Abstract
Grb2/Ash and Shc are the adapter proteins that link tyrosine-kinase receptors to Ras and make tyrosine-kinase functionally associated with receptors and Ras in fibroblasts and hematopoietic cells. Grb2/Ash and Shc have the SH3, SH2, or phosphotyrosine binding domains. These domains bind to proteins containing proline-rich regions or tyrosine-phosphorylated proteins and contribute to the association of Grb2/Ash and Shc with other signaling molecules. However, there could remain unidentified signaling molecules that physically and functionally interact with these adapter proteins and have biologically important roles in the signaling pathways. By using the GST fusion protein including the full length of Grb2/Ash, we have found that c-Cbl and an unidentified 135-kD protein (pp135) are associated with Grb2/Ash. We have also found that they become tyrosine-phosphorylated by treatment of a human leukemia cell line, UT-7, with granulocyte-macrophage colony-stimulating factor (GM-CSF ). We have purified the pp135 by using GST-Grb2/Ash affinity column and have isolated the full-length complementary DNA (cDNA) encoding the pp135 using a cDNA probe, which was obtained by the degenerate polymerase chain reaction based on a peptide sequence of the purified pp135. The cloned cDNA has 3,958 nucleotides that contain a single long open reading frame of 3,567 nucleotides, encoding a 1,189 amino acid protein with a predicted molecular weight of approximately 133 kD. The deduced amino acid sequence reveals that pp135 is a protein that has one SH2, one SH3, and one proline-rich domain. The pp135, which contains two motifs conserved among the inositol polyphosphate-5-phosphatase proteins, was shown to have the inositol polyphosphate-5-phosphatase activity. The pp135 was revealed to associate constitutively with Grb2/Ash and inducibly with Shc using UT-7 cells stimulated with GM-CSF. In the cell lines derived from human chronic myelogenous leukemia, pp135 was constitutively tyrosine-phosphorylated and associated with Shc and Bcr-Abl. These facts suggest that pp135 is a signaling molecule that has a unique enzymatic activity and should play an important role in the signaling pathway triggered by GM-CSF and in the transformation of hematopoietic cells caused by Bcr-Abl.
MANY GROWTH FACTORS, including hematopoietic growth factors, regulate the proliferation, differentiation, and metabolic activities of their target cells by binding to their receptors on the cell surface. These stimuli cause tyrosine phosphorylation of the receptors including the common β chain of granulocyte-macrophage colony-stimulating factor (GM-CSF )/interleukin-3 (IL-3)/IL-5 receptors and several cellular proteins such as Shc, Vav, Raf, and mitogen-activated protein (MAP) kinase by the activation of receptor type and non–receptor type tyrosine kinases.1-3 It is considered that the tyrosine phosphorylation of these proteins is needed for the generation of the binding site for SH2 or phosphotyrosine binding (PTB) domains and to form signal transduction complexes including the adapter proteins.4 5
The adapter protein Shc is widely expressed in a variety of tissues and encodes three forms: p46, p52, and p66. The Shc contains the SH2 and PTB domains and can bind to the tyrosine-phosphorylated receptors such as receptors for epidermal growth factor (EGF ), nerve growth factor, and insulin.6-12 At the same time, Shc becomes tyrosine-phosphorylated on Tyr-317 by stimulation with growth factors or cytokines (EGF, IL-2, erythropoietin [Epo], GM-CSF, thrombopoietin [TPO], and others) and subsequently interacts with the SH2 domain of Grb2/Ash.13-19 This binding leads to the activation of Ras pathway, via the guanine nucleotide exchange factor, Sos, which constitutively binds to the SH3 domains of Grb2/Ash.
Grb2/Ash is a 25-kD protein composed of one SH2 and two SH3 domains in the following order: SH3-SH2-SH3.20,21 The SH2 domain of Grb2/Ash binds to tyrosine-phosphorylated proteins such as EGF receptor, Shc, IRS-1, and Syp.16,22-27 On the other hand, the SH3 domains of Grb2/Ash bind to the proline-rich domain of Sos,28,29 dynamin,30,31 and C3G,32 which regulate Ras or Ras-related proteins. Recently, it was reported that the SH3 domain of Grb2/Ash is also associated with c-Cbl, which becomes tyrosine-phosphorylated by a variety of stimulations such as Epo, GM-CSF, TPO, T-cell receptor cross-linking, and EGF.17 33-36
However, there could remain unidentified signaling molecules that physically and functionally interact with these adapter proteins and have biologically important roles in the signaling pathways. In this work, we have purified unidentified pp135, which is inducibly tyrosine-phosphorylated by the stimulation with GM-CSF and constitutively binds to Grb2/Ash in a human leukemia cell line, UT-7. We have cloned the complementary DNA (cDNA) for pp135 and determined the complete nucleotide sequence, which has shown that pp135 contains several known functional motifs such as two motifs conserved among the inositol polyphosphate-5-phosphatase proteins as well as one SH2, one SH3, and one proline-rich domain. Moreover, pp135 became associated with Shc in response to GM-CSF stimulation in UT-7 cells. In the cell lines derived from human chronic myelogenous leukemia (CML), pp135 was constitutively tyrosine-phosphorylated and associated with Shc and Bcr-Abl. These facts suggest that pp135 is a signaling molecule that has a unique enzymatic activity and may play an important role in the signaling pathway triggered by GM-CSF and in the oncogenic transformation of hematopoietic cells caused by Bcr-Abl.
MATERIALS AND METHODS
Cell lines and growth factors.UT-7 cells were maintained in RPMI 1640 medium containing 8% bovine serum and 10 ng/mL GM-CSF. Recombinant human GM-CSF and recombinant human Epo were supplied by Kirin Brewery Co Ltd (Tokyo, Japan) and Chugai Pharmacy Co Ltd (Tokyo, Japan), respectively.
Antibodies.Mouse monoclonal antibody to Grb2/Ash purchased from MBL (Nagoya, Japan) was used for the immunoblotting of Grb2/Ash. Mouse monoclonal antiphosphotyrosine antibody (anti-Py) 4G10 and rabbit polyclonal antibody to Shc were purchased from UBI (Lake Placid, NY). The anti-pp135 antibodies (anti-P6) were raised by immunizing rabbits with the GST-P6 fusion protein expressed in bacteria as described below and were used for immunoblotting and immunoprecipitation.
GST fusion proteins.The bacterial expression plasmids coding for GST fusion proteins containing the full-length, the N-terminal SH3 domain, or the SH2 domain of Grb2/Ash31 were generously provided by Dr T. Takenawa (University of Tokyo, Tokyo, Japan). The bacterial expression plasmids coding for GST fusion proteins containing the SH3 domains of Tec, Lyn, Fyn, or Abl were from Dr H. Mano, Dr T. Yamamoto, and Dr B. Mayer, respectively. The bacterial expression plasmids coding for GST fusion proteins containing the SH3 domains of Crk, Nck, Src, or Cas were also used in this study. The cDNA sequence encoding a polypeptide of amino acids 318 to 957 of pp135 was amplified by polymerase chain reaction (PCR) using the cDNA obtained as a template. The resulting PCR fragment was cloned into pGEX-2T vector (Pharmacia Inc, Uppsala, Sweden; GST-P6) and was expressed in XL-I Blue strain of Escherichia coli. Induction and purification of GST fusion proteins were performed as described before.33
Preparation of cell lysates.UT-7 cells were incubated in RPMI 1640 medium containing 0.1% bovine serum albumin (BSA) without serum and growth factors for 8 to 15 hours before stimulation with growth factors and then were resuspended in RPMI 1640 medium containing 100 μmol/L Na3VO4. The cells were treated with 10 ng/mL GM-CSF or 20 U/mL Epo for 5 minutes at 37°C and then lysed at 4°C for 20 minutes in the lysis buffer containing 20 mmol/L Tris/HCl (pH 8.0), 1% Nonidet P-40, 1 mmol/L phenylmethyl sulphonyl fluoride, 500 U/mL aprotinin, 2 mmol/L EDTA, 50 mmol/L NaF, and 1 μmol/L Na3VO4. Unsolubilized materials were removed by centrifugation at 15,000g at 4°C for 10 minutes.
Affinity precipitation with GST fusion proteins and immunoprecipitation.Lysates from 1 × 107 cells were mixed with 40 mg of the fusion protein noncovalently coupled to glutathione-agarose beads (Sigma Chemical Co, St Louis, MO) for 3 hours at 4°C. Beads were washed with the lysis buffer before resuspension in Laemmli's sample buffer. To immunoprecipitate the proteins, lysates from 1 × 107 cells were incubated with the antibody for 3 hours at 4°C. The immunoprecipitates were collected with protein A-sepharose (Sigma Chemical Co). All the immunoprecipitates were washed intensively with the lysis buffer before resuspension in Laemmli's sample buffer.
Immunoblotting.Samples were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene difluoride filters (Millipore, Bedford, MA). Filters were blocked with the buffer containing 10 mmol/L Tris/HCl (pH 7.4), 150 mmol/L NaCl, 5% BSA, and 0.05% Triton X-100. Filters were sequentially incubated with the specific antibody and with horseradish peroxidase–coupled second antibodies (antirabbit or antimouse Fc; Amersham, Buckinghamshire, UK). Development by enhanced chemiluminescence was performed using the standard protocol.
Purification of pp135.After the lysates from 1 × 109 UT-7 cells were pretreated with GST protein noncovalently coupled to glutathione-agarose beads, the unbound fractions were mixed with the GST-Grb2/Ash fusion protein noncovalently coupled to glutathione-agarose beads. Successively, the eluent from the GST-Grb2/Ash column containing pp135 was subjected to SDS-PAGE and blotted onto the polyvinylidene difluoride membrane. After being visualized by ponceau-S, an isolated 135-kD band (approximately 20 pmol) was cut out and in situ–digested with lysylendopeptidase Achromobactor protease I (Wako, Tokyo, Japan) or endproteinase Asp-N (Boehringer Mannheim, Mannheim, Germany). Sequences of six digested fragments were determined by amino acid sequence analysis.
Cloning of pp135.For preparing the screening probes, RNA-based PCR (reverse transcriptase PCR [RT-PCR]) was performed by oligo(dT)-priming cDNA synthesis from the total RNA of UT-7 cells, followed by PCR with degenerate sense and antisense oligonucleotide primers synthesized based on the three-peptide sequence. One pair of primers was DN-11 sense (5′-GAYCARYTIATHGARTTYTA-3′ ) and DN-9 antisense (5′-AAICCDATIACRTADATRTC-3′ ), and the other was DN-11 sense and AP-6 antisense (5′-TCIGCRAAIACRTAYTCYTT-3′ ). The PCR fragment 1 (1,117 bp) and fragment 2 (898 bp) were subcloned into pCRTMII vector using Original TA Cloning Kit (Invitrogen, San Diego, CA) and sequenced, and the nucleotide sequences were confirmed to match with the corresponding original amino acid sequences. The EcoRI fragment of pCRTMII vector containing PCR fragment 1 was labeled with Megaprime DNA labeling system (Amersham) and was used as the probe to screen the cDNA library of SKH1, a human leukemic cell line.37 Six overlapping clones were isolated, and nested deletions of two clones were generated using exonuclease III according to the manufacturer's instructions (Pharmacia) and sequenced by the cycle sequencing method using Thermo Sequenase Kit (Amersham), Cy5-labeled M13-reversal and universal primers, and A.L.F. DNA Sequencer II (Pharmacia). The sequence in this report has been deposited in the GenBank database (accession no. U53470).
Assay of inositol-5-phosphatase activity.Inositol-5-phosphatase activity was assayed at 37°C for 15 minutes in 100 μL of 50 mmol/L Tris/HCl buffer (pH 7.4)/3 mmol/L MgCl2/20 nCi [3H]-inositol (1,3,4,5)-P4 (Dupont-New England Nuclear, Boston, MA)/10 mmol/L inositol (1,3,4,5)-P4 (Boehringer Mannheim) containing immunoprecipitates from UT-7 cells. The reaction was initiated by the addition of the substrate and was terminated by adding 1 mL of 0.1 mol/L ammonium formate/0.1 mol/L formic acid. The [3H]-inositol polyphosphates were then separated on Dowex 1 (BioRad Laboratories, Richmond, CA) anion exchange chromatography columns38 and quantified by liquid scintillation spectrometry.
RESULTS
As shown in Fig 1A, several tyrosine-phosphorylated proteins, including pp130 and pp135, in the lysates of GM-CSF–treated UT-7 cells were found to bind to GST-Grb2/Ash in vitro, and their phosphorylation was dependent on GM-CSF stimulation. Because these tyrosine-phosphorylated bands were not observed when GST protein was used instead of GST-Grb2/Ash (data not shown), these tyrosine-phosphorylated proteins were thought to bind specifically to Grb2/Ash protein in vitro. Using anti-Shc polyclonal antibody, the 52-kD (pp52) and 66-kD proteins (pp66) were identified as Shc (data not shown), which is one of the well-known molecules that binds to Grb2/Ash and has an important role in the signal transduction of many cytokines including EGF, Epo, GM-CSF, TPO, and IL-3.13,16,17 As previously reported, the 130-kD protein was identified as c-Cbl, which becomes tyrosine-phosphorylated with stimulation of cytokines such as GM-CSF, Epo, IL-3, TPO, and EGF,17,33-35 and binds to the many signaling molecules including EGF-receptor (EGFR), Grb2/Ash, Crk, Nck, p85 PI3 kinase, and v-Src.17,33-36 39-44 Both c-Cbl and pp135 constitutively bound to the SH3 domain but not to the SH2 domain of Grb2/Ash and became tyrosine-phosphorylated with stimulation of GM-CSF in UT-7 cells (Fig 1B). However, the nature of pp135 was still unknown. To identify the Grb2/Ash-binding protein, pp135, we purified this protein and cloned its cDNA.
(A) Proteins associated with Grb2/Ash in vitro and their tyrosine phosphorylation. The lysates from UT-7 cells unstimulated (lane 2) or stimulated with GM-CSF (lane 1) were mixed with the GST fusion protein including the full-length Grb2/Ash noncovalently coupled to glutathione-agarose beads. Beads were resuspended in Laemmli's sample buffer, subjected to SDS-PAGE, and immunoblotted with anti-Py (4G10). Molecular weight markers, indicated at the left, are given in kilodaltons. The arrows indicate the position of pp52, pp66, pp130, and pp135. (B) In vitro association of pp130 and pp135 with the SH3 domain of Grb2/Ash and tyrosine phosphorylation of pp130 and pp135. The lysates from UT-7 cells unstimulated (lanes 1 and 3) or stimulated with GM-CSF (lanes 2 and 4) were mixed with GST fusion protein including the SH2 domain (lanes 1 and 2) or the N-terminal SH3 domain (lanes 3 and 4) of Grb2/Ash noncovalently coupled to glutathione-agarose beads. The resulting precipitates were resuspended in Laemmli's sample buffer, subjected to SDS-PAGE, and immunoblotted with anti-Py (4G10). Molecular weight markers, indicated at the left, are given in kilodaltons. The arrows indicate the position of pp52, pp66, pp130, and pp135.
(A) Proteins associated with Grb2/Ash in vitro and their tyrosine phosphorylation. The lysates from UT-7 cells unstimulated (lane 2) or stimulated with GM-CSF (lane 1) were mixed with the GST fusion protein including the full-length Grb2/Ash noncovalently coupled to glutathione-agarose beads. Beads were resuspended in Laemmli's sample buffer, subjected to SDS-PAGE, and immunoblotted with anti-Py (4G10). Molecular weight markers, indicated at the left, are given in kilodaltons. The arrows indicate the position of pp52, pp66, pp130, and pp135. (B) In vitro association of pp130 and pp135 with the SH3 domain of Grb2/Ash and tyrosine phosphorylation of pp130 and pp135. The lysates from UT-7 cells unstimulated (lanes 1 and 3) or stimulated with GM-CSF (lanes 2 and 4) were mixed with GST fusion protein including the SH2 domain (lanes 1 and 2) or the N-terminal SH3 domain (lanes 3 and 4) of Grb2/Ash noncovalently coupled to glutathione-agarose beads. The resulting precipitates were resuspended in Laemmli's sample buffer, subjected to SDS-PAGE, and immunoblotted with anti-Py (4G10). Molecular weight markers, indicated at the left, are given in kilodaltons. The arrows indicate the position of pp52, pp66, pp130, and pp135.
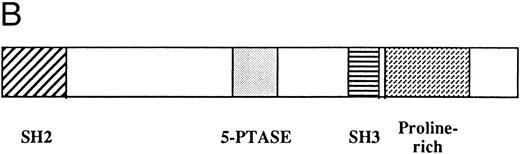
The pp135 protein was purified from UT-7 cell lysates using the GST-Grb2/Ash affinity column by the procedure described in the Materials and Methods. The six peptide sequences derived from the purified pp135 were determined by the amino acid sequence analysis. None of the peptide sequences were identical to the proteins in the database of the National Center of Biotechnology Information (N.C.B.I.). Using the DN-11, DN-9, and AP-6 oligonucleotide sequences as PCR primers, we obtained two DNA fragments from the cDNA prepared from UT-7 cells. The amino acid sequences deduced from the nucleotide sequences of the two DNA fragments contained the sequences completely identical to the original peptide sequences. The longer DNA fragment was used as a probe to screen a cDNA library of a human leukemic cell line, SKH1. Two cDNAs of the six obtained clones were sequenced and revealed to contain 3,958 nucleotides, 3,567 of which encoded an open reading frame of 1,189 amino acids with a predicted molecular size of approximately 133 kD (Fig 2). The deduced amino acid sequence contained the sequences of the six obtained peptide sequences. The cDNA sequence around the first methionine was in good agreement with Kozak's consensus sequence, and therefore, the first methionine is considered to be the translation initiation one.45,46 The homology search analysis based on the deduced amino acid sequence revealed that there were several interesting motifs within pp135. There was a consensus sequence for the SH2 domain (amino acids 5 to 102) that was most similar to that of Abl. The consensus sequence for the SH3 domain was also identified within pp135 (amino acids 861 to 909),4,47 but it is necessary to confirm whether this region is, in fact, capable of binding to some proline-rich polypeptides. Two sequences, INPNY and ENPLY, within pp135 matched the consensus NPXY motif to which the PTB domain binds.6-8,48 In the proline-rich domain near the C-terminus and at amino acids 123 to 128, there were several sequences that match the binding sequences for the SH3 domain (eg, PRKEPPPCP for class I and PELPPR, PISPKK, PKMPRK, and PRPPRR for class II)49,50 and were probably responsible for the purification of pp135 with GST-Grb2/Ash affinity chromatography. The homology search with the N.C.B.I. database revealed that pp135 shares a similarity with human inositol polyphosphate-5-phosphatase (29.1% identity in 326 amino acid overlap)51 and OCRL (29.9% identity in 351 amino acid overlap), which is encoded by the causative gene for human Low's oculocerebrorenal syndrome and is a homologue of inositol polyphosphate-5-phosphatase.52,53 The members of inositol polyphosphate-5-phosphatase (5-phosphatase) proteins have two conserved motifs,54 and the amino acid sequences at 583 to 594 and 665 to 692 of pp135 were matched to these motifs. These results suggest that pp135 could have the inositol polyphosphate-5-phosphatase activity. The various domains of pp135 are shown in Fig 2B.
(A) Amino acid sequence and known motifs of pp135. The underlined amino acids are the SH2 domain, SH3 domain, 5-phosphatase conserved motifs, and proline-rich domain. The hatched-underlined amino acids are the two binding sites for PTB domain. The hatched-overlined amino acids are the six peptides obtained from the amino acid sequence of purified pp135. (B) Diagrammatic representation of the various domains within pp135.
(A) Amino acid sequence and known motifs of pp135. The underlined amino acids are the SH2 domain, SH3 domain, 5-phosphatase conserved motifs, and proline-rich domain. The hatched-underlined amino acids are the two binding sites for PTB domain. The hatched-overlined amino acids are the six peptides obtained from the amino acid sequence of purified pp135. (B) Diagrammatic representation of the various domains within pp135.
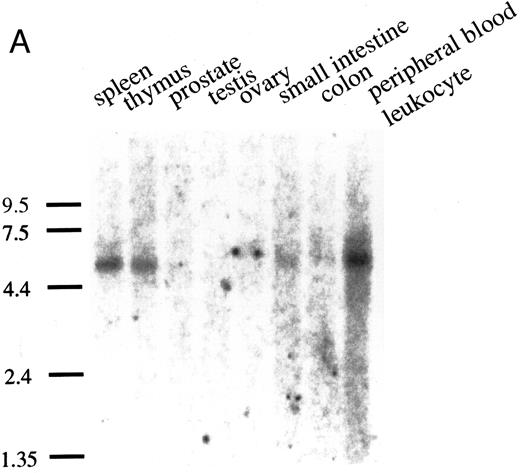
Northern blot analysis was performed with poly(A)+ RNAs from various human tissues (human multiple tissue Northern blot; Clontech, Palo Alto, CA). As shown in Fig 3A, pp135 mRNA (5.4 kb) was obviously expressed in spleen, thymus, and peripheral blood leukocytes, but in other tissues, the expression of pp135 mRNA was very low or undetectable. Next, we examined the expression of the mRNA of pp135 in myelocytic/monocytic cell lines (HL60, U937, KG1, KU812, and SKH1), erythroid cell lines (HEL and K562), and megakaryocytic cell lines (UT-7, CMK, and MOLM1) as described previously.55 As shown in Fig 3B, pp135 mRNA was ubiquitously expressed in all these cell lines. These results indicate that the pp135 might play an important role in the signal transduction in hematopoietic cells.
The expression of the pp135 mRNA in human tissues and leukemic cell lines. Poly (A)+ RNAs from various human tissues (A) or leukemic cell lines (B) were blot-hybridized with the PCR fragment probe used for cloning cDNA. The migration position of RNA size makers (in kilobases) is indicated on the left.
The expression of the pp135 mRNA in human tissues and leukemic cell lines. Poly (A)+ RNAs from various human tissues (A) or leukemic cell lines (B) were blot-hybridized with the PCR fragment probe used for cloning cDNA. The migration position of RNA size makers (in kilobases) is indicated on the left.
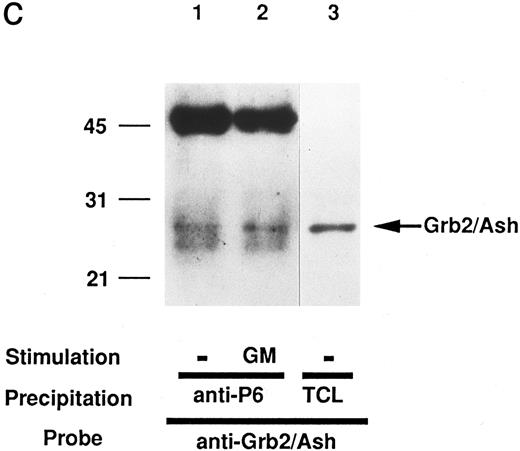
We then tried to confirm that we had indeed purified the pp135 and cloned the cDNA for the pp135 originally identified. After the lysates from UT-7 cells stimulated with or without GM-CSF were mixed with rabbit antiserum to pp135 (anti-P6) generated against the GST-P6 fusion protein, the resulting immunoprecipitates, in combination with the precipitates bound to GST-SH3 domain of Grb2/Ash, were subjected to SDS-PAGE and immunoblotted with anti-Py (4G10) or anti-p6. The pp135 immunoprecipitated with anti-P6 showed the same electrophoretic mobility as that of pp135 bound to GST-SH3 domain of Grb2/Ash, whether probed with anti-Py or anti-P6 (Fig 4A and B). The pp135 immunoprecipitated with anti-p6 and probed with anti-Py was shown to become tyrosine-phosphorylated in vivo, depending on GM-CSF stimulation (lanes 3 and 4 of Fig 4A). Reprobing with anti-P6 has shown that pp135 constitutively associates with GST-Grb2/Ash-SH3 (lanes 1 and 2 in Fig 4B) and that the amount of pp135 was not altered with GM-CSF stimulation (lanes 3 and 4 in Fig 4B). To confirm the association of pp135 and Grb2/Ash in vivo, immunoprecipitates with anti-P6 from lysates of the UT-7 cells were subjected to SDS-PAGE and immunoblotted with anti-Grb2/Ash antibody. Grb2/Ash was shown to be coimmunoprecipitated with pp135 from both lysates of GM-CSF–stimulated or –unstimulated cells (Fig 4C), suggesting the constitutive association of pp135 and Grb2/Ash in vivo. From these findings, we concluded that we had correctly cloned the cDNA for the pp135 originally identified.
The pp135 constitutively binds to the SH3 domain of Grb2/Ash and inducibly becomes tyrosine-phosphorylated with the stimulation of GM-CSF. The lysates from UT-7 cells stimulated with GM-CSF (lanes 2 and 4) or unstimulated (lanes 1 and 3) were mixed with GST-Grb2/Ash SH3 (lanes 1 and 2) or with anti-P6 (lanes 3 and 4). The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-Py (A) and reprobed with anti-P6 (B). The arrow indicates the position of pp135. Molecular weight markers, indicated at the left, are given in kilodaltons. (C) In vivo association of pp135 with Grb2/Ash. The lysates from UT-7 cells stimulated with GM-CSF (lane 2) or unstimulated (lane 1) were mixed with anti-P6. The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-Grb2/Ash. The total UT-7 cell lysate (TCL, lane 3) is also applied for reference. Molecular weight markers, indicated at the left, are given in kilodaltons. The arrow indicates the position of Grb2/Ash.
The pp135 constitutively binds to the SH3 domain of Grb2/Ash and inducibly becomes tyrosine-phosphorylated with the stimulation of GM-CSF. The lysates from UT-7 cells stimulated with GM-CSF (lanes 2 and 4) or unstimulated (lanes 1 and 3) were mixed with GST-Grb2/Ash SH3 (lanes 1 and 2) or with anti-P6 (lanes 3 and 4). The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-Py (A) and reprobed with anti-P6 (B). The arrow indicates the position of pp135. Molecular weight markers, indicated at the left, are given in kilodaltons. (C) In vivo association of pp135 with Grb2/Ash. The lysates from UT-7 cells stimulated with GM-CSF (lane 2) or unstimulated (lane 1) were mixed with anti-P6. The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-Grb2/Ash. The total UT-7 cell lysate (TCL, lane 3) is also applied for reference. Molecular weight markers, indicated at the left, are given in kilodaltons. The arrow indicates the position of Grb2/Ash.
So far, it has been shown that the signaling pathways downstream of GM-CSF receptor and Epo receptor are closely related to each other. These observations motivated us to investigate the tyrosine-phosphorylation profile of pp135 in the signaling pathway triggered by Epo. The tyrosine phosphorylation of pp135 was also induced in UT-7 cells stimulated with Epo (data not shown), indicating that pp135 has an important role in a variety of cytokine-triggered signaling pathways.
It is well known that Shc also associates with a highly tyrosine-phosphorylated protein of approximately 145-kD in several cell types activated by a variety of stimuli such as GM-CSF, Epo, IL-3, IL-2, Steel factor, EGF, and platelet-derived growth factor,9,55-61 or in cells transformed by activated oncogenes.62 To assess whether pp135 also binds to Shc, immunoprecipitates with anti-Shc from lysates of the UT-7 cells with or without the stimulation of GM-CSF were subjected to SDS-PAGE and immunoblotted with anti-Py or anti-P6 antibody (Fig 5). Anti-Shc immunoprecipitants contained several tyrosine-phosphorylated proteins including pp135 in GM-CSF–treated cells, and pp135 in the anti-Shc immunoprecipitants was also recognized with the anti-P6 antibody (Fig 5). These results have shown that the pp135 associates with Shc, depending on GM-CSF stimulation in vivo.
GM-CSF induces the association of pp135 with Shc. The lysates from UT-7 cells stimulated with GM-CSF (lanes 1 and 3) or unstimulated (lanes 2 and 4) were mixed with anti-Shc. The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-Py (lanes 1 and 2) and reprobed with anti-P6 (lanes 3 and 4). Molecular weight markers, indicated at the left, are given in kilodaltons. The arrow indicates the position of pp135.
GM-CSF induces the association of pp135 with Shc. The lysates from UT-7 cells stimulated with GM-CSF (lanes 1 and 3) or unstimulated (lanes 2 and 4) were mixed with anti-Shc. The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-Py (lanes 1 and 2) and reprobed with anti-P6 (lanes 3 and 4). Molecular weight markers, indicated at the left, are given in kilodaltons. The arrow indicates the position of pp135.
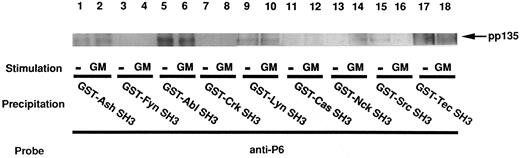
As described above, pp135 has a proline-rich domain and associates with the SH3 domain of Grb2/Ash. Therefore, we next examined whether pp135 binds to the SH3 domains of other proteins. The cell lysates from UT-7 cells unstimulated or stimulated with GM-CSF were mixed with a variety of GST-SH3 fusion proteins. The resulting complexes were retrieved with glutathione-agarose beads, subjected to SDS-PAGE, and immunoblotted with anti-P6. As shown in Fig 6, pp135 bound to the GST fusion proteins containing the SH3 domain of Abl, Lyn, and Src as well as Grb2/Ash. The association with GST-SH3 fusion proteins was independent of GM-CSF stimulation in UT-7 cells. These findings indicate that the proline-rich region of pp135 has some specificity for the association with the SH3 domains.
pp135 associates with the SH3 domains of different proteins. The lysates from UT-7 cells stimulated with GM-CSF (lanes 2, 4, 6, 8, 10, 12, 14, 16, and 18) or unstimulated (lanes 1, 3, 5, 7, 9, 11, 13, 15, and 17) were mixed with each GST-SH3 fusion protein noncovalently coupled to glutathione-agarose beads. The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-P6. The arrow indicates the position of pp135.
pp135 associates with the SH3 domains of different proteins. The lysates from UT-7 cells stimulated with GM-CSF (lanes 2, 4, 6, 8, 10, 12, 14, 16, and 18) or unstimulated (lanes 1, 3, 5, 7, 9, 11, 13, 15, and 17) were mixed with each GST-SH3 fusion protein noncovalently coupled to glutathione-agarose beads. The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-P6. The arrow indicates the position of pp135.
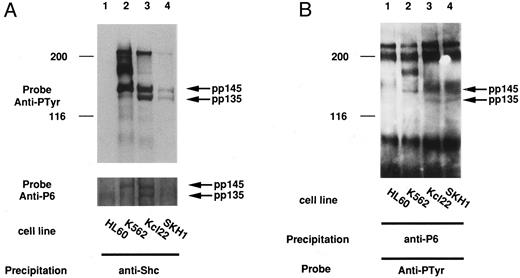
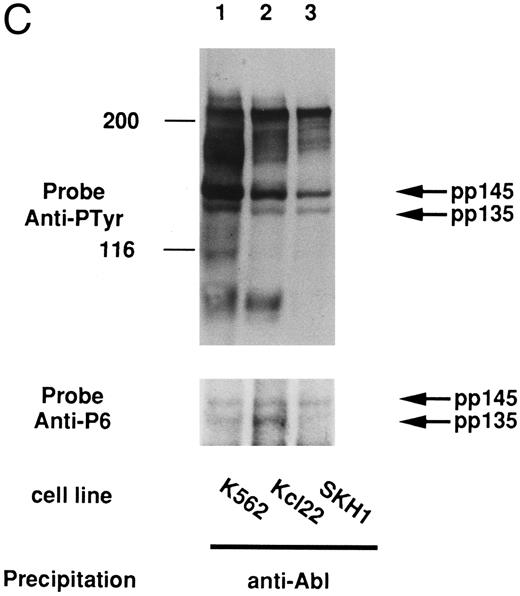
In some tumor cells, it is considered that the signal for cell growth is constitutively transduced to the nucleus and that many cellular proteins are constitutively tyrosine-phosphorylated. In the case of cell lines derived from CML, these phenomena could be caused by the Bcr-Abl oncogene. We then examined if pp135 was tyrosine-phosphorylated in CML cell lines. The cell lysates from K562, KCl22, SKH1 (CML), and HL-60 (acute myelogenous leukemia [AML]) cell lines were immunoprecipitated with anti-P6 antibody and immunoblotted with anti-Py. As shown in Fig 7A and B, pp135 was constitutively tyrosine-phosphorylated in all three CML cell lines but not in HL-60. Moreover, pp135 was coimmunoprecipitated with Shc and Bcr-Abl in Bcr-Abl–expressing cells (Fig 7A and C). At the same time, tyrosine-phosphorylated 145-kD protein (pp145) was also recognized by anti-P6 antibody and was found to be associated with Shc and Bcr-Abl. Although the nature of p145 is not clear, pp145 may be an alternative–splicing form or a posttranslationally modified form of pp135. These results suggest that constitutive tyrosine phosphorylation of pp135 could be caused by Bcr-Abl in CML cells and might be involved in neoplastic transformation of hematopoietic cells.
pp135 was constitutively tyrosine-phosphorylated and associated with Shc and Bcr-Abl in CML cell lines. The lysates from three human CML (K562, Kcl22, and SKH1) and human AML (HL-60) cell lines were mixed with anti-Shc (A) or anti-P6 antibody (B). The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-Py (top panels) and reprobed with anti-P6 (bottom panel of A). The lysates from these CML cell lines were also mixed with anti-Abl antibody (C). The precipitates were subjected to SDS-PAGE and immunoblotted with anti-Py (top panel) and reprobed with anti-P6 (bottom panel). The arrow indicates the position of pp135 and pp145. Molecular weight markers, indicated at the left, are given in kilodaltons.
pp135 was constitutively tyrosine-phosphorylated and associated with Shc and Bcr-Abl in CML cell lines. The lysates from three human CML (K562, Kcl22, and SKH1) and human AML (HL-60) cell lines were mixed with anti-Shc (A) or anti-P6 antibody (B). The resulting precipitates were subjected to SDS-PAGE and immunoblotted with anti-Py (top panels) and reprobed with anti-P6 (bottom panel of A). The lysates from these CML cell lines were also mixed with anti-Abl antibody (C). The precipitates were subjected to SDS-PAGE and immunoblotted with anti-Py (top panel) and reprobed with anti-P6 (bottom panel). The arrow indicates the position of pp135 and pp145. Molecular weight markers, indicated at the left, are given in kilodaltons.
As described above, pp135 has two motifs conserved among the inositol polyphosphate-5-phosphatase proteins. We next analyzed the phosphatase activity of pp135 using inositol (1,3,4,5)-P4 as the substrate. The inositol-5-phosphatase can remove the 5-phosphate and produce inositol (1,3,4)-P3 from inositol (1,3,4,5)-P4. After the total lysates, anti-P6 immunoprecipitates, or anti-Shc immunoprecipitates were incubated with [3H]-inositol (1,3,4,5)-P4, the products and substrates were separated with Dowex 1 ion-exchange resin and the radioactivity of the [3H]-inositol (1,3,4)-P3 was measured. As shown in Fig 8, anti-P6 immunoprecipitates hydrolyzed inositol (1,3,4,5)-P4 regardless of GM-CSF stimulation. Moreover, anti-Shc immunoprecipitates from GM-CSF–stimulated cell lysates but not from unstimulated cell lysates have shown the inositol-5-phosphatase activity. These results suggest that pp135 has inositol-5-phosphatase activity and confirmed that pp135 associates with Shc, depending on the stimulation of GM-CSF.
Inositol-5-phosphatase activity of pp135. The lysates from UT-7 cells with (+) or without (−) GM-CSF stimulation were mixed with normal rabbit serum (NRS), anti-P6, or anti-Shc. The inositol-5-phosphatase activity of the resulting immunoprecipitates was measured using [3H]inositol (1,3,4,5)-P4 as the substrate. Results are expressed as picomoles of hydrolysis product per 15 minutes.
Inositol-5-phosphatase activity of pp135. The lysates from UT-7 cells with (+) or without (−) GM-CSF stimulation were mixed with normal rabbit serum (NRS), anti-P6, or anti-Shc. The inositol-5-phosphatase activity of the resulting immunoprecipitates was measured using [3H]inositol (1,3,4,5)-P4 as the substrate. Results are expressed as picomoles of hydrolysis product per 15 minutes.
DISCUSSION
In this work, we have purified pp135, which bound GST-Grb2/Ash and became tyrosine-phosphorylated with stimulation of GM-CSF in UT-7 cells. Moreover, we cloned the full-length cDNA for pp135, which has the SH2, SH3, and inositol-5-phosphatase domains. Recently, cDNA for the 145- to 150-kD protein associated with Shc was cloned from the cDNA library of murine hematopoietic cell lines.63,64 This protein has an SH2 domain and the consensus motifs for the 5-phosphatase, as is the case with pp135, and was named SHIP for SH2-containing inositol-5-phosphatase. We compared the amino acid sequences of the two proteins and found that overall 85% of the amino acids of pp135 were identical with those of SHIP. From this observation, our pp135 can be considered to be the human counterpart of SHIP. On the other hand, Kavanaugh et al65 have reported that the partial amino acid sequence of human 130-kD protein (SIP-130)65 and this sequence was included within that of pp135. Moreover, SIP130 has the SH2 and inositol-5-phosphatase domains and shows inositol-5-phosphatase activity as pp135 does. These results suggest that pp130 and SIP130 are probably identical.
In UT-7 cells, anti-P6 antibody recognized mainly pp135 in GST-Grb2/Ash binding proteins and anti-Shc immunoprecipitates (Fig 4). On the other hand, anti-P6 antibody recognized both pp135 and pp145 in KCL22 and SKH1 cells, but mainly pp145 in K562 (Fig 8). The nature of pp145 is unclear, but it was suggested that three alternative splicing forms of SIP (SIP-110, SIP-130, and SIP-140) were generated.65 Therefore it may be reasonable to consider that pp145 is an alternative splicing form of pp135 and that pp135 and pp145 are identical with SIP-130 and SIP-140, respectively. The differences in the expression patterns of pp135 and pp145 among the cell lines remain to be elucidated.
We have shown that pp135 associates with Shc, constitutively in CML cell lines and inducibly in UT-7 cells with GM-CSF stimulation. Although the mechanism for the association between Shc and pp135 or SHIP has not been clarified, we could consider that tyrosine-phosphorylated proteins can bind to the PTB domain of Shc,9,62 which may interact with the tyrosine-phosphorylated NPXY motifs within SHIP or pp135. Our result that GST fusion protein with the SH2 domain of Shc did not bind to pp135 (data not shown) and the reported result that p150SHIP was cloned by the yeast two-hybrid screening using the PTB domain of Shc strongly support this mechanism.64
Although the SH3 domains of Grb2/Ash bind to the proline-rich domains of dynamin,30,31 C3G,32 and Sos,28,29 which regulate the small G-proteins, pp135 has no homologous regions with guanine nucleotide exchange factors or GTPase-activating proteins. Therefore, pp135, which constitutively associates with Grb2/Ash through its SH3 domain, could be involved in the signal transduction pathway different from the Ras pathway. As shown in Fig 8, it has been demonstrated that pp135 has the 5-phosphatase activity, as was reported with SHIP/SIP.63-65 However, the 5-phosphatase activity of pp135 was not altered by the GM-CSF stimulation and the tyrosine phosphorylation of pp135. A number of cellular proteins are tyrosine-phosphorylated, depending on extracellular signals including growth factors, and begin to form complexes with other signaling molecules.4,5 In the case of pp135, tyrosine-phosphorylated pp135 may form a complex with Grb2/Ash, Shc, and other molecules containing SH2, SH3, or PTB domain. Furthermore, pp135 associated with the SH3 domains of the different proteins in vitro (Fig 6) and was coimmunoprecipitated with Bcr-Abl in CML cell lines (Fig 7). The formation of such a complex is probably important for the regulation of the enzymatic activity of pp135. Theoretically, the candidates for the substrates of 5-phosphatases are inositol 1,4,5-trisphosphate, inositol 1,3,4,5-tetrakisphosphate, phosphatidylinositol 4,5-bisphosphate, and phosphatidylinositol 3,4,5-trisphosphate,38,66 but SHIP and SIP hydrolyzed only the polyphosphate-containing phosphate in the 3 position of the inositol ring.63-65 These substances are produced by activated PI3 kinase, depending on the stimulation, such as growth factors, and the production of these substances is correlated with Ca2+ mobilization, Ras, PKCλ, or c-Akt regulation.67-71 These observations suggest that pp135 and SHIP could play an important role in the signal transduction pathways triggered by hematopoietic growth factors, especially in the pathway in which PI3 kinase is involved and in hematopoietic cell transformation caused by Bcr-Abl.
In summary, we have purified the 135-kD protein that associates constitutively with Grb2/Ash but inducibly with Shc. Moreover, pp135 becomes tyrosine-phosphorylated with the stimulation of GM-CSF or Epo in hematopoietic cells. In CML cell lines, pp135 was constitutively tyrosine-phosphorylated and associated with Shc and Bcr-Abl. The deduced amino acid sequence has shown that pp135 has the SH2, SH3, and 5-phosphatase domains. Indeed, it has been shown that pp135 has the 5-phosphatase activity. We propose that the cloned isoform pp135 should be designated as SHIP-135 for Src homology containing inositol phosphatase, 135-kD protein.
ACKNOWLEDGMENT
UT-7 was a kind gift from Dr Norio Komatsu (Jichi Medical School, Tochigi, Japan). We thank Dr Tomoyuki Tanaka, Dr Seishi Ogawa, and Dr Naoto Hirano for helpful discussions and Naoko Nishimura and Rie Mitsumori for technical assistance.
Supported in part by Grants-in-Aids from the Ministry of Education, Science and Culture of Japan, and from the Ministry of Health and Welfare of Japan.
Address reprint requests to Hisamaru Hirai, MD, Third Department of Internal Medicine, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113, Japan.








![Fig. 8. Inositol-5-phosphatase activity of pp135. The lysates from UT-7 cells with (+) or without (−) GM-CSF stimulation were mixed with normal rabbit serum (NRS), anti-P6, or anti-Shc. The inositol-5-phosphatase activity of the resulting immunoprecipitates was measured using [3H]inositol (1,3,4,5)-P4 as the substrate. Results are expressed as picomoles of hydrolysis product per 15 minutes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2745/4/m_bl_0035f8.jpeg?Expires=1765956847&Signature=eQH3J~JY5h~ZxYosBeUXz9saSE212qgZRhxPGNMDZiDqc0yEC6NZ3OfgmmBDsORmjmHKoBj6XRezgojfVJSw-Rena67N4CxaUv5M3Xu~NSu18qZTsWzjZ1NQN2-zMTxKV5IgFApocgXPT~VEqgsUu~ewo4jhyUkCe4WCz47a~AmB4Bt4Eb~r1kVV5~zy0S~B1Bb9Xh5bSpSbaCCiU-mruoVyl4yoWcOxN7s6IXND2oqrdftWCWyt1OBV7nnC3vFxyptCqkQutExxEwkOjuKUT3kxaWFR3EUgzShoPLBGZhHyFM1bwSfzqjsKoPfPDm9Pwele0pFjSNxOgc7HwWkGsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal