Abstract
Fas (CD95) is a transmembrane molecule that induces programmed cell death (PCD) of lymphocytes. We examined its function in children with chronic thrombocytopenia, serum autoantibodies, and lymphadenopathy and/or splenomegaly. We found that T-cell lines from six of seven patients with this autoimmune/lymphoproliferative disease (ALD) were relatively resistant to PCD induced by monoclonal antibodies to Fas. By contrast, Fas function was normal in control patients with typical chronic idiopathic thrombocytopenic purpura (ITP) without lymphadenopathy. The defect was not due to decreased Fas expression, nor to over-production of soluble forms of Fas. Moreover, it specifically involved the Fas system because PCD was induced in the normal way by methylprednisolone. Complementary DNA sequencing of the Fas gene did not identify any causal mutation in patients with ALD. This distinguished them from patients with the human autoimmune lymphoproliferative syndrome (ALPS), who carry mutations of the Fas gene. Moreover, patients with ALD did not show the peripheral expansion of CD4/CD8 double-negative T cells that characterizes the ALPS phenotype. Fas signaling involves activation of a sphingomyelinase-catalyzing production of ceramide. We found that ceramide-induced PCD was defective in patients with ALD and not in patients with typical chronic ITP. These data suggest that the ALD patient defect involves the Fas signaling pathway downstream from the sphingomyelinase and that Fas gene mutations and double-negative T-cell expansion are not the only signs of a defective Fas system.
FAS (CD95) IS A transmembrane molecule belonging to the tumor necrosis factor (TNF ) receptor superfamily. It interacts with the Fas ligand (FasL), a type II transmembrane protein belonging to the TNF cytokine superfamily.1-4 Fas ligation by monoclonal antibodies (MoAbs) or FasL induces programmed cell death (PCD) in several lymphoid cell lines and may play a role in immune response control, lymphocyte life span regulation, and induction of peripheral tolerance. Moreover, Fas is involved in cytotoxic T-lymphocyte and TH1 cell cytotoxicity, which is partially due to the interaction of FasL expressed by activated cytotoxic cells with Fas expressed by target cells. In lpr and gld mice, homozygous mutations of the Fas or FasL gene respectively affect Fas-induced PCD and cause lymphoproliferation and a generalized autoimmune disease, with hypergammaglobulinemia, autoantibody production, glomerulonephritis, arthritis, vasculitis, and accumulation of nonmalignant TCRαβ+ CD4/CD8 double-negative (DN) T cells in secondary lymphoid organs.5-8
In humans, a rare disease reminiscent of that displayed by lpr mice has been independently described by Fisher et al9 and Rieux-Laucat et al,10 and more recently by Bettinardi et al.11 Designated autoimmune lymphoproliferative syndrome (ALPS) by Fisher et al, it is characterized by nonmalignant lymphoadenopathy with an expanded DN population and autoimmune phenomena with hemolytic anemia, thrombocytopenia, neutropenia, or some combination of these, recurrent urticaria consistent with immune vasculitis, and glomerulonephritis. Defective Fas-mediated T-cell PCD due to single deleterious Fas gene mutation is always observed.
In our work, we identified seven unrelated patients with lymphoadenopathy and/or splenomegaly and recurrent episodes of thrombocytopenia, neutropenia, and/or autoimmune hemolytic anemia, but with no DN cell expansion. T cells from six patients displayed reduced Fas capacity to induce PCD, but no Fas gene mutation. By contrast, the Fas defect seemed to involve the Fas signaling pathway because ceramide, a second messenger for Fas signaling,12 did not overcome the PCD defect. These data show that lymphoproliferative/autoimmune diseases may involve not only Fas itself, as in patients with ALPS, but also its signaling pathway, and suggest that Fas gene mutations and DN cell expansion are not the only signs of a defective Fas system.
MATERIALS AND METHODS
Patients.Seven unrelated patients with several symptoms suggestive of an ALPS condition were selected from those followed by the Hematology Unit of the Pediatric Department of the University of Torino. They showed lymphadenopathy and/or splenomegaly, several combinations of peripheral cytopenias, and several types of serum autoantibodies. Common causes of lymphadenopathy were ruled out in all subjects. At onset, all patients were negative for immunoglobulin (Ig)M to Epstein-Barr virus (EBV), cytomegalovirus (CMV), and rubella. Patients 1 and 4 were weakly positive for IgG to EBV, but were always negative for EBV by polymerase chain reaction (PCR). Patients 1, 2, and 5 were positive for IgG to rubella. Patient 1 was positive for IgG to CMV. This pattern of IgG antibodies was similar to that of age-matched normal controls. Biopsies of four particularly impressive lymphadenopathies showed nonspecific hyperplasia (patients 1, 2, 4, and 5). No patient showed renal or cardiopulmonary manifestations or rash. Two patients had hypergammaglobulinemia, two were hypogammaglobulinemic, and three showed fluctuations in gammaglobulin levels. Bone marrow specimens were normal or showed a reduction in mature myeloid cells. Clinical data are summarized in Table 1.
Clinical Data
| Patient . | Sex . | Age at Onset . | Age at Study . | Platelets . | Hb (g/dL)* . | ANC . | Serum IgG (mg/dL)* . | DN T cells† (μL) . | LN‡ . | Spleenρ . | LAC . | ANA . | a-DNA . | Clinical Status at Study . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | (×103/μL)* . | . | (×103/μL)* . | . | . | . | . | . | . | . | . |
| 1 | M | 13 yr | 22 yr | 5-480 | 7.4-12.8 | 0.18-4.5 | 1,150-1,600 | 5% (130) | +++ | +++ | + | + | − | Neutropenia, mild thrombocytopenia, no therapy |
| 2 | M | 7 yr | 16 yr | 2-160 | 8.2-13.5 | 1.8-8.6 | 1,500-2,410 | 4% (100) | +++ | + | + | + | − | Severe thrombocytopenia, in therapy with methyl-PDN 10/mg/kg every 4 wk |
| 3 | F | 8 mo | 8 yr | 91-480 | 7.4-11.5 | 0.17-4.5 | 2,120-3,510 | 13% (140) | + | ++++ | + | + | − | Mild anemia and thrombocytopenia, no therapy |
| 4 | M | 4 yr | 14 yr | 5-924 | 5.5-14.5 | 0.38-7.8 | 345-640 | 5% (100) | +++ | ++ | + | − | − | Severe neutropenia and thrombocytopenia, in therapy with PDN 1 mg/kg/d |
| 5 | M | 3 yr | 8 yr | 8-167 | 11.6-14.1 | 1.0-6.8 | 550-922 | 9% (150) | +++ | ++ | + | − | − | In remission, no therapy |
| 6 | M | 4 mo | 3 yr | 17-267 | 7.3-12.7 | 0.7-5.6 | 408-760 | 4% (120) | + | +++ | + | − | − | In remission, no therapy |
| 7 | M | 2 yr | 3 yr | 45-168 | 9.7-11.8 | 1.6-4.7 | 670-795 | 2% (53) | + | +++ | + | − | − | Mild thrombocytopenia, no therapy |
| Patient . | Sex . | Age at Onset . | Age at Study . | Platelets . | Hb (g/dL)* . | ANC . | Serum IgG (mg/dL)* . | DN T cells† (μL) . | LN‡ . | Spleenρ . | LAC . | ANA . | a-DNA . | Clinical Status at Study . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | (×103/μL)* . | . | (×103/μL)* . | . | . | . | . | . | . | . | . |
| 1 | M | 13 yr | 22 yr | 5-480 | 7.4-12.8 | 0.18-4.5 | 1,150-1,600 | 5% (130) | +++ | +++ | + | + | − | Neutropenia, mild thrombocytopenia, no therapy |
| 2 | M | 7 yr | 16 yr | 2-160 | 8.2-13.5 | 1.8-8.6 | 1,500-2,410 | 4% (100) | +++ | + | + | + | − | Severe thrombocytopenia, in therapy with methyl-PDN 10/mg/kg every 4 wk |
| 3 | F | 8 mo | 8 yr | 91-480 | 7.4-11.5 | 0.17-4.5 | 2,120-3,510 | 13% (140) | + | ++++ | + | + | − | Mild anemia and thrombocytopenia, no therapy |
| 4 | M | 4 yr | 14 yr | 5-924 | 5.5-14.5 | 0.38-7.8 | 345-640 | 5% (100) | +++ | ++ | + | − | − | Severe neutropenia and thrombocytopenia, in therapy with PDN 1 mg/kg/d |
| 5 | M | 3 yr | 8 yr | 8-167 | 11.6-14.1 | 1.0-6.8 | 550-922 | 9% (150) | +++ | ++ | + | − | − | In remission, no therapy |
| 6 | M | 4 mo | 3 yr | 17-267 | 7.3-12.7 | 0.7-5.6 | 408-760 | 4% (120) | + | +++ | + | − | − | In remission, no therapy |
| 7 | M | 2 yr | 3 yr | 45-168 | 9.7-11.8 | 1.6-4.7 | 670-795 | 2% (53) | + | +++ | + | − | − | Mild thrombocytopenia, no therapy |
Abbreviations: Hb, hemoglobin; ANC, absolute neutrophil count; LAC, lupus anticoagulant (anticardiolipin antibodies); ANA, antinuclear antibodies; a-DNA, anti-DNA antibodies; PDN, prednisone.
Range observed since onset.
CD4 CD8 double negative T cells expressed as percentage of total TCRαβ+ lymphocytes and, in brackets, as absolute counts/μL of PB. DN T cells (mean ± SD) were 7 ± 4% (165 ± 118/μL) in patients with chronic ITP, 7 ± 5% (170 ± 25/μL) in age-matched normal controls, 6 ± 4% (113 ± 33/μL) in patients with ALD, and 22 ± 2% (452 ± 28/μL) in three patients with ALPs.
Lymphoadenopathy: +, 1-2 cm nodes; ++, 2-4 cm nodes; +++, one or more nodes >4 cm.
ρ Splenomegaly: +, 1-2 cm; ++, 3-4 cm; +++, 5-6 cm; ++++, >6 cm below costal margin.
Patient 1 is a 22-year-old man observed at 13 years of age for thrombocytopenia. Shortly thereafter he presented with splenomegaly and jugular and axillary lymph node enlargement. He has received many courses of intravenous (IV) immunoglobulins and steroids, with transient effects. Neutropenia, thrombocytopenia, and lymphoadenopathy persisted after splenectomy. His paternal grandfather was repeatedly tranfused for “anemia.”
Patient 2 is a 16-year-old boy admitted at 7 years of age for thrombocytopenia. At the age of 3, biopsy of a large inguinal lymph node showed nonspecific hyperplasia. Steroids and IV immunoglobulins yielded minor effects; thrombocytopenia persisted after splenectomy performed when the patient was 15 years of age.
Patient 3 is a 10-year-old girl who presented with prolonged fever and splenomegaly at the age of 8 months. Immunoglobulin levels were high. Further episodes of fever followed in the next 2 years; results of viral and bacterial serology were consistently negative. In the following years, she showed episodes of hemolytic anemia and thrombocytopenia. At present, she has mild cytopenia and relevant splenomegaly. Her parents are second cousins. A maternal aunt with splenomegaly, hypergammaglobulinemia, and mild autoimmune thrombocytopenia requiring hospitalization was reported; the maternal grandfather of the patient and all three of his brothers died from malignancies.
Patient 4 is a 14-year-old boy admitted in our department at 4 years of age with autoimmune pancytopenia, splenomegaly, and jugular, axillary, and inguinal lymph node enlargement. Many courses of treatment, including cyclophosphamide and cyclosporin, produced only transient responses. Splenectomy was not effective. The patient died in the course of the present study during an episode of uncontrolled hemolysis. His father died from acute autoimmune hemolytic anemia and a maternal uncle from B-cell lymphoma.
Patient 5 is an 8-year-old boy who was referred for treatment at 3 years of age because of jugular, cervical, and inguinal lymph node enlargement concurrent with thrombocytopenia and neutropenia. Mild hepatosplenomegaly developed subsequently. Platelets reached a normal value after a second course of 1 g/kg IV immunoglobulins 2 years ago. Mild splenomegaly and lymphadenopathy persist.
Patient 6 is a 3-year-old boy who was referred for treatment at the age of 4 months because of anemia, thrombocytopenia, and splenomegaly. He was treated with steroids and 1 g/kg IV immunoglobulins. Thereafter, platelets and hemoglobin have been normal, but splenomegaly has persisted.
Patient 7 is a 3-year-old boy who was referred for examination at 2 years of age for mild thrombocytopenia and relevant splenomegaly. No treatment was given, and his clinical status has been stable. His paternal grandfather had chronic autoimmune thrombocytopenia and vasculitis.
Six male and six female subjects 18 months to 16 years of age were selected from patients with typical chronic idiopathic thrombocytopenic purpura (ITP) without lymphadenopathy and splenomegaly. None of them had a family history positive for autoimmunity and/or splenomegaly and lymphoma.
Controls were selected from age-matched children who underwent hematologic controls before surgery.
Patients with ALPS were recruited from the Pediatric Division of the Ospedale S.S. Annunziata of Naples and have been described previously.11 They were three siblings carrying two distinct missense mutations in the Fas gene and showing defective Fas-induced PCD.
Informed consent was obtained from the parents of all patients.
Because our results suggest that the disease observed in the seven patients forming the subject of this study is different from that caused by Fas gene mutations, it will be referred to as autoimmune/lymphoproliferative disease (ALD).
Cells and antibodies.Peripheral blood mononuclear cells (PBMCs) were prepared by gradient centrifugation (Lymphoprep, Nycomed, Oslo, Norway) of venous blood. Immunophenotype was determinated by direct immunofluorescence using the following (MoAbs) conjugated with either fluorescein or phycoerythrin: Leu2 (CD8), Leu3 (CD4), Leu4 (CD3), Leu15 (CD11b), LeuM7 (CD13), LeuM3 (CD14), LeuM9 (CD33), anti–HLA-DR, and TCRαβ (Becton Dickinson), and Dako-CD5, Dako-CD19, Dako-CD11b (Dako Immunoglobulins, Copenhagen, Denmark). FITC-conjugated anti-Fas MoAb (Immunotech, Marseille, France) was used to evaluate Fas expression. Lymphocyte staining was evaluated using a fluorescence-activated cell-sorting (FACS) cytofluorimeter (Becton Dickinson).
Function of Fas and the Fas pathway.The ability of Fas to induce cell death was determined on T-cell lines obtained by activating PBMCs with phytohemagglutinin (PHA) at days 0 (1 μg/mL) and 15 (0.2 μg/mL) and cultured in RPMI 1640 plus 10% fetal calf serum plus recombinant interleukin-2 (IL-2; 5 U/mL; Biogen, Geneva, Switzerland). Fas function was assessed 6 days after the second stimulation. Cells were incubated with control medium or anti-Fas MoAb (IgM isotype; 1 μg/mL; UBI, Lake Placid, NY) or anti-Fas MoAb plus anti-IgM rabbit antimouse serum (1 μg/mL; Serotec, Oxford, UK) in the presence of IL-2 (5 U/mL) to minimize spontaneous cell death. Cell survival was evaluated after 18 and 48 hours by counting live cells in each well via the trypan blue exclusion test. The same conditions were used to measure cell death induced by methylprednisolone (Upjohn, Puurs, Belgium) or C2-ceramide (N-acetyl-D-sphingosine; Sigma, St Louis, MO). This protocol was chosen in preliminary experiments performed on T-cell lines from 20 healthy donors when several anti-Fas MoAb concentrations (10, 1, and 0.1 μg/mL) and incubation times (1, 4, 8, 18, 48, and 72 hours) were used to induce cell death in T-cell lines cultured for 3, 6, 9, 15, 18, 21, and 24 days with PHA plus IL-2. Cell death was evaluated both indirectly, by counting total surviving cells via the trypan blue exclusion test, or directly, via FACS determination of the proportion displaying shrunken/hypergranular morphology, using the forward scatter/side scatter (FSC/SSC) parameters, or those displaying DNA fragmentation after staining with propidium iodide. The protocol chosen was found to give the most reproducible results. Fas-induced cell death was always less striking in these polyclonal PHA-derived T-cell lines than in stabilized tumor cell lines because it was slower and more asynchronous.
In the trypan blue exclusion test, Fas function was expressed as percentage of specific cell survival:
In the FACS assay, Fas function was expressed as percentage specific cell death:
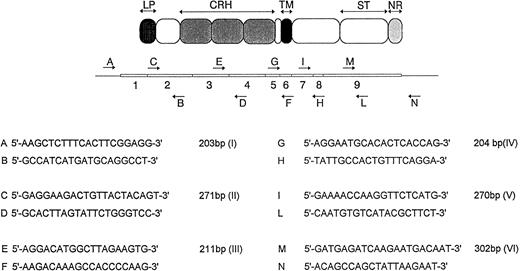
Analysis of the Fas gene.Mutation analysis of the Fas gene was performed by single-strand conformation polymorphism (SSCP)13 and complementary DNA (cDNA) sequencing. Total RNA was extracted from fresh PBMCs using the Ultraspec kit (Biotex). Total RNA (2 μg) was used as a template for cDNA synthesis with the Promega cDNA synthesis kit. The entire coding sequence of the Fas gene was amplified in a single 1,114-bp segment and in overlapping 203- to 302-bp segments (Fig 1). The size was selected to allow optimal SSCP resolution. PCR was performed with 25 pmol of each primer and one fourth of the cDNA synthesis reaction (35 cycles per fragment). SSCP was performed as previously reported.14 Because different SSCP conditions are required to allow 100% detection of DNA changes, all patients were studied with three or more SSCP protocols. The following parameters were varied: glycerol concentration (0% to 10%), electrophoresis temperature (4°C to 20°C), silver staining, or radioactivity as a means to detect band shifts. Normal controls were included in each experiment. All the samples showing abnormal shifts were purified using the Qiaex kit (Qiagen) and subjected to sequencing analysis with the Sequenase vs2 kit (US Biochemical Corporation). Moreover, because four of the seven mutations reported in Fas-deficient patients occur in the 3′ region of the gene (exon 9), we sequenced the corresponding PCR products (fragment V and VI) from all patients. The possibility that mutation might have been missed was completely ruled out by determining the entire coding sequences of the six patients who were found to have the Fas defect, namely patients 1, 2, 3, 5, 6, and 7.
Schematic representation (not to scale) of the approach used for PCR amplification of the human Fas gene. The exon organization of the cDNA and the relationship of the exons to the domain structure of the protein are indicated. LP, leader peptide; CRH, cystein-rich subdomains; TM, transmembrane domain; ST, apoptotic signal transduction domain; NR, negative regulatory domain. (Below) PCR primers used for amplification of the Fas gene and the relative length of PCR products. Primers were designed according to the cDNA sequence reported by Itoh et al.4
Schematic representation (not to scale) of the approach used for PCR amplification of the human Fas gene. The exon organization of the cDNA and the relationship of the exons to the domain structure of the protein are indicated. LP, leader peptide; CRH, cystein-rich subdomains; TM, transmembrane domain; ST, apoptotic signal transduction domain; NR, negative regulatory domain. (Below) PCR primers used for amplification of the Fas gene and the relative length of PCR products. Primers were designed according to the cDNA sequence reported by Itoh et al.4
RESULTS
T-cell subset distribution and response to activation stimuli.Peripheral blood lymphocyte (PBL) subset distribution shown by two-color immunofluorescence was the same in patients with ALD and those with ITP. The CD19+, CD3+, CD4+, and CD8+ cell counts were comparable with those displayed by normal healthy controls, and both patient groups displayed increased counts of T cells expressing the activation markers HLA-DR and CD38. T-cell responsiveness to activation stimuli was evaluated from the PBMC proliferative response to titrated amounts of anti-CD3 MoAb, anti-CD3 plus phorbol myristate acetate (PMA), and PHA. The mean proliferative response was not significantly different in the two patient groups, or from that in normal healthy donors (data not shown).
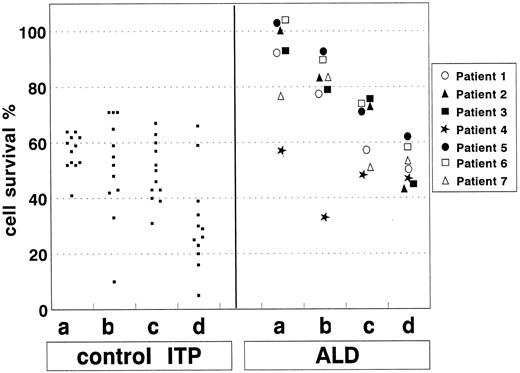
Functional analysis of the Fas pathway.The Fas apoptosis pathway was analyzed by triggering the Fas molecule with anti-Fas MoAb or anti-Fas plus anti-Ig serum and counting surviving cells via the trypan blue exclusion test. It was significantly lower in the patients with ALD than in those with ITP (Fig 2 and Table 2). The defect was always significant and maximally evident at 18 hours after triggering with the anti-Fas MoAb alone. Only one patient with ALD (patient 4) displayed Fas activity in the normal range. In the patients with ITP, it was comparable with that displayed by healthy donors (Table 2). Fas function was not abolished in the patients with ALD because cell death increased when incubation was prolonged to 48 hours and/or Fas triggering was potentiated by the anti-IgM serum. Similar results were obtained when Fas-induced PCD was evaluated by detecting the proportion of cells with shrunken/hypergranular morphology (Fig 3). Each patient was evaluated three to five times over a 1-year period. Results were always consistent, and no correlation was found with the clinical status.
Defective cell death induction by Fas triggering in T cells from patients with ALD and not in patients with typical chronic ITP. Fas was triggered with anti-Fas MoAb (lanes a and b) or anti-Fas MoAb + anti-IgM serum (lanes c and d), and cell survival was assessed after 18 (lanes a and c) or 48 hours (lanes b and d) by the trypan blue exclusion test. Results are expressed as percentage of specific cell survival (see the Materials and Methods). Statistical analysis is reported in Table 2.
Defective cell death induction by Fas triggering in T cells from patients with ALD and not in patients with typical chronic ITP. Fas was triggered with anti-Fas MoAb (lanes a and b) or anti-Fas MoAb + anti-IgM serum (lanes c and d), and cell survival was assessed after 18 (lanes a and c) or 48 hours (lanes b and d) by the trypan blue exclusion test. Results are expressed as percentage of specific cell survival (see the Materials and Methods). Statistical analysis is reported in Table 2.
Defective Cell Death Induction by Fas Triggering in T Cells From Patients With ALD and Not in Patients With Typical Chronic ITP
| Treatment . | Incubation . | Specific Cell Survival %* . | Statistical Analysis† . | ||||
|---|---|---|---|---|---|---|---|
| . | Time (hr) . | I . | II . | III . | II v III . | I v III . | I v II . |
| . | . | Normal Controls‡ . | Patients with Chronic ITP . | Patients with ALD . | P < . | P < . | P < . |
| . | . | (n = 16) . | (n = 12) . | (n = 7) . | . | . | . |
| Anti-Fas | 18 | 59 ± 9 | 56 ± 6 | 89 ± 17 | .01 | .001 | NS |
| Anti-Fas | 48 | 54 ± 10 | 52 ± 18 | 77 ± 26 | .01 | .01 | NS |
| Anti-Fas ± anti-Ig | 18 | 53 ± 13 | 49 ± 10 | 64 ± 12 | .05 | .05 | NS |
| Anti-Fas ± anti-Ig | 48 | 34 ± 12 | 31 ± 17 | 51 ± 7 | .01 | .01 | NS |
| Treatment . | Incubation . | Specific Cell Survival %* . | Statistical Analysis† . | ||||
|---|---|---|---|---|---|---|---|
| . | Time (hr) . | I . | II . | III . | II v III . | I v III . | I v II . |
| . | . | Normal Controls‡ . | Patients with Chronic ITP . | Patients with ALD . | P < . | P < . | P < . |
| . | . | (n = 16) . | (n = 12) . | (n = 7) . | . | . | . |
| Anti-Fas | 18 | 59 ± 9 | 56 ± 6 | 89 ± 17 | .01 | .001 | NS |
| Anti-Fas | 48 | 54 ± 10 | 52 ± 18 | 77 ± 26 | .01 | .01 | NS |
| Anti-Fas ± anti-Ig | 18 | 53 ± 13 | 49 ± 10 | 64 ± 12 | .05 | .05 | NS |
| Anti-Fas ± anti-Ig | 48 | 34 ± 12 | 31 ± 17 | 51 ± 7 | .01 | .01 | NS |
Cell death was induced as reported in Fig 2. Results are expressed as means ± SD of the percentage of specific cell survival.
Statistical analysis was performed using the nonparametric Mann-Whitney test.
Age-matched normal controls.
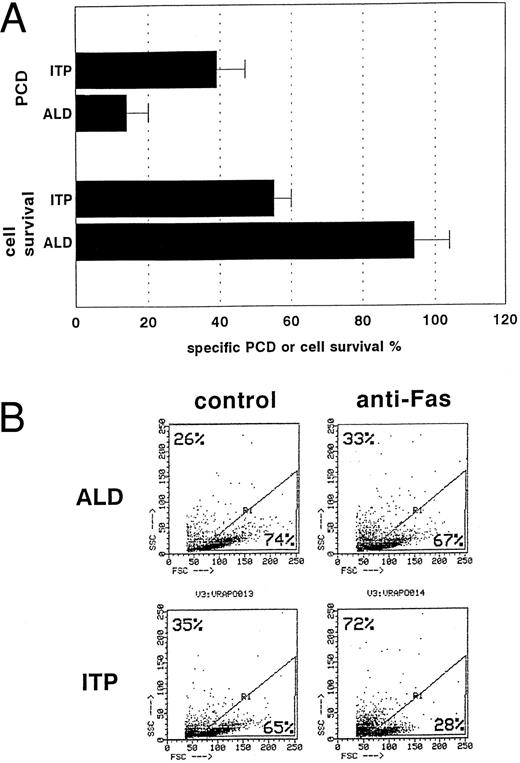
Comparison bewteen Fas-induced cell death detected as percentage of specific cell survival by the trypan blue exclusion test and as percentage of specific cell death detected by FACS analysis of shrunken/hypergranular cells. Both methods detected defective Fas activity in patients with ALD (n = 6) and not in those with typical chronic ITP (n = 6) (P < .01 using the nonparametric Mann-Whitney test). T cells were incubated for 18 hours in the presence of anti-Fas MoAb (A). Total cell survival was then assessed by the trypan blue exclusion test, and the proportion of apoptotic cells was detected by FACS analysis of shrunken/hypergranular cells using the FSC/SSC FACS parameters. Results are expressed as means ± SD. A significant inverse correlation was found between the two methods (r = −.84, P < .001). (B) FACS analysis of cultured T cells derived from a patient with ALD and one with ITP and treated with control medium or the anti-Fas MoAb for 18 hours. In each plot, the same gate (R1) was set between apoptotic and live cells, and the proportion of these cells is shown in the upper left and lower right corners, respectively.
Comparison bewteen Fas-induced cell death detected as percentage of specific cell survival by the trypan blue exclusion test and as percentage of specific cell death detected by FACS analysis of shrunken/hypergranular cells. Both methods detected defective Fas activity in patients with ALD (n = 6) and not in those with typical chronic ITP (n = 6) (P < .01 using the nonparametric Mann-Whitney test). T cells were incubated for 18 hours in the presence of anti-Fas MoAb (A). Total cell survival was then assessed by the trypan blue exclusion test, and the proportion of apoptotic cells was detected by FACS analysis of shrunken/hypergranular cells using the FSC/SSC FACS parameters. Results are expressed as means ± SD. A significant inverse correlation was found between the two methods (r = −.84, P < .001). (B) FACS analysis of cultured T cells derived from a patient with ALD and one with ITP and treated with control medium or the anti-Fas MoAb for 18 hours. In each plot, the same gate (R1) was set between apoptotic and live cells, and the proportion of these cells is shown in the upper left and lower right corners, respectively.
The decreased Fas activity was not due to decreased Fas expression by ALD patient T cells because direct immunofluorescence and FACS analysis showed that this was not different in patients with ALD or ITP, or in healthy donors, both in fresh peripheral blood T cells (PBT) and in the PHA-derived T-cell lines (data not shown). Moreover, the defect was not due to hyperproduction of soluble forms of Fas because supernatants of the ALD T-cell cultures did not inhibit cell death induced by anti-Fas MoAb in T-cell cultures from healthy donors (Table 3).
Supernatants From T-Cell Cultures From Patients With ALD and From Normal Donors Do Not Affect Fas-Mediated Cell Death in T-Cell Cultures
| Cells3-151 . | Medium . | Supernatant3-150 . | |||||
|---|---|---|---|---|---|---|---|
| . | . | Patient 1 . | Patient 5 . | Patient 6 . | Control 1 . | Control 2 . | Control 3 . |
| Experiment 1 | |||||||
| Patient 1 | 99c | 100 | 99 | 100 | 99 | 99 | 100 |
| Control 1 | 67 | 61 | 61 | 62 | 66 | 62 | 62 |
| Control 2 | 56 | 52 | 56 | 56 | 50 | 53 | 55 |
| Control 3 | 55 | 52 | 50 | 53 | 50 | 55 | 52 |
| Experiment 2 | Medium | Patient 3 | Control 4 | Control 5 | Control 6 | ||
| Patient 3 | 100 | 100 | 91 | 90 | 98 | ||
| Control 4 | 60 | 57 | 60 | 55 | 54 | ||
| Control 5 | 26 | 33 | 32 | 35 | 30 | ||
| Control 6 | 56 | 51 | 51 | 57 | 54 | ||
| Experiment 3 | Medium | Patient 2 | Control 7 | ||||
| Patient 2 | 100 | 91 | 95 | ||||
| Control 7 | 52 | 53 | 58 | ||||
| Cells3-151 . | Medium . | Supernatant3-150 . | |||||
|---|---|---|---|---|---|---|---|
| . | . | Patient 1 . | Patient 5 . | Patient 6 . | Control 1 . | Control 2 . | Control 3 . |
| Experiment 1 | |||||||
| Patient 1 | 99c | 100 | 99 | 100 | 99 | 99 | 100 |
| Control 1 | 67 | 61 | 61 | 62 | 66 | 62 | 62 |
| Control 2 | 56 | 52 | 56 | 56 | 50 | 53 | 55 |
| Control 3 | 55 | 52 | 50 | 53 | 50 | 55 | 52 |
| Experiment 2 | Medium | Patient 3 | Control 4 | Control 5 | Control 6 | ||
| Patient 3 | 100 | 100 | 91 | 90 | 98 | ||
| Control 4 | 60 | 57 | 60 | 55 | 54 | ||
| Control 5 | 26 | 33 | 32 | 35 | 30 | ||
| Control 6 | 56 | 51 | 51 | 57 | 54 | ||
| Experiment 3 | Medium | Patient 2 | Control 7 | ||||
| Patient 2 | 100 | 91 | 95 | ||||
| Control 7 | 52 | 53 | 58 | ||||
Supernatants from T-cell culture obtained from patients with ALD and age-matched normal donors (controls) were added to the Fas-mediated cell death assay at a final dilution of 1:4.
Cells used as targets in the Fas-mediated cell death assay. They were PHA-derived T-cell lines obtained from patients with ALD and normal controls as described in Materials and Methods.
Cells were incubated for 18 hours with control medium or anti-Fas MoAb (1 μg/mL). Then total cell survival was assessed by the trypan blue exclusion test. Results are expressed as percentage of cell survival.
In lpr and gld mice, as well as in patients with ALPS, decreased Fas function is accompanied by expansion of TCRαβ+ DN T cells. Two-color immunofluorescence on PBMCs simultaneously stained with FITC-conjugated anti-TCRαβ MoAb and phycoerythrin conjugated anti-CD4 and anti-CD8 MoAbs did not detect any expansion of these cells in patients with ALD (Table 1), nor were they detected in the spleen of one patient with ALD splenectomized during the study whose spleen T cells displayed the same phenotypic and functional features as peripheral T cells and were resistant to Fas-induced cell death (data not shown).
Analysis of the Fas gene.By analogy with patients with ALPS, the functional defect of Fas in ALD could be due to mutations of the Fas gene. Therefore, a search for mutations of Fas was performed by SSCP and cDNA sequencing of the entire coding sequence of the Fas gene in patients 1, 2, 3, 5, 6, and 7. These patients showed PCR products normal both in size and amount, thus ruling out small deletions, splicing defects, or reduced expression. Two PCR products were obtained both in the full length (1,114 and 1,051 bp) and in fragment IV (204 and 140 bp). This is due to a known alternative splicing that removes exon 6 and generates an mRNA transcript variant, which encodes a human Fas molecule lacking the transmembrane domain and present as a soluble, secreted protein.15
SSCP showed abnormal shifts in three patients and normal controls in fragment IV. cDNA sequencing showed that they were due to the common silent polymorphism at codon 198 reported by Fiucci and Ruberti.16 Patients 2, 5, and 7 showed genotype A/C, and patients 1, 3, and 6 showed A/A according to Fiucci and Ruberti's classification. These genotypes are the most frequent in the Italian population.16
A further band shift detected in a normal control in fragment II was shown to correspond to a previously unreported silent mutation at codon 45 (AAG-AAA). This change does not alter the encoded amino acid (lysine). None of the other subjects showed this change. No other mutation was identified.
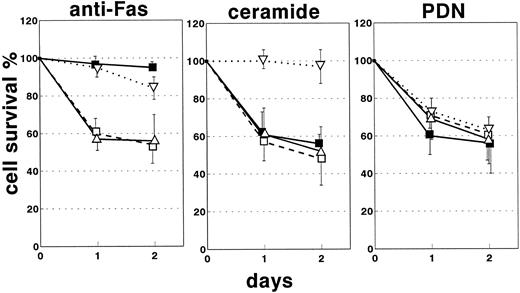
Cell death induction by ceramide and methylprednisolone.Another possible explanation was that Fas function in ALD was affected by alterations in the signal transduction pathway used by Fas. Fas signaling involves activation of a sphingomyelinase-catalyzing production of ceramide, which acts as the second messenger in the induction of PCD.12 To determine whether this pathway was altered downstream from Fas, the ability of ceramide to induce T-cell death was assessed in the six patients with ALD who had the Fas function defect (patients 1, 2, 3, 5, 6, and 7), as well as in the patients with ITP, the three patients with ALPS, and the normal controls. The ability of methylprednisolone to induce cell death was also assessed to determine the specificity of the Fas defect (Fig 4). Fas-induced and ceramide-induced cell death were significantly reduced in patients with ALD compared with patients with ITP and in normal controls (P < .001 using the nonparametric Mann-Whitney test). The patients with ALPS showed reduced Fas-induced cell death, whereas the incidence of ceramide-induced cell death was normal. Response to methylprednisolone was not significantly different in the four groups. Fas-induced and ceramide-induced cell death were not significantly different in patients with ITP and in normal controls.
Cell death induction by several stimuli in T cells from patients with ALD, typical chonic ITP, and ALPS, as well as in age-matched normal controls. Anti-Fas MoAb and ceramide displayed decreased capacity to induce cell death in patients with ALD (n = 6) (dotted lines, ▿) and not in patients with typical chronic ITP (n = 10) (continuous lines, ▵) or in normal controls (n = 13) (dashed lines, □). Patients with ALPS (n = 3) (continuous lines, ▪) displayed decreased Fas-induced but normal ceramide-induced cell death. Cell death induced by methyl-prednisolone (PDN) was not different in patients with ALD or ITP or in normal controls. PHA-derived T cells were cultured as reported in Fig 2 for 18 or 48 hours in the presence of either anti-Fas MoAb (1 μg/mL), ceramide (50 μmol/L), or PDN (1 μmol/L). Cell death was then assessed by the trypan blue exclusion test. The stimuli were simultaneously tested in each patient. Results are expressed as means ± SD of the specific cell survival percentage.
Cell death induction by several stimuli in T cells from patients with ALD, typical chonic ITP, and ALPS, as well as in age-matched normal controls. Anti-Fas MoAb and ceramide displayed decreased capacity to induce cell death in patients with ALD (n = 6) (dotted lines, ▿) and not in patients with typical chronic ITP (n = 10) (continuous lines, ▵) or in normal controls (n = 13) (dashed lines, □). Patients with ALPS (n = 3) (continuous lines, ▪) displayed decreased Fas-induced but normal ceramide-induced cell death. Cell death induced by methyl-prednisolone (PDN) was not different in patients with ALD or ITP or in normal controls. PHA-derived T cells were cultured as reported in Fig 2 for 18 or 48 hours in the presence of either anti-Fas MoAb (1 μg/mL), ceramide (50 μmol/L), or PDN (1 μmol/L). Cell death was then assessed by the trypan blue exclusion test. The stimuli were simultaneously tested in each patient. Results are expressed as means ± SD of the specific cell survival percentage.
DISCUSSION
We report a defective Fas ability to induce T-cell death in children with ALD, which is characterized by recurrent thrombocytopenia, serum autoantibodies, and lymphadenopathy/splenomegaly. This defect was specific because cell death induced by Fas-independent stimuli, namely methylprednisolone, was unaltered. Reduced Fas function due to overproduction of a soluble form of Fas has been suggested to play a role in pathogenesis of systemic lupus erythematosus (SLE).15 This overproduction was not involved in our patients with ALD because supernatants of their lymphocyte cultures did not inhibit cell death induced by anti-Fas MoAb in control lymphocytes. Moreover, the defect was different than that displayed by SLE because Fas function was in the normal range in T cells from three patients with SLE (data not shown). Extensive analysis of the Fas gene did not detect any causal mutation in patients with ALD, whereas extended rearrangements of the Fas gene or mutations in unscreened regions affecting the Fas expression (such as changes in the regulatory regions) were ruled out by the normal amount of protein.
Our data do not rule out the possibility that the Fas defect was the outcome of a lymphocyte functional state induced by unknown environmental factors. However, the pediatric onset, the similarities with ALPS, the consanguineous parents of patient 3, and especially the family history with autoimmunity of four patients favor a genetic component. Moreover, a maternal uncle of patient 4 died from B-cell lymphoma, which recalls the family history of lymphoma reported by Fisher et al for one of their patients with ALPS whose father and paternal uncle died from lymphoma.9 By contrast, none of the patients with typical chronic ITP had a family history with autoimmunity and/or splenomegaly and lymphoma. It is noteworthy that six of our seven patients with ALD were male, whereas fewer than one third of patients with typical chronic ITP were male.17 This bias might be due to hormone influence on the immune response or to some X-linked gene that regulates Fas function because the Fas gene has been mapped on human chromosome 10.18 These data are in line with those of Calderwood et al, who showed a significant predominance of males in patients with thrombocytopenia and neutropenia associated with lymphoadenopathy/splenomegaly.19
Despite the clinical pattern and the resemblance between the functional Fas defect of six of seven patients with ALD and that of ALPS, the lack of both Fas mutations and DN T-cell expansion indicates that the two diseases are different, but probably related. It is noteworthy that seven of 11 patients with ALPS were heterozygous for Fas mutations,9-11 whereas the parents heterozygous for the Fas mutation were generally healthy. Rieux-Laucat et al described two siblings with ALPS whose mother carried the Fas mutation, whereas the father showed decreased Fas ability to trigger cell death in vitro, but no Fas mutations. Both parents were healthy. Therefore, it has been suggested that heterozygous symptomatic patients with ALPS inherit a single mutation in the Fas gene from one parent and a second abnormality, not directly involving the Fas gene, from the other. Interaction of the two abnormalities can generate the overt disease. The ALD abnormality seems to involve the Fas signaling pathway downstream from sphingomyelinase because ceramide, the second messenger produced by this enzyme, did not overcome the inability of Fas to induce cell death in patients with ALD. It may be speculated that the ALD mutation may be similar to the “second mutation” thought to be involved in expression of ALPS in heterozygotes. Ceramide-induced cell death was normal in the three patients with ALPS that we analyzed. However, they were homozygous for Fas mutations and presumably did not need any further alteration of the Fas-induced PCD pathway.
An increasing number of molecules, namely hematopoietic cell phosphate (HCP), FAST, TIA-1, ICE, Ich-1, bcl-x, FAP-1, RIP, and FADD,20-27 have been involved in signaling via Fas, and their mutation could generate an lpr-like disease. In line with this possibility, HCP-mutant mev/mev mice exhibit increased Fas expression and decreased Fas-induced PCD, associated with development of an lpr-like autoimmune disease.20 However, these mice also develop severe combined immunodeficiency, which suggests that signaling mediated through the HCP pathway may be important at an early stage of hematopoietic cell development. Interestingly, mev/mev mice do not show the expansion of DN T cells that characterizes the disease showed by lpr mice and patients with ALPS, but which is absent in patients with ALD.
The several pathways used by Fas signaling could be the source of the different effects of Fas ligation in several cell types. For instance, Fas triggering mainly induces PCD in activated T cells, whereas it is costimulatory in resting T cells.1,2 Moreover, molecules involved in Fas signaling also may participate in signaling via other surface receptors. For instance, sphingomyelinase has been involved in signaling via several cytokine receptors.1 Therefore, it may be postulated that alterations at different levels in the Fas signaling pathway may induce clinical patterns that only partially overlap, as shown by mice carrying the lpr or the mev mutations. Patients with ALD may be a heterogeneous group of patients carrying mutations at different levels of the Fas signaling pathway. Expression of ALD might depend on the type and severity of mutations and the concomitant presence of mutations at different levels of the pathway.
ACKNOWLEDGMENT
We thank Professor L. Notarangelo (University of Brescia) for allowing us to read his paper before publication and for helpful discussion.
Supported by TELETHON (Rome) Grant E160, Associazione Italiana Ricerca sul Cancro (AIRC, Milan), AIDS Project (Istituto Superiore di Sanità, Rome), and CNR (Rome).
Address reprint requests to Umberto Dianzani MD, PhD, Dipartimento di Scienze Mediche, Università di Torino sede di Novara, via Solaroli 17, 28100 Novara, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal