Abstract
Primary interleukin-3 (IL-3)–dependent mast cell cultures from bone marrow of p53-null mice and littermate controls were established. Both p53-null and wild-type cells entered apoptosis on IL-3 removal, showing that p53 is not required for entry into apoptosis after factor deprivation. After X-irradiation, a lower proportion of the p53-null than wild-type cells underwent G2 arrest, but their radiosensitivity was similar. An IL-3–dependent cell line expressing wild-type p53 was used to show that cells die at a fixed time after X-irradiation rather than from a specific cell cycle point.
THE p53 PROTEIN functions to suppress tumor formation; p53 is frequently mutated in human tumors,1 and inheritance of a mutated p53 allele predisposes individuals to a variety of cancers.2,3 Mutant p53 probably acts to block the function of wild-type p53,4,5 because mice homozygously deleted for p53 show the mutant phenotype of enhanced tumor formation.6 p53 acts, at least in part, by regulating gene expression. On binding a specific DNA sequence it transactivates target genes7; it also represses transcription of genes lacking a p53 target sequence by interacting with the basal transcription machinery.8
One response of cells to ionizing radiation and other DNA-damaging treatments is the induction of G1 and G2 cell cycle arrest. p53 expression is upregulated after exposure of cells to DNA-damaging agents9 and the G1 checkpoint can be controlled by the p53-dependent induction of expression of cell cycle inhibitors.10-12 Control of the G2 checkpoint in mammalian cells is believed to be p53 independent, although its mechanism remains unclear. Cells may also be induced to enter apoptosis after ionizing radiation.13 In some cell populations, such as thymocytes,14,15 but not mature lymphocytes,16 this radiation-induced apoptosis is p53-dependent. p53 has also been reported to be required for apoptosis induced by disregulation of expression of the cell cycle regulators c-Myc17 or E2F18 or by expression of adenovirus E1A protein.19 In the case of DNA-damage–induced or E1A-induced apoptosis, the transactivation of target genes by p53 has been shown to be unnecessary.20 21
Diverse cell types in culture and in vivo are dependent on growth factors or cytokines for their survival. When such trophic factors are removed, cell death follows with the morphologic and biochemical features of apoptosis.22,23 There is some evidence to suggest that this form of apoptosis after factor deprivation requires the action of p53. In 2 tumor cell lines that lacked wild-type p53, the introduction of a wild-type p53 allele led to apoptosis, which could be inhibited by interleukin-6 (IL-6),24 erythropoietin, IL-3,25 or Steel factor.25,26 Furthermore, in IL-3–dependent cell lines expressing wild-type p53, a dominant interfering p53 molecule slowed the onset of apoptosis on IL-3 removal.27,28 However, development of p53-null mice proceeds normally.6 Many steps in development are controlled by the limiting supply of trophic factors leading to apoptosis of unwanted cells.22 This suggested to us that apoptosis of normal, primary cells after trophic factor removal could proceed in the absence of p53. In this report, we use primary IL-3–dependent cultures of bone marrow cells from p53-null mice and littermate controls to show that apoptosis on IL-3 withdrawal does not require p53. Furthermore, X-irradiation–induced apoptosis and its inhibition by IL-3 is also independent of p53 in this cell population.
MATERIALS AND METHODS
Cell culture.BAF3 cells29 and bone marrow cultures were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 5% WEHI-3B conditioned medium, which was used throughout as a source of IL-3. The generation of p53-null mice was previously described.14 Bone marrow was obtained from homozygously deleted mice or wild-type littermate controls. Primary IL-3–dependent cultures of bone marrow cells were prepared as previously described.30 After 15 days in culture, staining with toluidine showed that greater than 90% of cells had a mast cell phenotype.31 Viable cells were determined by counting cells able to exclude trypan blue on a hemocytometer; duplicate samples of at least 50 cells were analyzed. X-irradiation was performed using a Pantac x-ray machine (Chester Beatty Laboratories, London, UK) with an output of 240 KeV at a dose rate of 3 to 4 Gy/min. Plating efficiency was measured after serial twofold dilution of cells from 500 cells per well of a 96-well plate. After 15 days, positive wells were evaluated by incorporation of 0.5 μCi 3HTdR for 12 hours, cell harvesting, and determination of incorporated radioactivity using scintillation counting in a Beckman β-counter (Beckman, Fullerton, CA). Wells with counts greater than threefold standard deviation above background were scored as positive. Plating efficiency was calculated from the dilutions in which less than 30% of wells were positive, in which the positives can be assumed to have derived from single cell clones. The method of Herrmann et al32 was used to measure DNA fragmentation.32 Valinomycin was obtained from Sigma (Poole, UK).
Cell cycle analysis.A total of 106 cells were suspended in 200 μL phosphate-buffered saline (PBS) and fixed with 2 mL of ice-cold 75% ethanol/25% PBS. Fixed cells were pelleted and vigorously resuspended in PBS and then incubated for 30 minutes at 37°C with 100 μg/mL ribonuclease and 40 μg/mL propidium iodide (PI). The fluorescence of stained cells was analyzed using a FACScan (Becton Dickinson and Co, Mountain View, CA); subdiploid cells were used as a measure of apoptotic cells.30 To analyze cell cycle progression, cells were prelabeled with 10 μmol/L BrdU (Sigma). After 2 hours, cells were washed twice in DMEM/10% FBS and cultured under the conditions shown. Cells that incorporated BrdU were detected with fluorescein isothiocyanate (FITC)-labeled anti-BrdU, as previously described.33 Sorting of cells according to anti-BrdU and PI staining was performed using a FACStar (Becton Dickinson); sorted cells were photographed by fluorescent microscopy using 400 ASA Ektachrome 35-mm film (Kodak, Stuttgart, Germany).
RESULTS
IL-3 inhibition of apoptosis and radioprotection in the absence of p53.Culture of bone marrow with no stromal cells in the presence of IL-3 results in a homogeneous population of IL-3–dependent mast cells.31 To examine the role of p53 in IL-3 regulation of cell death, such populations were derived from homozygous p53-null mice and wild-type littermate controls. After 1 month, bulk cultures that proliferated at similar rates in the presence of IL-3 were obtained and used for further experiments. Figure 1A and B shows that both wild-type and p53-null cultures began to lose viability 12 hours after IL-3 removal; thereafter, the p53-null cultures died at a slightly slower rate, with 50% cells remaining viable at 30 hours, compared with 20 hours in the wild-type cultures. X-irradiation of wild-type cells accelerated the loss of viability. This acceleration was not observed for the p53-null cells (Fig 1A and B). Wild-type and p53-null cells were resistant to X-irradiation in the presence of IL-3 (Fig 1A and B). Similar results were obtained with five independent bone marrow preparations from different p53-null and littermate wild-type mouse marrow (data not shown). Both the wild-type and p53-null cells died by apoptosis on IL-3 removal, as characterized by DNA laddering (Fig 1C). Furthermore, rapid apoptosis could be induced in both populations by valinomycin34 when equivalent DNA fragmentation could be seen after 6 hours (Fig 1C). These data show that the p53-null cells were fully competent to rapidly enter apoptosis on an appropriate signal.
IL-3 regulation of apoptosis in the absence of p53. IL-3–dependent bone marrow cells from wild-type (A) or p53-null (B) mice, were plated at 2 × 105 cell/mL in a 24-well plate in the presence or absence of IL-3 after X-irradiation with 4 Gy as indicated. Cell viability was measured by trypan blue exclusion at the times shown. ([○] + IL-3; [□] + IL-3 + 4 Gy; [•] − IL-3; [▪] − IL-3 + 4 Gy.) (C) 5 × 106 wild-type or p53-null cells were analyzed for DNA fragmentation after X-irradiation as indicated with 4 Gy for 20 hours (wild-type) or 50 hours (p53-null), with or without IL-3, or after 6 hours of treatment with 10 μg/mL valinomycin in the presence of IL-3.
IL-3 regulation of apoptosis in the absence of p53. IL-3–dependent bone marrow cells from wild-type (A) or p53-null (B) mice, were plated at 2 × 105 cell/mL in a 24-well plate in the presence or absence of IL-3 after X-irradiation with 4 Gy as indicated. Cell viability was measured by trypan blue exclusion at the times shown. ([○] + IL-3; [□] + IL-3 + 4 Gy; [•] − IL-3; [▪] − IL-3 + 4 Gy.) (C) 5 × 106 wild-type or p53-null cells were analyzed for DNA fragmentation after X-irradiation as indicated with 4 Gy for 20 hours (wild-type) or 50 hours (p53-null), with or without IL-3, or after 6 hours of treatment with 10 μg/mL valinomycin in the presence of IL-3.
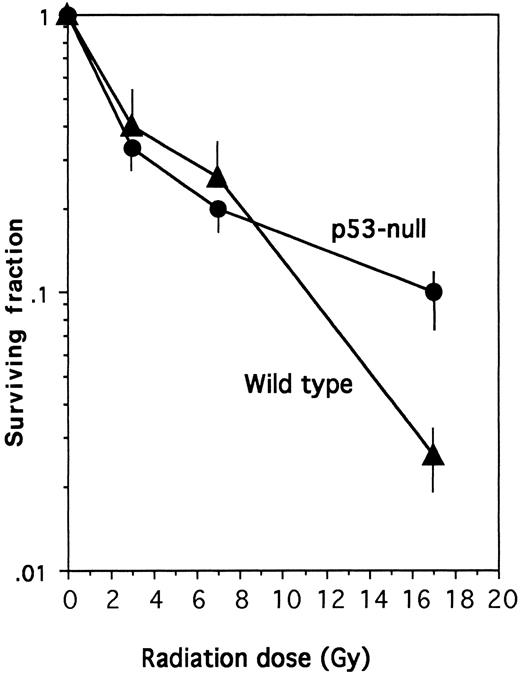
These results are similar to those obtained with thymocytes from homozygous p53-null mice, in which irradiation did not accelerate loss of viability.14 15 They also extend results obtained with a dominant-negative p5327 by showing that the rate of apoptosis after IL-3 removal was only slightly slower in the complete absence of p53. Therefore, p53 is clearly not required for the inhibition of apoptosis by IL-3 or cell death in its absence. The data in Fig 1B also argued that the radioprotective effect of IL-3 might be independent of p53. To confirm this, it was necessary to measure the long-term survival of wild-type and p53-null cells in the presence of IL-3 after exposure to increasing radiation doses. Figure 2 shows that the two cell populations showed similar radiosensitivity, with 50% loss of clonigenicity after exposure to 2.4 Gy and 90% loss at 78 Gy. Thus, although the lack of p53 slowed the initial rate of apoptosis on irradiation without IL-3, the absence of p53 neither enhanced nor inhibited the long-term radioprotective action of IL-3.
IL-3 radioprotection in the absence of p53. The plating efficiency of wild-type or p53-null cells was measured after X-irradiation at the indicated dose as described in the Materials and Methods. In the absence of radiation, the plating efficiency of the wild-type cells was 9.5% and that of the p53-null cells was 12.5%.
IL-3 radioprotection in the absence of p53. The plating efficiency of wild-type or p53-null cells was measured after X-irradiation at the indicated dose as described in the Materials and Methods. In the absence of radiation, the plating efficiency of the wild-type cells was 9.5% and that of the p53-null cells was 12.5%.
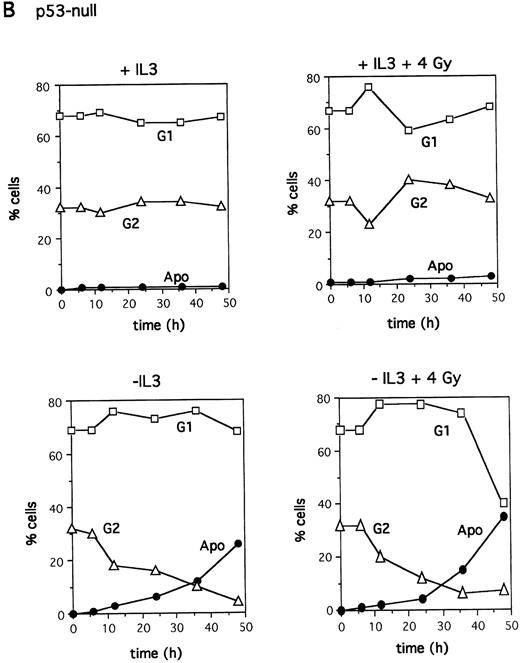
Cell cycle kinetics after irradiation. IL-3–dependent bone marrow cells from wild-type (A) or p53-null (B) mice were plated at 2 × 105 cell/mL in a 24-well plate in the presence or absence of IL-3 after X-irradiation with 4 Gy as indicated. Cell cycle analysis was performed by labeling the cells with PI and analyzing DNA content using Lysis II software (Becton Dickinson, San Jose, CA) on the FACScan.
Cell cycle kinetics after irradiation. IL-3–dependent bone marrow cells from wild-type (A) or p53-null (B) mice were plated at 2 × 105 cell/mL in a 24-well plate in the presence or absence of IL-3 after X-irradiation with 4 Gy as indicated. Cell cycle analysis was performed by labeling the cells with PI and analyzing DNA content using Lysis II software (Becton Dickinson, San Jose, CA) on the FACScan.
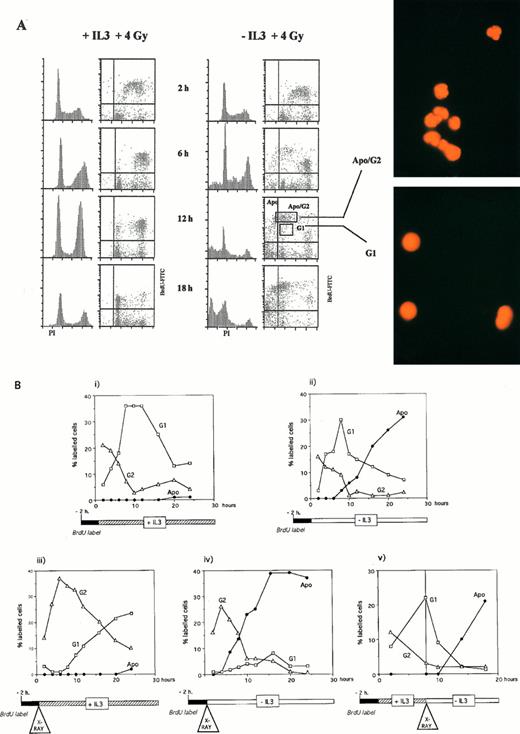
Entry into apoptosis irrespective of cell cycle position. (A) Cell cycle of BAF3 cells prelabeled for 2 hours with BrdU, irradiated with 4 Gy, and incubated in the absence or presence of IL-3. After the times shown, cells were fixed and labeled with PI and anti-BrdU as described in the Materials and Methods. Squares marked G1 and Apo/G2 represent the gates set to sort populations that were then photographed using fluorescent microscopy. (B) BAF3 cell cycle analysis after the treatments indicated below each panel.
Entry into apoptosis irrespective of cell cycle position. (A) Cell cycle of BAF3 cells prelabeled for 2 hours with BrdU, irradiated with 4 Gy, and incubated in the absence or presence of IL-3. After the times shown, cells were fixed and labeled with PI and anti-BrdU as described in the Materials and Methods. Squares marked G1 and Apo/G2 represent the gates set to sort populations that were then photographed using fluorescent microscopy. (B) BAF3 cell cycle analysis after the treatments indicated below each panel.
Entry into apoptosis irrespective of cell cycle position.G2 arrest was previously observed after X-irradiation in the IL-3–dependent cell line BAF3, which expresses wild-type p53, and in the absence of IL-3 apoptotic BAF3 cells appeared in parallel with loss of G2 cells.35 Both G1 and G2 arrest were observed in another study after X-irradiation of BAF3.36 To explore further the relationship between p53, cell cycle arrest, and apoptosis, the cell cycle distribution of bone marrow cultures during entry into apoptosis was monitored. Because these cells cycle slowly, few cells in either culture (<10% at any time) were in S phase; for clarity, they are omitted from the figure. Figure 3A shows that bone marrow cultures with wild-type p53 behaved in a similar manner to the BAF3 cell line.30 35 In the absence of irradiation when IL-3 was removed, no cell cycle arrest occurred, and apoptotic cells appeared concomitant with loss of both G1 and G2 cells. After irradiation, some accumulation in G2 was observed and cells entered apoptosis in the absence of IL-3; again, a parallel loss of both G1 and G2 cells was seen. Less G2 accumulation occurred in the p53-null cells after irradiation, and cells entered apoptosis with a loss of cells from both G1 and G2 (Fig 3B). These data showed that p53 function is required for optimal G2 arrest in these cells. However, the kinetics of both wild-type and p53-null cell loss from G1 and G2 argued that they entered apoptosis from both points after irradiation.
To prove this, it was necessary to track labeled cohorts of cells through the cell cycle and observe from which point they entered apoptosis. It was also necessary to clearly identify cells entering apoptosis from G2, which will have a sub-tetraploid DNA content, and thus distinguish them from S and G1 cells. To do this we used the BAF3 cell line, which expresses wild-type p53, because it was not possible to track the slowly cycling primary bone marrow cultures. Figure 4 shows cells prelabeled for 2 hours with BrdU and then irradiated and incubated in the absence or presence of IL-3. In the presence of IL-3, both G1 and G2 arrest were seen; the BrdU-positive cells accumulated in G2 and then resumed cycling. In the absence of IL-3, the disappearance of labeled cells from this G2 arrest was accompanied by their appearance in a new brightly staining population. Sorting of cells within a region containing this population (Apo/G2, Fig 4A) demonstrated that they showed the characteristic morphology of apoptotic cells (Fig 4A). These cells therefore correspond to those entering apoptosis from G2. We quantitated Apo/G2 cells (Fig 4A) and the subdiploid-labeled cells (Apo, Fig 4A) as a total measure of apoptotic-labeled cells. Quantitation of unlabeled cells was not undertaken because of the problem of identifying apoptotic G2 cells.
Figure 4B (iii and iv) shows that 2 hours of prelabeling with BrdU followed by X-irradiation resulted in a labeled cohort of cells in G2. In the presence of IL-3 (iii), they re-entered the cell cycle after a delay, compared with control unirradiated cells (Fig 4B, i). In the absence of IL-3, labeled apoptotic cells started to appear from the G2 population 6 hours after irradiation (iv). Using prelabeling for 2 hours with BrdU and then preculturing for 8 hours in the presence of IL-3 before irradiation, a labeled cohort of cells in G1 was obtained (Fig 4B, v). These cells also entered apoptosis 6 hours after irradiation, without passing into G2 (v). Therefore, entry into apoptosis after irradiation occurred after a fixed lag time, regardless of cell cycle position. For the BAF3 cell line, this lag was only 6 hours, whereas for the primary marrow cultures, it was approximately 12 hours (wild-type) or 24 hours (p53-null). These tracking experiments show that our previous assumption, ie, that G2 arrest was critical for entry into apoptosis after irradiation, was erroneous35; timing, rather than cell cycle position, dictates entry into apoptosis.
DISCUSSION
The use of IL-3–dependent mast cell cultures from p53-null mice has allowed us to establish that p53 is not required for apoptosis on IL-3 withdrawal. p53-null cells entered apoptosis on removal of IL-3 with a slight (10-hour) delay, compared with wild-type control cells. Expression of dominant-negative p53 in IL-3–dependent cells caused a prolonged (72-hour) delay in entry into apoptosis, although the investigators comment that this effect is most marked in one cell line.27 Our results in a mixed population of primary cells show that apoptosis after IL-3 removal is largely p53 independent. Such p53-independent death has been described for other cell types from the p53-null mouse.16 Furthermore, the radiosensitivity of the p53-null cells in the presence of IL-3 was identical to wild-type control cells. Thus, the death of these cells in response to ionizing radiation is also p53 independent. This differs from hematopoietic progenitor colony formation, in which p53-null cells have been reported to be less sensitive to ionizing radiation.37 Clearly the radioprotective effect of IL-3 also cannot depend on the regulation of p53-responsive genes as was previously proposed.36
Using the BAF3 cell line, which expresses wild-type p53, we have shown that entry into apoptosis occurs at a fixed time after irradiation, irrespective of cell cycle position. This might be expected for a p53-independent mechanism. In contrast, mouse erythroleukemia cells, in which apoptosis can be induced in a p53-dependent manner, die predominantly from G1.26 The overexpression of HPV16 E6 in BAF3 cells inhibits G1 arrest after irradiation and blocks apoptosis.36 However, this cannot be used as an argument for the importance of G1 arrest in the induction of apoptosis. Whereas the effect of E6 on G1 arrest is probably caused by its targeting of p53 for degradation,38 its effect on apoptosis might be caused by a p53-independent mechanism.39 It is now critical to identify the p53-independent signal that directs entry into apoptosis and to determine how it is regulated by IL-3.
ACKNOWLEDGMENT
We are indebted to Ian Titley for sorting cells and fluorescence-activated cell sorted analysis.
Supported by the Cancer Research Campaign.
Address reprint requests to Mary K.L. Collins, PhD, CRC Centre for Cell and Molecular Biology, Institute of Cancer Research, Chester Beatty Laboratories, 237 Fulham Rd, London SW3 6JB, UK.

![Fig. 1. IL-3 regulation of apoptosis in the absence of p53. IL-3–dependent bone marrow cells from wild-type (A) or p53-null (B) mice, were plated at 2 × 105 cell/mL in a 24-well plate in the presence or absence of IL-3 after X-irradiation with 4 Gy as indicated. Cell viability was measured by trypan blue exclusion at the times shown. ([○] + IL-3; [□] + IL-3 + 4 Gy; [•] − IL-3; [▪] − IL-3 + 4 Gy.) (C) 5 × 106 wild-type or p53-null cells were analyzed for DNA fragmentation after X-irradiation as indicated with 4 Gy for 20 hours (wild-type) or 50 hours (p53-null), with or without IL-3, or after 6 hours of treatment with 10 μg/mL valinomycin in the presence of IL-3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2717/4/m_bl_0010f1a.jpeg?Expires=1769206604&Signature=RbaFLSHQceBgq40MOD1AMbxgQN7mIWfxY-xxuqqAHJDis5qkEJaawVqk1mOnYqJTGiksGUBKMZsJphOGiDwk7E8wjho3vS4fMx-EPhARRm3he1XDyJOv4jXodMBpNsCUT6FjkgFZCgJHmmL8c4nZLa2TKaNe71KaRMZFTEW2joPgOaO3d2cEa0oQQmLsejn8l0drWxm5T0db8dbYzMEGyPvUQBaW5l4ZmUkAaoPGzH7Tki8FuojMhJifKtcplE-rL3ieLRaGUzQ0I2AIkgOFHepa7GfUMbgYeb1LP-nxKA6duT2i0lODpN2kjtl-6vwsdECU966tDFrFjF81XJR8Cg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. IL-3 regulation of apoptosis in the absence of p53. IL-3–dependent bone marrow cells from wild-type (A) or p53-null (B) mice, were plated at 2 × 105 cell/mL in a 24-well plate in the presence or absence of IL-3 after X-irradiation with 4 Gy as indicated. Cell viability was measured by trypan blue exclusion at the times shown. ([○] + IL-3; [□] + IL-3 + 4 Gy; [•] − IL-3; [▪] − IL-3 + 4 Gy.) (C) 5 × 106 wild-type or p53-null cells were analyzed for DNA fragmentation after X-irradiation as indicated with 4 Gy for 20 hours (wild-type) or 50 hours (p53-null), with or without IL-3, or after 6 hours of treatment with 10 μg/mL valinomycin in the presence of IL-3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2717/4/m_bl_0010f1c.jpeg?Expires=1769206604&Signature=u1cWIkoIwfY4JtmcbM266rPZcbba-gDcOQ5zZWkSNh~xw91uLHV~1pFQIkfowMmdZlgXVHo8DOgVBdChEOPmlqrjGt7yzc9ooFPN~E-zCJRAvUMBOx1ynPwk8jT4Xk6pqDBiT2mDbH6Bap2TLhIK31JvKDQx0HIUSsoCDH6ck7Jm-hHC8elNY4tb5BgSNdN7WJxotp~3SEnKBiwnEV3ejtqmEmCYwxWizQCpRtR1XsHXhoO64ueTaAhwUZ5CDfDTGBKfDCv8v9Nr4cO~yZT2~2TeCXA9X~-o6iLZiKT-WMWdhoPTNLqXhFKRKfLRaTk7A1cUFatWZOHRnJgzpsIJ8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal