivo. Lysates of parental 32D cells transfected with the naked vector (lanes 2 and 3) or of clone 2C cells (lanes 4 and 5) either untreated or treated for 5 hours with 75 μmol/L zinc (as indicated above the lanes) were immunoprecipitated with antisera to p19 or to cyclin D3 (designated by IP, right margin) and then blotted with antibodies to p19, cyclin D3, or CDK4 (as indicated by Blot, right margin). Lysates from control cells were precipitated with nonimmune serum (NRS, lanes 1). Recombinant proteins produced in Sf9 cells were loaded as positive controls (lane 6) for the immunoblots.
p19-mediated inhibition of cyclin D-dependent kinases.Like many immature myeloid cells, 32D cells express cyclins D2 and D3, but not D1, together with the two major cyclin D–dependent catalytic subunits, CDK4 and CDK6.26,27 The expression of the D-type cyclins and their associated kinases is highly IL-3–dependent. When 32D cells are switched to medium containing G-CSF, cyclin D2 is degraded within 24 hours and cyclin D3 by 72 hours after IL-3 withdrawal, resulting in the complete loss of cyclin D–dependent kinase activity and G1 phase arrest.27 The arrested cells then terminally differentiate to granulocytes over the next 7 to 10 days and ultimately die.29
Because combinatorial assembly generates four distinct cyclin D-CDK holoenzymes in cells grown in IL-3, we estimated the relative expression of cyclins D2 and D3, and CDK4 and CDK6 in parental 32D cells and in representative p19-transfected subclones maintained in IL-3, both in the presence or absence of zinc. Using specific, well-characterized antibodies directed to the individual components (see Materials and Methods), the relative concentration of each protein in lysates from equivalent numbers of cells was quantitated by comparing their immunoblot signals with those generated by known quantities of purified, recombinant proteins run side by side on the same gels (Fig 2). The calculated quantity (ng) of each cyclin (panels A and B) or CDK (panels C and D) is denoted beneath lanes 2 to 4 of each panel. This experimental approach negates any requirement for using antisera of similar titers or for matching the autoradiographic exposure times of different panels.
Quantitation of D-type cyclins and their CDK subunits in 32D cells. Lysates from equal numbers of parental 32D cells (lanes 1 and 2) or from clone 2C cells grown in the absence (lane 3) or presence (lane 4) of zinc were precipitated with nonimmune rabbit serum (lane 1, NRS) or with antisera to various cyclins or CDKs (lanes 2 to 4) as indicated at the right of each panel. Immune precipitates were electrophoretically separated on denaturing gels together with the indicated amounts (ng) of corresponding recombinant proteins and were then blotted with the same antiserum as used for immunoprecipitation. The quantities (in ng) of cyclin D2 (A), D3 (B), CDK4 (C), and CDK6 (D) present in the cell lysates were estimated by comparative densitometry and are indicated by the numbers below lanes 2 to 4 at the left of each panel. Note that with the antisera used, D2 was detected at greater sensitivity than D3, and CDK6 was somewhat more readily detected than CDK4; nonetheless, D3 and CDK4 were the predominant proteins in the lysates (see text).
Quantitation of D-type cyclins and their CDK subunits in 32D cells. Lysates from equal numbers of parental 32D cells (lanes 1 and 2) or from clone 2C cells grown in the absence (lane 3) or presence (lane 4) of zinc were precipitated with nonimmune rabbit serum (lane 1, NRS) or with antisera to various cyclins or CDKs (lanes 2 to 4) as indicated at the right of each panel. Immune precipitates were electrophoretically separated on denaturing gels together with the indicated amounts (ng) of corresponding recombinant proteins and were then blotted with the same antiserum as used for immunoprecipitation. The quantities (in ng) of cyclin D2 (A), D3 (B), CDK4 (C), and CDK6 (D) present in the cell lysates were estimated by comparative densitometry and are indicated by the numbers below lanes 2 to 4 at the left of each panel. Note that with the antisera used, D2 was detected at greater sensitivity than D3, and CDK6 was somewhat more readily detected than CDK4; nonetheless, D3 and CDK4 were the predominant proteins in the lysates (see text).
In proliferating parental 32D cells, cyclin D3 was expressed at 10- to 15-fold higher levels than cyclin D2 (compare Fig 2A and B), whereas the level of CDK6 was about half that of CDK4 (Figs 2C v D). Therefore, cyclin D3–dependent kinases should predominate in these cells. In addition, the levels of D-type cyclins and their associated CDKs were not greatly different (within a factor of 2) in parental versus p19-transfected cells, regardless of whether p19 was induced by zinc or not (for exemplary control data for D3 expression in zinc-treated parental cells, see Fig 3C). The steady-state concentrations of the CDKs were significantly greater than those of the D-cyclins (Fig 2C and D v A and B), consistent with data indicating that the D-cyclins are the rate-limiting components.13 48
Using a monoclonal antibody to mouse cyclin D3, pRb kinase activity was readily detected in immune complexes precipitated from cells grown in IL-3 (Fig 1C; anti-D3, time = 0), whereas no significant kinase activity was obtained using an irrelevant, isotype-matched control antibody (lane C). Following treatment of the cells with zinc for different times, cyclin D3–dependent kinase activity recovered from the lysates decreased rapidly and approached background levels by 2.5 hours of zinc addition (anti-D3, remaining time points to 55 hours), correlating with the rapid kinetics of p19 induction (Fig 1B). When a recombinant glutathione S-transferase (GST)-p19 fusion protein19 was added to lysates of untreated cells before immunoprecipitation, pRb kinase activity was directly inhibited (Fig 1C, +GST-p19), whereas GST itself was without effect (+GST). Similar data were obtained using antisera directed to carboxylterminal epitopes of CDK4 (Fig 1D), where in matched exposures, the CDK4-dependent kinase activity approached that of the cyclin D3–dependent kinase (Fig 1D v C). The remaining D3-associated Rb kinase activity was caused by CDK6 (data not shown), consistent with the relative abundance of the two cyclin D–dependent catalytic subunits (Fig 2C and D). In contrast, negligible pRb kinase activity was detected under these conditions using antibodies to cyclin D2 (data not shown), in agreement with data indicating that cyclin D3 was present in more than 10-fold excess in these cells (Fig 2A and B).
As predicted, when p19 was induced, it moved into complexes with both CDK4 and CDK6 in vivo. The level of p19 expressed in a transfected clone grown in IL-3 was approximately twofold higher than that of parental 32D cells (Fig 3A, lane 4 v lane 2). Whereas zinc treatment for 5 hours did not affect p19 expression in control transfectants (Fig 3A, lane 3 v lane 2), it led to marked induction of the protein in the INK4d-transfected clone (lane 5). When lysates from an equivalent number of zinc-induced cells were immunoprecipitated with antiserum to p19 and the precipitated proteins were separated on denaturing gels, transferred to nitrocellulose, and blotted with antiserum to CDK4, CDK4 subunits could now be readily visualized in complexes with p19 (Fig 3B, lane 5). Similar results were obtained using antiserum to CDK6 (not shown). Cyclin D3 was expressed at the same levels in control and INK4d-transfected cells, whether or not they were treated for 5 hours with zinc (Fig 3C), but complexes between cyclin D3 and CDK4 were largely disrupted in cells induced to overexpress p19 (Fig 3D, lane 5). Notably, immunoprecipitates prepared with antibody to cyclin D3 contained p19 (Fig 3E, lane 5), even though p19 does not directly associate with D-type cyclins.19 Hence, some p19 must have entered into ternary complexes with residual cyclin D3-CDK4 (Fig 3D and E, lane 5), consistent with previous observations that such complexes can form in vitro and are enzymatically inactive.19 We can therefore conclude that induction of p19 (Fig 1A and B) and formation of p19-CDK complexes, either containing or lacking cyclin D (Fig 3), resulted in a loss of cyclin D–dependent kinase activity (Fig 1C and D), even though cyclins D2 and D3 and CDKs 4 and 6 were continuously expressed (Fig 2). Similar data to those shown with clone 2C were obtained with other individually derived p19-inducible subclones.
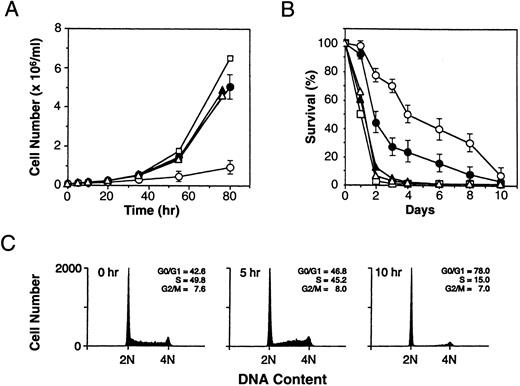
Induction of p19 induces cell cycle arrest and prolongs viability in the absence of IL-3.In each of six independent clones tested, the induction of p19 and the resulting inhibition of cyclin D-dependent kinase activity induced cell cycle arrest. When cells were transferred to medium containing IL-3 and zinc, the growth rate of the p19 overexpressors was retarded, as compared with transfected clones grown in IL-3 alone, which proliferated at rates indistinguishable from those of parental cells or of neo-resistant derivatives transfected with the naked vector (Fig 4A). Flow cytometric analysis of their DNA content showed that most cells expressing high levels of p19 accumulated rapidly in G1 phase within a single cell cycle (Fig 4C). Although the percentage of cells in G1 phase almost doubled within 10 hours of zinc addition to these cultures, no further increases in the G1 fraction were observed at later times, implying that growth arrest was incomplete. This was consistent with the fact that some zinc-treated cells continued to proliferate at a slow rate (Fig 4A).
Effect of p19-overexpression on cell proliferation and viability. (A) The indicated cells were plated (1 × 105/mL) in growth medium containing IL-3 and incubated with or without zinc. Cell numbers were determined in triplicate cultures of parental 32D cells and cells transfected with the empty vector, as well as the means and standard errors of cell counts of the six clones with zinc-inducible p19. Symbols are: (□), parental 32D cells; (▴), cells transfected with empty vector (no zinc treatment); (▵), cells transfected with empty vector (plus zinc); (•), cells transfected with p19-inducible vector (no zinc); and (○); cells transfected with p19-inducible vector (plus zinc). (B) Cells grown in IL-3 were shifted to medium lacking growth factors and zinc or containing zinc alone. The number of viable cells remaining in the cultures was determined daily by enumerating cells that excluded trypan blue vital dye. Symbols are the same as in (A). (C) Clone 2C cells growing in medium containing IL-3 were treated with zinc for the indicated times, stained with propidium iodide, and their DNA content was determined. The percentage of cells in different phases of the cell cycle was calculated and is given in the upper right of each panel.
Effect of p19-overexpression on cell proliferation and viability. (A) The indicated cells were plated (1 × 105/mL) in growth medium containing IL-3 and incubated with or without zinc. Cell numbers were determined in triplicate cultures of parental 32D cells and cells transfected with the empty vector, as well as the means and standard errors of cell counts of the six clones with zinc-inducible p19. Symbols are: (□), parental 32D cells; (▴), cells transfected with empty vector (no zinc treatment); (▵), cells transfected with empty vector (plus zinc); (•), cells transfected with p19-inducible vector (no zinc); and (○); cells transfected with p19-inducible vector (plus zinc). (B) Cells grown in IL-3 were shifted to medium lacking growth factors and zinc or containing zinc alone. The number of viable cells remaining in the cultures was determined daily by enumerating cells that excluded trypan blue vital dye. Symbols are the same as in (A). (C) Clone 2C cells growing in medium containing IL-3 were treated with zinc for the indicated times, stained with propidium iodide, and their DNA content was determined. The percentage of cells in different phases of the cell cycle was calculated and is given in the upper right of each panel.
Like parental 32D cells, the viability of the p19-inducible subclones was strictly dependent on IL-3, but their rate of cell death after IL-3 withdrawal was significantly slower than that of parental 32D cells or of neo-resistant control transfectants (Fig 4B). When shifted into medium lacking both zinc and IL-3, the p19-inducible cells, which express higher basal levels of p19 than parental cells (Figs 1A and 3A), showed extended survival. Induction of p19 in response to zinc treatment further prolonged their viability (Fig 4B). Thus, induction of p19 protects against the rapid cell death that normally accompanies growth factor withdrawal.
Cells overexpressing p19 acquire macrophage characteristics.May-Grünwald-Giemsa staining of cytocentrifuged preparations showed that p19-transfected 32D clones exhibited a myeloblastic phenotype similar to that of parental 32D cells when grown in the presence of IL-3 (Fig 5A v B). However, after 4 days of zinc-treatment, the p19 inducible cells appeared larger, developed a higher cytoplasmic to nuclear ratio with conspicuous cytoplasmic vacuoles, and became somewhat adherent (Fig 5C). The cytoplasm of zinc treated cells also stained for acid phosphatase whereas untreated cells did not (data not shown), consistent with increased lysosomal activity following induction. No such effects were observed in zinc-treated control cells that had been transfected with the naked vector. Parental 32D cells and p19-inducible clones, whether treated with zinc or not, expressed equivalent numbers of FcRs, but FcR-dependent immunophagocytosis was also observed in approximately 6% of zinc-treated cells that overexpressed p19 (Fig 5D).
Differentiation of p19-induced 32D cells. Parental 32D cells (A) and the 2C subclone (B) maintained in medium containing IL-3 but lacking zinc have the appearance of myeloid blast cells. When shifted to medium containing 75 μmol/L zinc (C, D) and examined 4 days later, the cells acquired a macrophage-like morphology (C) and a fraction of them exhibited Fc-receptor-dependent immunophagocytosis (D). When transferred to medium containing G-CSF instead of IL-3, p19-inducible clones exhibited nuclear changes typical of neutrophils after only 3 days of culture (E). Under identical conditions in medium containing both G-CSF and zinc, some macrophage-like cells also emerged (arrowheads) (F ). If clone 2C cells were grown in G-CSF plus zinc for 4 days and shifted back to medium containing IL-3 plus zinc, macrophage-like cells but not neutrophils persisted, and by 7 days after medium change, the cultures resembled those illustrated in (C). No macrophage-like cells were ever observed in cultures of parental 32D cells grown in G-CSF plus zinc (negative data not shown).
Differentiation of p19-induced 32D cells. Parental 32D cells (A) and the 2C subclone (B) maintained in medium containing IL-3 but lacking zinc have the appearance of myeloid blast cells. When shifted to medium containing 75 μmol/L zinc (C, D) and examined 4 days later, the cells acquired a macrophage-like morphology (C) and a fraction of them exhibited Fc-receptor-dependent immunophagocytosis (D). When transferred to medium containing G-CSF instead of IL-3, p19-inducible clones exhibited nuclear changes typical of neutrophils after only 3 days of culture (E). Under identical conditions in medium containing both G-CSF and zinc, some macrophage-like cells also emerged (arrowheads) (F ). If clone 2C cells were grown in G-CSF plus zinc for 4 days and shifted back to medium containing IL-3 plus zinc, macrophage-like cells but not neutrophils persisted, and by 7 days after medium change, the cultures resembled those illustrated in (C). No macrophage-like cells were ever observed in cultures of parental 32D cells grown in G-CSF plus zinc (negative data not shown).
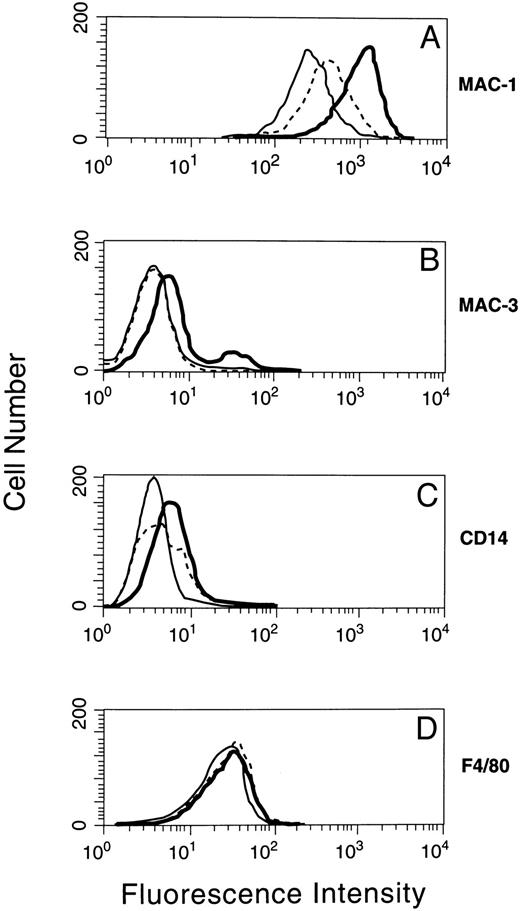
The expression of macrophage-specific antigens on the surfaces of induced and uninduced cells was examined using monoclonal antibodies against Mac-1,49 Mac-3,50 CD14,51 and F4/80.52 As shown in Fig 6, parental 32D cells stained positively in different degrees for Mac-1, Mac-3, and F4/80 antigens.53 54 Overexpression of p19 led to a pronounced and generally uniform increase in Mac-1 and Mac-3 fluorescence, with a lesser effect on CD14 expression, but with no detectable change in F4/80. Therefore, cells induced to express p19 acquired certain properties characteristically found in more differentiated cells of the monocyte/macrophage lineage.
Macrophage lineage antigen expression in 32D cells induced to synthesize p19. A p19-inducible 32D subclone grown in IL-3 plus zinc for 4 days was stained with fluorescent antibodies directed to MAC-1 (A), MAC-3 (B), CD14 (C), and F4/80 (D) as detected by flow cytometry. The staining profiles of parental 32D cells (solid line) and of clone 2C cells grown in the absence (dotted line) or presence (bold line) of zinc are indicated in each panel.
Macrophage lineage antigen expression in 32D cells induced to synthesize p19. A p19-inducible 32D subclone grown in IL-3 plus zinc for 4 days was stained with fluorescent antibodies directed to MAC-1 (A), MAC-3 (B), CD14 (C), and F4/80 (D) as detected by flow cytometry. The staining profiles of parental 32D cells (solid line) and of clone 2C cells grown in the absence (dotted line) or presence (bold line) of zinc are indicated in each panel.
Although expression of the CSF-1R is one of the hallmarks of mononuclear phagocyte maturation,55-58 inducible clones remained unresponsive to CSF-1, whether treated with zinc or not. When exposed to CSF-1 instead of IL-3, all clones failed to survive, suggesting that they expressed negligible or no CSF-1R (data not shown). Clones were metabolically labeled with [35S]methionine both before and at various times after zinc treatment, and lysates were immunoprecipitated with two different high titer rabbit antisera reactive to murine CSF-1R. As a positive control, lysates of metabolically labeled BAC1.2F5 macrophages were analyzed in parallel. Again, no CSF-1R was detected in any of the 32D derivatives, although receptor expression was readily documented in control macrophages (Fig 7A). Cytohistochemical staining for murine CSF-1R confirmed that no p19-inducible 32D clones expressed receptors after zinc treatment, whereas BAC1.2F5 macrophages were uniformly positive (data not shown).
Absence of CSF-1R expression in p19-inducible 32D cells. (A) Parental 32D cells and the p19-inducible 2C subclone were grown in medium containing IL-3 in the presence or absence of zinc for 4 days, as indicated at the top of the panel. BAC1.2F5 macrophages were grown in medium containing CSF-1. Cells metabolically labeled for 90 minutes with [35S]-methionine were lysed and precipitated with nonimmune rabbit serum (NRS), or with rabbit antisera directed to human CSF-1R (c-fms ) or to the feline v-fms oncogene product, as indicated below the panel. The precipitated labeled proteins were electrophoretically separated on denaturing polyacrylamide gels and detected by autoradiography (exposure time 36 hours). The position of CSF-1R is indicated at the right. (B) RNAs extracted from the same cells as in A were copied into DNA by reverse transcription and amplified by PCR using internal primers based on the mouse CSF-1R cDNA sequence. For two reactions, RNA from CSF-1R-positive BAC1.2F5 cells (lane 6) was mixed as 1% (lane 4) and 5% (lane 5) of the total together with RNA extracted from subclone 2C cells that had been grown in the presence of zinc. RT-PCR products were separated on nondenaturing agarose gels using markers of known complexity (lane M). The position of the expected 554 base-pair CSF-1R product is indicated at the right.
Absence of CSF-1R expression in p19-inducible 32D cells. (A) Parental 32D cells and the p19-inducible 2C subclone were grown in medium containing IL-3 in the presence or absence of zinc for 4 days, as indicated at the top of the panel. BAC1.2F5 macrophages were grown in medium containing CSF-1. Cells metabolically labeled for 90 minutes with [35S]-methionine were lysed and precipitated with nonimmune rabbit serum (NRS), or with rabbit antisera directed to human CSF-1R (c-fms ) or to the feline v-fms oncogene product, as indicated below the panel. The precipitated labeled proteins were electrophoretically separated on denaturing polyacrylamide gels and detected by autoradiography (exposure time 36 hours). The position of CSF-1R is indicated at the right. (B) RNAs extracted from the same cells as in A were copied into DNA by reverse transcription and amplified by PCR using internal primers based on the mouse CSF-1R cDNA sequence. For two reactions, RNA from CSF-1R-positive BAC1.2F5 cells (lane 6) was mixed as 1% (lane 4) and 5% (lane 5) of the total together with RNA extracted from subclone 2C cells that had been grown in the presence of zinc. RT-PCR products were separated on nondenaturing agarose gels using markers of known complexity (lane M). The position of the expected 554 base-pair CSF-1R product is indicated at the right.
We therefore undertook RT-PCR analysis to detect CSF-1R mRNA, reasoning that this would be the most sensitive test for CSF-1R expression that we could apply. No PCR products were documented in parental or p19-transfected 32D cells, whether they were treated with zinc or not (Fig 7B, lanes 1 to 3). However, control BAC1.2F5 macrophages yielded the expected 554 base-pair CSF-1R PCR product, as did those that had been mixed with p19-inducible 32D cells at 5% or 1% levels (Fig 7B, lanes 4 to 6). Because the majority of zinc-treated p19-inducible 32D cells expressed increased Mac-1 antigen on their surface (Fig 6A), whereas a minimum of 6% were phagocytic (Fig 5D), we should have readily detected CSF-1R mRNA products had they been represented by at least 1% of cells in the population.
The discussed results raised the intriguing possibility that p19-induced 32D cells might not terminally differentiate because they lacked CSF-1R. Therefore, we transfected one of the p19-inducible clones with a vector encoding human CSF-1R. Transfection of immature, IL-3–dependent myeloid cell lines with CSF-1R cDNA can confer CSF-1 responsiveness on a small percentage of derived subclones, and a fraction of these surviving variants are able to differentiate toward macrophages.31,53,54 59 Nonetheless, in the experiments here, selected drug-resistant 32D cell lines uniformly expressing CSF-1R could not be maintained in medium containing human recombinant CSF-1, and they failed to upregulate their macrophage markers when treated with human CSF-1 plus zinc (negative data not shown).
When transferred from IL-3–containing medium to that containing G-CSF but lacking zinc, clones capable of expressing p19 retained their capacity to differentiate to neutrophils in response to G-CSF, and no macrophage-like cells were observed in the cultures. After only a 3-day exposure to G-CSF alone, p19-inducible clones already had begun to exhibit neutrophilic morphology, as marked by the formation of ring-shaped nuclei and band forms (Fig 5E). When such cells were treated with both G-CSF and zinc, the majority still formed neutrophils, but a minority (∼5%) acquired macrophage characteristics (Fig 5F ). Induction of p19 accelerated granulocyte formation in response to G-CSF by the majority of cells (Fig 5F v E). Hence, although cells differentiating in G-CSF alone lose cyclin D–dependent kinase activity because of cyclin degradation upon IL-3 withdrawal,27 more rapid p19-induced inhibition of these enzymes appeared to accelerate differentiation. In addition, the ability of zinc plus G-CSF to selectively support the acquisition of macrophage markers by a small fraction of the p19-inducible cells argues that p19, and not IL-3, triggers this alternative response. Cultures that were treated for 4 days with IL-3 and zinc and had acquired macrophage characteristics (Fig 5C) also retained the ability to differentiate to neutrophils when shifted to medium containing G-CSF alone, in agreement with the concept that the differentiation of these cells in the macrophage lineage is incomplete and reversible (see above).
DISCUSSION
Negative regulation of cell cycle progression may help reinforce the proper execution of differentiation programs and coordinate the number of mature cells that develop from uncommitted progenitors in response to a particular differentiation stimulus. Universal CDK inhibitors of the Cip1/Kip1 family as well as specific inhibitors of cyclin D–dependent kinases (the INK4 proteins) have been broadly implicated in such processes.60 Not surprisingly, many CDK inhibitors are specifically upregulated during differentiation of various cell types in vivo.61-68 However, their exact biological functions remain a mystery.
In normal tissue development, the functions of particular CDK inhibitors may well be redundant, so that the individual loss of one such regulator might be compensated by the presence of another. Nullizygous animals unable to synthesize p21Cip1 or p16INK4a develop entirely normally69-71 even though both proteins have the capability to govern differentiation-specific decisions in cultured cell lines (discussed below). On the other hand, mice lacking p27Kip1 develop generalized organomegaly being 60% larger than their wild-type littermates, and their tissues are hyperplastic and contain cells that are unusually small in size.72-74 The latter results are in good agreement with observations made with cultured cell lines, in which cells overexpressing G1 cyclins were reduced in size and had a decreased dependency on mitogens,13,75 and where inhibition of p27Kip1 inhibitory function prevented the immediate cell cycle exit usually seen upon mitogen withdrawal.76 77
Overexpression of cyclin D–dependent kinases in cultured myoblasts can cancel the ability of the MyoD transcription factor to promote muscle cell differentiation; conversely, enforced expression of CDK inhibitors, including p21Cip1 or p16INK4a, can potentiate muscle cell differentiation even in the presence of otherwise overriding mitogenic signals.64-66 Transient overexpression of p21Cip1 triggers differentiation of U937 cells to macrophages, suggesting that G1 arrest in this system might itself be sufficient to activate the program.78 Similarly, enforced expression of cyclins D2 and D3 in 32Dcl3 myeloid cells prevented their differentiation in G-CSF,27 whereas, as shown here, p19INK4d overexpression and the resulting inhibition of cyclin D–dependent kinase activity induced differentiation, albeit in an unusual manner.
When cultured in IL-3, induction of p19 by addition of zinc to the medium led to the expression of macrophage-specific markers. The morphology of virtually all of the cells changed dramatically, and the vast majority expressed considerably higher levels of particular cell surface antigens, such as Mac-1. In contrast, a smaller fraction of zinc-treated cells expressed higher levels of Mac-3 or CD14, and only about 6% exhibited frank Fc-dependent phagocytic activity. The nonuniformity of the differentiative response, despite the fact that the cells were clonally derived, may reflect the variability in the magnitude of p19INK4d induction or extent of cyclin D–dependent kinase inhibition in individual cells. Although most cells showed greatly reduced cyclin D3- and CDK4-dependent pRb kinase activity within 2.5 hours of zinc treatment and arrested in G1 phase within 12 hours, a fraction continued to proliferate at a reduced rate, indicating that, for whatever reason, their response was not complete. Moreover, differentiation toward macrophage-like cells in each of six individually derived clones was not terminal, and the cells reverted to an immature phenotype within 3 to 4 days after zinc was removed from the cultures. Although we cannot exclude the possibility that this in part reflected the regrowth of less differentiated 32D precursors that remained in the zinc-induced cultures, the uniformity of the observed changes in cell morphology and Mac-1 expression following zinc addition and its subsequent withdrawal argues that the more differentiated cells were not committed to the macrophage lineage and were capable of reversion.
Although 32D cells do not normally differentiate toward macrophages, ectopic expression of the CSF-1 receptor54 or of the Egr-1 transcription factor in the presence of CSF-179 was previously shown to induce expression of macrophage-specific markers. In our case, however, differentiation within the mononuclear phagocyte lineage was completely independent of CSF-1, as the cells lacked any detectable CSF-1 receptors and did not respond to the exogenously added growth factor. Neither did the subsequent introduction and expression of the human CSF-1 receptor gene (FMS ) in the p19-inducible 32D cells enable them to respond further to recombinant human CSF-1.
Like parental 32D cells, most p19-overexpressing clones terminally differentiated to neutrophils in G-CSF, whether induced by zinc or not. The parental cells and the p19-inducible subclones synthesize cyclins D2 and D3 in response to IL-3, but they terminate cyclin D synthesis when transferred to medium containing G-CSF.26 27 Cyclin D2 is degraded very rapidly under these conditions, whereas cyclin D3, the major D-type cyclin expressed in this cell line, persists for 2 to 3 days. The cells arrest in G1 within this 2- to 3-day time frame and then differentiate toward granulocytes. When p19-inducible clones were cultured in IL-3 and zinc, the vast majority arrested in G1 phase within a single cell cycle, even though cyclin D was not yet degraded. By 5 hours after zinc treatment, p19 had disrupted most cyclin D-CDK complexes and had moved into inactive, ternary complexes with the remainder, effectively inhibiting all detectable cyclin D–dependent kinase activity. Similarly, p19-induced G1 arrest occurred much more rapidly in cells transferred to G-CSF plus zinc than in those cultured in G-CSF alone. The fact that some zinc treated cells acquired macrophage markers when grown in either IL-3 or G-CSF must, therefore, reflect an activity of p19INK4d itself. Clearly, cell cycle arrest per se cannot account for the observed macrophage differentiation, as G-CSF-treated cells also arrested in G1 , but at a slower rate. Nor was IL-3 required, because p19-induced macrophage differentiation occurred as long as the viability of zinc-treated cells was ensured by either IL-3 or G-CSF. Indeed, induction of p19INK4d extended the survival of these cells in the complete absence of hemopoietins.
Taken together, these results suggest that a specific inhibitor of cyclin D–dependent kinases can induce G1 arrest, cell survival, and a partial differentiative response, possibly through independent biochemical pathways. The simplest interpretation is that cyclin D–dependent kinases can phosphorylate substrates other than Rb to influence gene expression. We suggest that one role of cyclin D–dependent kinases may be to cancel differentiation programs in cycling cells, thereby coupling their execution with cell cycle arrest. Attractive substrates for these kinases might include differentiation-inducing transcription factors whose activities might be negatively regulated through such a mechanism.80
ACKNOWLEDGMENT
We thank Hiroshi Hirai and Dawn E. Quelle for constructive suggestions during the course of this work; Richard A. Ashmun for flow cytometric analyses of DNA content; and Shawn Hawkins, Carol Bockhold, and Joseph Watson for excellent technical assistance.
Supported by the Howard Hughes Medical Institute, by Cancer Center Core Grant CA-21765, and by the American Lebanese Syrian Associated Charities (ALSAC) of St Jude Children's Research Hospital.
Address reprint requests to Charles J. Sherr, MD, PhD, Department of Tumor Cell Biology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN, 38105.



![Fig. 7. Absence of CSF-1R expression in p19-inducible 32D cells. (A) Parental 32D cells and the p19-inducible 2C subclone were grown in medium containing IL-3 in the presence or absence of zinc for 4 days, as indicated at the top of the panel. BAC1.2F5 macrophages were grown in medium containing CSF-1. Cells metabolically labeled for 90 minutes with [35S]-methionine were lysed and precipitated with nonimmune rabbit serum (NRS), or with rabbit antisera directed to human CSF-1R (c-fms ) or to the feline v-fms oncogene product, as indicated below the panel. The precipitated labeled proteins were electrophoretically separated on denaturing polyacrylamide gels and detected by autoradiography (exposure time 36 hours). The position of CSF-1R is indicated at the right. (B) RNAs extracted from the same cells as in A were copied into DNA by reverse transcription and amplified by PCR using internal primers based on the mouse CSF-1R cDNA sequence. For two reactions, RNA from CSF-1R-positive BAC1.2F5 cells (lane 6) was mixed as 1% (lane 4) and 5% (lane 5) of the total together with RNA extracted from subclone 2C cells that had been grown in the presence of zinc. RT-PCR products were separated on nondenaturing agarose gels using markers of known complexity (lane M). The position of the expected 554 base-pair CSF-1R product is indicated at the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.126/5/m_bl_0050f7.jpeg?Expires=1769081552&Signature=Nu-i6tQhbMKyzOG8Br~qfytqVoGUUPdp6Al67HhXj3gbHsN6NytFx2L9uAD8j9pEkSbj8eYMB4oGQw0wqhTypjCywstCngZ9vI8t3n79cuh4n-XOaM7ko9Lct-0zRaM5XDeEoUSE3wayWm-oqkWvPR7onBJb6WhZhZbvOsIYdrcqo5TQAkHaiAflco5Q8IDKx~eVBYU0c04tT~7uUrtO8nvpH2CrY4Pfs1MABxjtXISCG9~csyUu3mujxI2DFGrCapzX4arWKK50Lpm4hthiINNn30dYFJ4YZNvKgvLpUV0~kyfsybbWAg8M9y44VtGanzPNdthrEkbbW0KGj2nrVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal