Abstract
The factors that control migration of mast cells to sites of inflammation and tissue repair remain largely undefined. Whereas several recent studies have described chemotactic factors that induce migration of murine mast cells, only stem cell factor (SCF ) is known to induce migration of human mast cells. We report here that the anaphylatoxins C3a and C5a are chemotactic factors for the human mast cell line HMC-1, human cord blood-derived mast cells (CBMC) and cutaneous mast cells in vitro. The presence of an extracellular matrix protein, laminin, was required for chemotaxis in response to complement peptides. Migration of mast cells towards C3a and C5a was dose-dependent, peaking at 1 μg/mL (100 nmol/L), and was inhibited by specific antibodies. Pretreatment with pertussis toxin inhibited the anaphylatoxin-mediated migration of HMC-1 cells, indicating that Gi proteins are involved in complement-activated signal transduction pathways in human mast cells. Both C3a and C5a also induced a rapid and transient mobilization of intracellular free calcium ([Ca2+]i ) in HMC-1 cells. Besides SCF, other chemotactic factors tested, such as interleukin-3, nerve growth factor, transforming growth factor β, RANTES (regulated upon activation, normal T cell expressed and secreted), monocyte chemotactic protein-1 (MCP-1), MCP-2, MCP-3, macrophage inflammatory protein-1α (MIP-1α), and MIP-1β, failed to stimulate migration of human mast cells. In summary, these findings indicate that C3a and C5a serve as chemotaxins for human mast cells. Anaphylatoxin-mediated recruitment of mast cells might play an important role in hypersensitivity and inflammatory processes.
MAST CELL ACCUMULATION has been described in different conditions, including allergic reactions, atopic diseases, rheumatoid arthritis, wound healing, angiogenesis, fibrosis, and host defense mechanisms against some parasites (for review, see Weber et al1). However, little is known concerning the mechanisms by which recruitment of mast cells to these sites is controlled. In murine mast cells, different factors, such as laminin,2 interleukin-3 (IL-3),3 the c-kit ligand stem cell factor (SCF ),4,5 transforming growth factor β (TGFβ),6 and monocyte chemotactic protein-1 (MCP-1)7 were found to promote mast cell migration. In contrast, in human mast cells, only SCF has been reported to activate chemotaxis,8 whereas other factors such as different growth factors and chemokines failed to stimulate migration of human mast cells.8 We propose here anaphylatoxins as an additional group of chemotactic factors through which human mast cells are recruited to sites of inflammation.
Activation of the complement system is a major defense mechanism of the immune system against microorganisms and antigen-antibody complexes (for review, see Burger and Zilow9 and Köhl and Bitter-Suermann10 ). Upon activation and subsequent cleavage of the third and fifth complement component, the anaphylatoxins C3a and C5a are generated. C3a and C5a are involved in a variety of inflammatory processes. Elevated body fluid levels of C3a or C5a have been reported in wasp-sting anaphylaxis,11 sepsis,12 systemic lupus erythematosus, rheumatoid arthritis, and other diseases.9,10 Biologic functions of C3a and C5a include contraction of smooth muscles,13 activation of guinea pig platelets,14 and different immunoregulatory effects.15,16 In human skin, intradermal injections of C3a, C5a, and, to a lesser extent, of C5a-des Arg induce a dose-dependent wheal and flare reaction.17-19 Histologically, these reactions are characterized by perivascular neutrophilic infiltrates and degranulation of dermal mast cells. In vitro, C5a is a potent chemotactic agent for neutrophils,20 eosinophils,21 basophils, and monocytes. C3a activates migration of eosinophils,22 but does not stimulate chemotaxis of neutrophils.20 Moreover, both anaphylatoxic fragments induce mediator release from basophils23,24 as well as from different subpopulations of mast cells.24-27
In the present study, we found that C3a and C5a are highly active mediators in stimulating chemotaxis and mobilization of cytosolic free [Ca2+]i of the human mast cell line HMC-1, cord blood-derived mast cells (CBMC), and also of cutaneous mast cells.
MATERIALS AND METHODS
Reagents.Laminin, collagen type I, vitronectin, and TGFβ were purchased from GIBCO BRL (Gaithersburg, MD); fibronectin and nerve growth factor (NGF ) from Boehringer Mannheim (Mannheim, Germany); SCF, IL-6, RANTES, MCP-1, MCP-2, MCP-3, macrophage inflammatory protein-1α (MIP-1α), and MIP-1β from Pepro Tech Inc (Rocky Hill, NJ); IL-3 from Hermann Biermann (Bad Nauheim, Germany); pertussis toxin from Sigma Chemical Co (St Louis, MO); and the anti-C5a receptor monoclonal antibody (MoAb) S5/128 from Serotec (distributed by Camon Labor-Service, Wiesbaden, Germany).
Preparation of complement peptides and MoAbs to C3a and C5a.Human C3a was purified from EGTA/plasma as previously described.29 Briefly, EGTA/plasma was activated with zymosan (Sigma). After centrifugation and cation exchange chromatography, C3a-containing fractions as identified in a dot-blot assay using the C3a/C3a-des Arg-specific MoAb H1330 were pooled and further purified by a Mono S-column. C3a fractions were tested for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biologic activity was determined by measurement of adenosine triphosphate release from guinea pig platelets. Preincubation with MoAb H13 completely blocked the C3a-mediated adenosine triphosphate release. There was no C5a detectable when the C3a preparations were controlled with an enzyme-linked immunosorbent assay using MoAbs to human C5a (kindly provided by Dr J. Oppermann, Göttingen, Germany). Recombinant human (rh) C5a and rhC5a-des Arg were produced and purified exactly as described.31 For several experiments, rhC5a was also obtained from Sigma. Mouse MoAbs to C3a (H13) and to C5a (561) were isolated as described earlier.29 32
Cells.HMC-1 is an immature mast cell line derived from a patient with mast cell leukemia (kindly provided by Dr J. H. Butterfield, Mayo Clinic, Rochester, MN).33 HMC-1 cells were maintained in Basal Iscove's medium, containing 10% fetal calf serum, 2 mmol/L L-glutamine, antibiotics (all from Seromed, Berlin, Germany), and 1.2 mmol/L monothioglycerol (Sigma).
Mononuclear cells were obtained from heparinized umbilical cord blood as described.8,34 35 Briefly, cells were suspended in RPMI 1640 medium (GIBCO BRL) supplemented with 10% fetal calf serum, 2 mmol/L L-glutamine, 50 μmol/L 2-mercaptoethanol (2-ME), and antibiotics. The cell suspensions were seeded in 10-mL plastic flasks at a density of 106 cells/mL and SCF (100 ng/mL) and IL-6 (70 ng/mL) were added to the cultures. One half of the culture medium was replaced once weekly with freshly prepared medium containing SCF and IL-6. Mast cells developed in the cultures were characterized by staining of metachromatic granules with toluidine blue. After 5 to 7 weeks, the cultures contained greater than 90% CBMC.
Cutaneous mast cells were dispersed from human chopped breast skin (surgical specimens removed for cosmetic surgery) by enzymatic digestion in PIPES (Sigma) buffer containing 0.03% human serum albumin (Pfrimmer, Erlangen, Germany), 0.1% glucose, 1.5 mg/mL collagenase (Sigma), 0.75 mg/mL hyaluronidase (Sigma), and antibiotics for 4 hours in a shaking water bath at 37°C.36 Mast cells were then separated by filtration through nylon gauze (150 μm), washed twice in PIPES buffer, and further purified by a multidensity Percoll (Seromed) gradient. Cell viability was estimated by trypan blue staining, and the percentage of mast cells was assessed by toluidine blue staining. Mast cell suspensions used in further experiments consisted of greater than 70% mast cells. Contaminating cells were mainly fibroblasts and endothelial cells. Before use in chemotaxis experiments, cutaneous mast cells were cultured in RPMI 1640 supplemented with SCF (100 ng/mL), 25 mmol/L HEPES, and antibiotics for 2 to 4 days.
Adhesion assay.Adhesion of HMC-1 cells was quantitated in vitro as previously described.37 Briefly, wells in 96-well plates were coated overnight at 4°C with various concentrations of human laminin, fibronectin, collagen type I, vitronectin, or phosphate-buffered saline (PBS)/3% bovine serum albumin (BSA) only. Plates were then gently washed and remaining binding sites were blocked by adding PBS/3% BSA for 1 hour at 37°C. A volume of 100 μL of HMC-1 cells (4 × 104 cells/mL) was added to each precoated well, and plates were further incubated for 1 hour at 37°C. Unbound cells were removed by gentle washing with PBS. The number of adherent cells was determined by measurement of the activity of the lysosomal enzyme N-acetyl-β-d-hexosaminidase, as described by Landegren.38 Absorbance was measured at 405 nm. Using a standard curve for HMC-1 cell suspensions (4 × 104 cells/well to 1.25 × 103 cells/well) as reference, the percentage of adhesion was calculated as follows: % Adhesion = 100 × (M − B)/(T − B), where M is the number of matrix-adherent cells, B is the number of BSA-adherent cells, and T is the total number of cells seeded. All experiments were performed in triplicate. Results are expressed as mean ± SD for the stated number of wells.
Chemotaxis assay.Migration was measured by Boyden's blind-well chamber technique using 48-well microchambers (Neuroprobe, Cabin John, MD). A volume of 30 μL of chemotactic stimuli in Basal Iscove/1% BSA or Basal Iscove/1% BSA alone was placed in the lower wells of the chamber. The lower compartment of the chamber was then separated from the upper compartment with an 8-μm pore size polycarbonate filter (Neuroprobe) coated for 1 hour at room temperature with 10 μg/mL laminin before use in the assay. HMC-1 cells and CBMC were suspended at 2 × 106 cells/mL and cutaneous mast cells at 5 × 105 cells/mL in Basal Iscove/1% BSA. A volume of 50 μL of mast cells was placed in each of the upper wells. For blocking experiments with the anti-C5a receptor MoAb, cells were first incubated with the MoAb for 90 minutes at 37°C, washed twice, and then used in the chemotaxis assay. The anticomplement MoAbs against C3a or C5a were instead added to the complement peptides in the lower wells of the chamber. The entire chamber was then incubated for 150 minutes at 37°C, and the filters were carefully scraped of nonmigrating cells, fixed, stained with Mayers Hemalaun solution (Merck Co, Darmstadt, Germany), and mounted between two glass slides by routine histologic methods. The number of cells migrating through the filter was counted microscopically. In experiments with suspensions of CBMC or cutaneous mast cells, only granule-containing mast cells were counted. Cell migration was calculated as the average number of five randomly selected high power fields per well. Spontaneous migration to Basal Iscove/1% BSA served as control and was designated as 100% migration for each experiment. All experiments were performed in triplicate wells. Results are expressed as the mean ± SEM for the stated number of experiments. For C5a and the anti-C5a receptor MoAb, less than three triplicate experiments were performed at one concentration. Results are then expressed as the mean ± SEM for the stated number of wells.
Pretreatment with pertussis toxin.HMC-1 cells were incubated with 2 μg/mL of pertussis toxin in complete medium for 90 minutes at 37°C. The cells were then washed twice and resuspended in complete medium before use in the chemotaxis and [Ca2+]i assay.
[Ca2+]i changes.HMC-1 cells were loaded with fura-2 by incubation for 30 minutes at room temperature with fura-2/AM (0.4 nmol/L/106 cells; Calbiochem-Novabiochem Co, San Diego, CA) in 25 mmol/L HEPES, pH 7.4, containing 125 mmol/L NaCl, 5 mmol/L KCl, 2 mmol/L CaCl2 , 1.2 mmol/L MgSO4 , 1.2 mmol/L KH2PO4 , and 6 mmol/L glucose. After washing, the cells were resuspended in the same buffer at 1 × 107 cells/mL, and [Ca2+]i-related fluorescence changes were determined after stimulation with anaphylatoxins. For blocking experiments, HMC-1 cells were either pretreated with the anti-C5a receptor MoAb for 90 minutes at 37°C or stimulated simultaneously with complement peptides and MoAbs against C3a or C5a. After each measurement, maximal and minimal fluorescence was calibrated by the addition of 1% Triton X-100 followed by 10 mmol/L EGTA, and the distance between minimal and maximal fluorescence was referred to as 100% fura-2 saturation. [Ca2+]i changes are expressed as the percentage of total fura-2 saturation.
Statistical analysis.The arithmetic means of percentage responses were compared with the control (100%) with the use of a one-sample t-test (two tail). For inhibition experiments, pairs of arithmetic means were compared with the use of the two sample t-test (two-tailed).
RESULTS
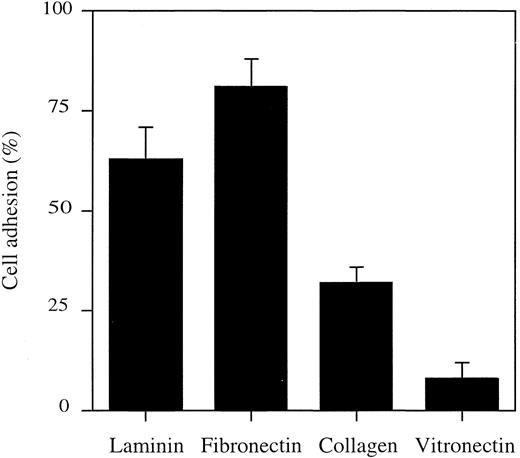
Adhesion of HMC-1 cells to extracellular matrix components.In initial experiments, HMC-1 cells failed to migrate through uncoated filters (data not shown). Because the migration of different cell types has been reported to depend on their adhesion to various ligands,7 the ability of HMC-1 cells to attach to different extracellular matrix components was tested (Fig 1). HMC-1 cells showed marked adhesion to laminin and fibronectin, whereas adhesion to collagen type I and vitronectin was less prominent. As we have recently shown,37 adhesion to fibronectin is accompanied by pronounced spreading of cells, whereas less spreading has been found upon adhesion to laminin. Because cell spreading might influence chemotaxis, laminin was chosen for precoating polycarbonate filters in all chemotaxis experiments.
Adhesion of HMC-1 cells to various extracellular matrix proteins. Wells in 96-well plates were coated with different extracellular matrix proteins at 10 μg/mL. HMC-1 cells were added, and the plates were incubated for 1 hour at 37°C. Plates were gently washed, and the number of adherent cells was determined by measurement of the endogenous hexosaminidase. Adhesion is expressed as the percentage of the total number of cells seeded, corrected for spontaneous adhesion. Data represent the mean ± SD of three experiments performed in triplicate (total n = 9). Spontaneous adhesion to BSA-coated wells was less than 5%.
Adhesion of HMC-1 cells to various extracellular matrix proteins. Wells in 96-well plates were coated with different extracellular matrix proteins at 10 μg/mL. HMC-1 cells were added, and the plates were incubated for 1 hour at 37°C. Plates were gently washed, and the number of adherent cells was determined by measurement of the endogenous hexosaminidase. Adhesion is expressed as the percentage of the total number of cells seeded, corrected for spontaneous adhesion. Data represent the mean ± SD of three experiments performed in triplicate (total n = 9). Spontaneous adhesion to BSA-coated wells was less than 5%.
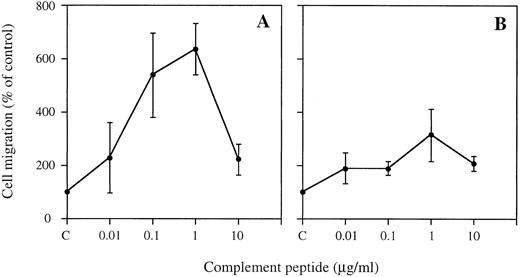
Migration of HMC-1 cells to complement peptides.The mean ± SEM of the spontaneous migration of HMC-1 cells to medium/1% BSA was 9.4 ± 2.1 cells/high power field (n = 22). Spontaneous migration to medium/1% BSA was designated as 100% migration. Both C3a and C5a stimulated migration of HMC-1 cells in a dose-dependent manner (Fig 2). Maximal responses were obtained at 1 μg/mL (100 nmol/L) of peptide. The overall mean response to the different concentrations of C3a was 494% ± 36% (P < .001), whereas the corresponding response to C5a was 222% ± 19% (P < .001). The desarginylated complement fragment C5a-des Arg also induced migration of HMC-1 cells, although migration towards C5a-des Arg was not significant (256% ± 60% at 1 μg/mL, data not shown). These results indicate that the anaphylatoxins C3a and C5a serve as chemotactic factors for HMC-1 cells.
Migration of HMC-1 cells to C3a (A) and C5a (B). Chemotactic response of HMC-1 cells to complement peptides was assayed in 48-well Boyden chambers. Various concentrations (1 nmol/L to 1 μmol/L) of C3a and C5a were placed in the lower wells of the chamber. The wells were covered with a laminin-coated (10 μg/mL) filter, and HMC-1 cells at 2 × 10 6 cells/mL were added to the upper wells of the chamber. Chambers were incubated for 150 minutes at 37°C, and filters were fixed, stained, and mounted using routine histologic methods. Mast cell migration was quantitated by counting the number of mast cells migrating through the filter. Spontaneous migration to medium/1% BSA served as control and was referred to as 100% migration. Data represent the mean ± SEM of 3 to 13 triplicate experiments for C3a (A; n = 3 to 13) and the mean ± SEM of 2 to 10 triplicate experiments for C5a (B; total n = 6 to 10).
Migration of HMC-1 cells to C3a (A) and C5a (B). Chemotactic response of HMC-1 cells to complement peptides was assayed in 48-well Boyden chambers. Various concentrations (1 nmol/L to 1 μmol/L) of C3a and C5a were placed in the lower wells of the chamber. The wells were covered with a laminin-coated (10 μg/mL) filter, and HMC-1 cells at 2 × 10 6 cells/mL were added to the upper wells of the chamber. Chambers were incubated for 150 minutes at 37°C, and filters were fixed, stained, and mounted using routine histologic methods. Mast cell migration was quantitated by counting the number of mast cells migrating through the filter. Spontaneous migration to medium/1% BSA served as control and was referred to as 100% migration. Data represent the mean ± SEM of 3 to 13 triplicate experiments for C3a (A; n = 3 to 13) and the mean ± SEM of 2 to 10 triplicate experiments for C5a (B; total n = 6 to 10).
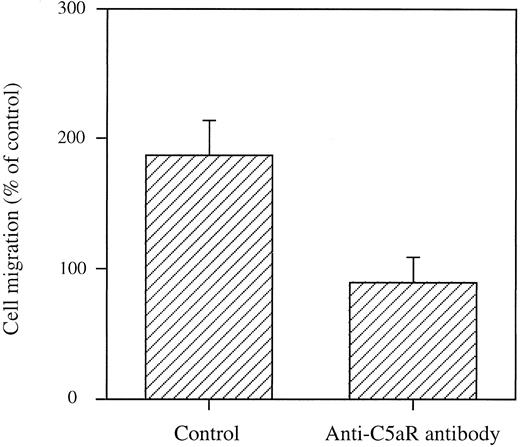
To determine the specificity of anaphylatoxin-induced chemotaxis of HMC-1 cells, we studied the effect of a MoAb against the C5a receptor (CD88) as well as the effect of MoAbs against C3a and C5a on migration in response to complement peptides. Both the anti-C5a receptor MoAb and the anti-complement MoAbs inhibited migration towards anaphylatoxins (Fig 3, data shown for the anti-C5a receptor MoAb, P < .05).
Inhibition of mast cell migration to C5a by an anticomplement receptor MoAb. HMC-1 cells were incubated for 90 minutes with the anti-C5a receptor MoAb S5/1 at 5 × 10−7 mol/L. Cells were then washed and migration to 0.1 μg/mL (10 nmol/L) C5a was examined in Boyden chambers. Results are expressed as mean ± SEM of 2 to 10 experiments performed in triplicate (total n = 6 to 10). No migration was observed when cells were stimulated with MoAbs alone.
Inhibition of mast cell migration to C5a by an anticomplement receptor MoAb. HMC-1 cells were incubated for 90 minutes with the anti-C5a receptor MoAb S5/1 at 5 × 10−7 mol/L. Cells were then washed and migration to 0.1 μg/mL (10 nmol/L) C5a was examined in Boyden chambers. Results are expressed as mean ± SEM of 2 to 10 experiments performed in triplicate (total n = 6 to 10). No migration was observed when cells were stimulated with MoAbs alone.
To evaluate whether anaphylatoxin-mediated migration of HMC-1 cells is due to a chemotactic (directional) rather than a chemokinetic (random) response, checkerboard analysis was performed with C3a and C5a. In limited experiments, checkerboard analysis showed chemotactic movement of HMC-1 cells in response to both C3a and C5a (data not shown).
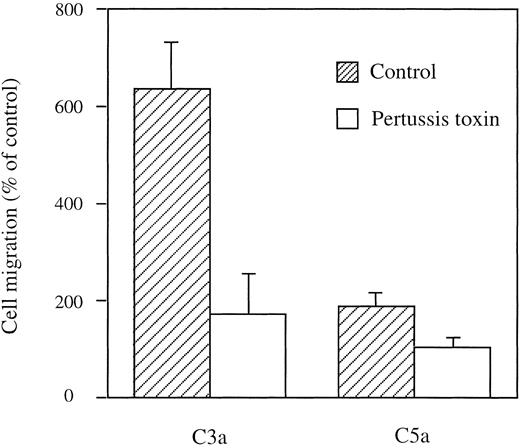
To examine whether complement-induced stimulation is mediated through guanine nucleotide-binding proteins (G proteins), HMC-1 cells were incubated with pertussis toxin before use in the chemotaxis assay. Pretreatment with pertussis toxin partially inhibited the migratory response to C3a (75% inhibition, P = .05) and completely inhibited migration to C5a (P < .05) compared with untreated controls (Fig 4).
C3a and C5a stimulate migration of HMC-1 cells via a pertussis toxin-sensitive G protein. After treatment of HMC-1 cells with pertussitoxin at 2 μg/mL for 90 minutes, cells were stimulated with C3a (1 μg/mL, 100 nmol/L) and C5a (0.1 μg/mL, 10 nmol/L) in Boyden chambers. Results are expressed as mean ± SEM of 3 to 13 experiments (n = 3 to 13).
C3a and C5a stimulate migration of HMC-1 cells via a pertussis toxin-sensitive G protein. After treatment of HMC-1 cells with pertussitoxin at 2 μg/mL for 90 minutes, cells were stimulated with C3a (1 μg/mL, 100 nmol/L) and C5a (0.1 μg/mL, 10 nmol/L) in Boyden chambers. Results are expressed as mean ± SEM of 3 to 13 experiments (n = 3 to 13).
In further experiments, other chemotactic factors, including the growth factors SCF, IL-3, NGF, and TGFβ, as well as the CC chemokines RANTES, MCP-1, MCP-2, MCP-3, MIP-1α, and MIP-1β were tested for their potential to induce migration of HMC-1 cells. Only SCF was found to weakly stimulate mast cell migration (Table 1, P > .2). All other growth factors and chemokines tested failed to promote chemotaxis of HMC-1 cells (data not shown). SCF, IL-3, NGF, and chemokines were tested within a concentration range of 0.1 to 1 μg/mL; TGFβ was used from 0.1 to 10 ng/mL.
Migration of HMC-1 Cells to C3a, C5a, and SCF
| Stimulus . | Migration (% of control) . | SEM . | n . |
|---|---|---|---|
| 1 μg/mL C3a | 633.14 | 96.68 | 13 |
| 100 ng/mL C3a | 536.50 | 159.36 | 8 |
| 1 μg/mL C5a | 313.47 | 98.39 | 7 |
| 100 ng/mL C5a | 187.27 | 26.98 | 10 |
| 100 ng/mL SCF | 137.56 | 32.91 | 4 |
| Stimulus . | Migration (% of control) . | SEM . | n . |
|---|---|---|---|
| 1 μg/mL C3a | 633.14 | 96.68 | 13 |
| 100 ng/mL C3a | 536.50 | 159.36 | 8 |
| 1 μg/mL C5a | 313.47 | 98.39 | 7 |
| 100 ng/mL C5a | 187.27 | 26.98 | 10 |
| 100 ng/mL SCF | 137.56 | 32.91 | 4 |
Migratory responses of HMC-1 cells to different chemotactic stimuli were assayed in Boyden chambers. All experiments were performed in triplicate.
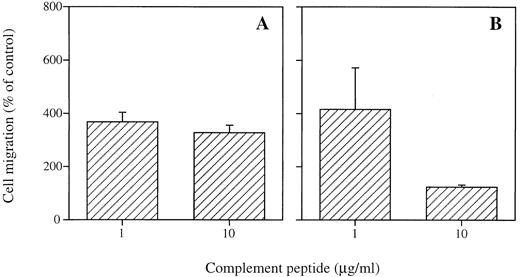
Migration of CBMC and purified skin mast cells to complement peptides.To investigate whether complement-mediated migration also occurred in other types of human mast cells, migratory activities of CBMC and purified skin mast cells towards C3a and C5a were determined (Fig 5, data shown for CBMC). As shown in Fig 5A, CBMC from two different cultures both showed chemotactic activity towards C3a at 1 μg/mL (P < .001) and 10 μg/mL (P < .001). C5a stimulated migration of CBMC at 1 μg/mL (P = .07), although migration at 10 μg/mL was less marked (P = .1, Fig 5B). Triplicate experiments were performed with highly purified skin mast cells from two different donors to evaluate migratory responses of cutaneous mast cells to C3a and C5a at 1 μg/mL (data not shown). In both experiments, skin mast cells migrated towards C5a (691% ± 129%). To a lesser extent, C3a also induced migration of cutaneous mast cells from one donor (445% ± 45%), whereas mast cells from the other donor did not respond to C3a (104% ± 45%).
Migration of CBMC to C3a (A) and C5a (B). CBMC were obtained from the mononuclear cell fraction of umbilical cord blood cultured for 5 to 7 weeks in RPMI 1640 supplemented with SCF (100 ng/mL) and IL-6 (70 ng/mL). Migration of CBMC to C3a (A) and C5a (B) was assayed in Boyden chambers. The mean ± SEM of the spontaneous migration of CBMC to medium/1% BSA was 13.1 ± 3.3 cells/high power field and was referred to as 100% migration. Data represent the mean ± SEM of two experiments performed in triplicate (total n = 6).
Migration of CBMC to C3a (A) and C5a (B). CBMC were obtained from the mononuclear cell fraction of umbilical cord blood cultured for 5 to 7 weeks in RPMI 1640 supplemented with SCF (100 ng/mL) and IL-6 (70 ng/mL). Migration of CBMC to C3a (A) and C5a (B) was assayed in Boyden chambers. The mean ± SEM of the spontaneous migration of CBMC to medium/1% BSA was 13.1 ± 3.3 cells/high power field and was referred to as 100% migration. Data represent the mean ± SEM of two experiments performed in triplicate (total n = 6).
[Ca2+]i mobilization of HMC-1 cells in response to complement peptides.In various cell types, migration was found to be associated with alterations in [Ca2+]i ,39 40 representing an early step in signal transduction. Therefore, [Ca2+]i changes of HMC-1 cells in response to anaphylatoxins were measured (Fig 6). C3a (Fig 6A) and C5a (Fig 6B) both induced a rapid and transient [Ca2+]i mobilization. Within a range from 0.01 to 1 μg/mL, these [Ca2+]i changes were concentration-dependent. C3a was more active than C5a with maximal [Ca2+]i elevations of 42.8% and 24.2% at 1 μg/mL, respectively. Again, C5a-des Arg also stimulated HMC-1 cells (data not shown). As shown in Fig 7, pretreatment with an anti-C5a receptor MoAb as well as the addition of MoAbs against C3a and C5a abrogated [Ca2+]i mobilization in response to 0.1 μg/mL of C3a and C5a in a dose-dependent manner (data shown for the anti-C3a MoAb), indicating a specific mechanism by which anaphylatoxins induce [Ca2+]i elevations in HMC-1 cells. In accordance with the chemotaxis studies, pretreatment with pertussis toxin partially abolished the [Ca2+]i response to C3a and completely abolished the C5a-mediated [Ca2+]i mobilization (data not shown).
Changes of the cytosolic free calcium concentration [Ca2+]i in HMC-1 cells induced by complement peptides. Fura-2–loaded HMC-1 cells were suspended at 1 × 107 cells/mL and monitored for fluorescence changes after stimulation with different concentrations of C3a (A) and C5a (B). [Ca2+]i changes are expressed as the percentage of total fura-2 saturation. Data represent the mean ± SEM of 3 to 11 experiments for C3a (A, n = 3 to 11) and the mean ± SEM of 4 to 12 experiments for C5a (B, n = 4 to 12).
Changes of the cytosolic free calcium concentration [Ca2+]i in HMC-1 cells induced by complement peptides. Fura-2–loaded HMC-1 cells were suspended at 1 × 107 cells/mL and monitored for fluorescence changes after stimulation with different concentrations of C3a (A) and C5a (B). [Ca2+]i changes are expressed as the percentage of total fura-2 saturation. Data represent the mean ± SEM of 3 to 11 experiments for C3a (A, n = 3 to 11) and the mean ± SEM of 4 to 12 experiments for C5a (B, n = 4 to 12).
Inhibition of [Ca2+]i changes in response to C3a by an anti-C3a MoAb. HMC-1 cells were stimulated simultaneously with C3a (0.1 μg/mL, 10 nmol/L) and anti-C3a MoAb H13 at different concentrations. Data represent the mean of 3 to 11 experiments (n = 3 to 11). No [Ca2+]i elevation was observed when cells were stimulated with MoAbs alone.
Inhibition of [Ca2+]i changes in response to C3a by an anti-C3a MoAb. HMC-1 cells were stimulated simultaneously with C3a (0.1 μg/mL, 10 nmol/L) and anti-C3a MoAb H13 at different concentrations. Data represent the mean of 3 to 11 experiments (n = 3 to 11). No [Ca2+]i elevation was observed when cells were stimulated with MoAbs alone.
DISCUSSION
In this study, we provide evidence that the anaphylatoxins C3a and C5a are potent chemotaxins for HMC-1 cells, CBMC, and human skin mast cells. Migration of these mast cells was dependent on the presence of an extracellular matrix protein such as laminin. Furthermore, C3a and C5a both induced a rapid and transient elevation of [Ca2+]i in HMC-1 cells.
C5a is one of the most potent chemotactic factors known for inflammatory cells.20,21 Recently, specific C5a receptors (CD 88) have been characterized on human neutrophils41 and eosinophils.42 In human mast cells, C5a has been shown to induce histamine release from skin and heart mast cells,24-27 but not from lung mast cells.24 A recent study by Füreder et al43 using indirect immunofluorescence has shown the expression of C5a receptors on skin and heart mast cells, as well as on HMC-1 cells, but not on lung, uterus, and tonsillar mast cells. This mast cell heterogeneity regarding anaphylatoxin-induced activation might be of particular clinical relevance. In contrast to the well-defined biologic effects of C5a on many different cell types, little is known about the effects of C3a. Specific activation by C3a has been shown primarily for guinea pig platelets,14 guinea pig macrophages,44 human basophils,23,45 and human eosinophils.22 In addition, C3a has also been reported to induce histamine release from human skin mast cells24,26,43 and peritoneal rat mast cells.27 46
The present study shows directional migration of mast cells towards C3a and C5a in different human mast cell systems (Figs 2 and 5). For most of the experiments, the immature human mast cell line HMC-1 was used as a model.33 Although HMC-1 cells have been reported not to express the high-affinity IgE receptor,47 this cell line resembles normal human mast cells in most other mast cell characteristics, including the expression of mast-cell related antigens such as c-kit (CD117) and the production of many cytokines such as tumor necrosis factor α, IL-1β, IL-3, IL-6, IL-8, MCP-1, and RANTES.47-49 In limited experiments, the results obtained with HMC-1 cells were confirmed with CBMC cultured in SCF and IL-634,35 as well as with purified skin mast cells. Whereas HMC-1 cells and skin mast cells are known to express the C5a receptor43,50 or, in addition, are able to be activated by anaphylatoxins,24,26,50 51 complement-mediated activation of CBMC has not been described before. Blocking experiments with an anti-C5a MoAb and anticomplement MoAbs confirmed the specificity of the anaphylatoxin-mediated stimulation of mast cells (Fig 3).
Pretreatment with pertussis toxin partially abolished the C3a-mediated activation of HMC-1 cells and completely abolished the C5a-mediated activation (Fig 4), indicating the involvement of Gi proteins in complement-activated signal transduction pathways in human mast cells. Whether C3a acts on mast cells via a specific receptor or whether nonspecific mechanisms, such as direct activation of G proteins,46 52 account for the stimulation by C3a remains to be studied in future experiments.
Anaphylatoxin-induced chemotaxis of mast cells was compared with migration towards the known chemotactic stimulus SCF (Table 1).8 Both complement peptides were more potent than SCF in inducing migration of HMC-1 cells, with C3a being the most potent chemoattractant. Other chemotactic stimuli tested, such as the CC chemokines RANTES, MCP-1, MCP-2, MCP-3, MIP-1α, and MIP-1β, as well as the growth factors IL-3, NGF, and TGFβ, failed to stimulate migration of HMC-1 cells. This, in part, is in agreement with the results of Nilsson et al,8 who have also found that IL-8, RANTES, MCP-1, MIP-1α, MIP-1β, IL-3, NGF, and the ligand to the tyrosine kinase receptor flt3/flk-2, flt3L, were unable to promote chemotaxis of HMC-1 cells and, with the exception of RANTES, of CBMC. In contrast, murine mast cells have been shown to migrate towards laminin,2 IL-3,3 SCF,4,5 TGFβ,6 platelet-derived growth factor-AB, vascular endothelial cell growth factor, basic fibroblast growth factor,53 C1q,54 RANTES, MCP-1, MIP-1α, and platelet factor-4.7 According to the different migratory responses to chemokines between human and murine mast cells, we and others have also observed species heterogeneity of mast cells regarding mediator release in response to chemokines.51,55 56
Chemotaxis of human mast cells towards anaphylatoxins was found to be dependent upon binding to an extracellular matrix protein, laminin. Nilsson et al8 have also shown that adhesion to matrix proteins was required for migration of human mast cells towards SCF. However, in the study of Nilsson et al,8 HMC-1 cells and CBMC have failed to adhere to laminin and also have not migrated on laminin-coated filters. Therefore, fibronectin has been used to coat the chemotaxis filters. Minor differences between the HMC-1 cells used in the two studies might explain the different results.37 By contrast, adhesion to laminin has been reported for human skin mast cells.57 Besides, in several studies investigating chemotaxis of murine mast cells, laminin has been proven to promote mast cell migration.3-5 7
In other cell types, migration was found to be associated with the mobilization of [Ca2+]i. Interactions with extracellular matrix components have been described to promote alterations in [Ca2+]i.39 Furthermore, [Ca2+]i changes directly affect the function of various actin-binding proteins, altering the organization of the actin network. Several Ca2+-sensitive kinases and phosphatases are associated with the regulation of the cytoskeletal structure.40 Integrin-mediated [Ca2+]i changes have been shown to facilitate migration of endothelial cells on vitronectin.39 Therefore, [Ca2+]i changes induced by complement fragments were measured in HMC-1 cells. C3a and C5a caused a dose-dependent rapid mobilization of [Ca2+]i (Fig 6). [Ca2+]i changes in response to complement fragments were transient, returning to the basic level often within 60 seconds. Kretzschmar et al45 have described two different patterns of [Ca2+]i mobilization for C3a and C5a in leukemia-derived basophils.45 Whereas they have noticed a rapid decrease of the [Ca2+]i response after stimulation with C3a, the C5a-induced [Ca2+]i elevation faded away slowly within several minutes. The characteristics of the [Ca2+]i changes that we observed in HMC-1 cells after stimulation with C3a and C5a were similar to C3a-induced [Ca2+]i changes in leukemia-derived basophils. Whether these different characteristics of [Ca2+]i mobilization in response to complement fragments are related to different binding and activation processes remains to be further investigated.
The present study shows that the anaphylatoxins C3a and C5a are highly active chemotactic factors for human mast cells. Directional migration of mast cells might play a critical role in a variety of pathologic processes, such as inflammation and fibrosis. In addition, migration of mast cells is presumably involved in the differentiation process from bone marrow-derived mast cell precursors to mature mast cells in peripheral tissues.58 Because anaphylatoxins are known to be elevated in the serum of patients with inflammatory diseases,10-12 mast cell migration towards complement might represent a crucial mechanism in inflammation rather than in the recruitment of mast cell precursors to tissue sites under physiologic conditions.
ADDENDUM
After the submission of this manuscript, two studies were published that partly confirm our data. Legler et al showed that HMC-1 cells bind 125I-C3a with low and high affinity, suggesting the existence of two receptors for C3a, and that C3a induces mobilization of [Ca2+]i in HMC-1 cells.59 Nilsson et al60 found that C3a and C5a induce chemotaxis and [Ca2+]i changes in HMC-1 cells. These investigators also showed that complement-mediated stimulation of HMC-1 cells is sensitive to pretreatment with pertussis toxin. Both reports are in accordance with our data describing anaphylatoxin-mediated chemotaxis and [Ca2+]i mobilization in HMC-1 cells. In this report, we provide evidence that complement-induced migration is not restricted to HMC-1 cells but also relevant to primary human mast cells such as CBMC and highly purified cutaneous mast cells.
ACKNOWLEDGMENT
We thank P. Dietl for excellent technical assistance and Dr D.W. Alling (National Institutes of Health, Clinical Center) for skillful assistance with statistical analysis.
Supported by a grant from the German Research Foundation, Deutsche Forschungsgemeinschaft (Zu 85/1-1).
Address reprint requests to Karin Hartmann, MD, National Institutes of Health, NIAID/Laboratory of Allergic Diseases, Bldg 10, Room 11C209, 10 Center Dr MSC 1881, Bethesda, MD 20892-1881.

![Fig. 6. Changes of the cytosolic free calcium concentration [Ca2+]i in HMC-1 cells induced by complement peptides. Fura-2–loaded HMC-1 cells were suspended at 1 × 107 cells/mL and monitored for fluorescence changes after stimulation with different concentrations of C3a (A) and C5a (B). [Ca2+]i changes are expressed as the percentage of total fura-2 saturation. Data represent the mean ± SEM of 3 to 11 experiments for C3a (A, n = 3 to 11) and the mean ± SEM of 4 to 12 experiments for C5a (B, n = 4 to 12).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2863/4/m_bl_0005f6.jpeg?Expires=1769088081&Signature=c9v9IR-Ox8YWXNnzKhMcVaaMvhZz1-YmqITswBGc0EQdJySt-ap8lPUY3plDsmIEUcqx3R8Ghwh18PCxF7mGPzJbDFA3HrWUjU-In8tsfkXgaPui~X2Y1BtVBxOqDasLZsjx3CeVgCOTUrB~GjocHrAKwD3YcwjJ74O2v837hW9mseIMUZ7iCKTy6V1a2M76wSLfkofcJihFp0xEZPsHjK0L2lqiMBSAZZc6HWpyyUuwFh~dUwMplR40v9e6xiC0z7td~b-AVjj7Jl2-xcSqDmI7dCTs0AQQZRVkvZIvfNB2olWNhyBSy6~kIZcDUO3OAOsyeOFMmCA1DjN8iyikBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Inhibition of [Ca2+]i changes in response to C3a by an anti-C3a MoAb. HMC-1 cells were stimulated simultaneously with C3a (0.1 μg/mL, 10 nmol/L) and anti-C3a MoAb H13 at different concentrations. Data represent the mean of 3 to 11 experiments (n = 3 to 11). No [Ca2+]i elevation was observed when cells were stimulated with MoAbs alone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2863/4/m_bl_0005f7.jpeg?Expires=1769088081&Signature=FhguCvPl7ZkA2RQPsR0G~CHFpN3h8raNF4qjIdsd04gZYr2~PcwUcB4PF8pnpzpoZb7-NZgDLtPIIkaHDFKSqVDffibXXY3dZsFw-QAUuEPtlE3~w18TLIyVrYLqu3wgNRqtxrPRSYN0RC53TmyXYB8zpqI2ibmkgHYWBMP3S0Tk~2jbUcFin4VIx2-r5DLP96yquHeTplZo4p63xa5v8SwYAO0za1n-z81nUqThzaWmpxosIQeLdG4FCcRuFWKIIU8oyP~tFS~4-Ro4cEl~b6pGkAVV1W5LR9TF2kwr-YGLGHkRFINcwwEqWEBbdBQi61XX2SoBwZLWqUDOJSByZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal