Abstract
A variety of environmental stresses, such as osmotic shock, UV radiation, and heat shock, or the proinflammatory cytokines tumor necrosis factor-α and interleukin-1 reportedly induce activation of c-Jun amino-terminal kinases (JNK), which are usually activated by SEK1/MKK4. We report here that the hematopoietic cytokines interleukin-3 (IL-3), erythropoietin (Epo), and thrombopoietin (Tpo), which regulate growth and differentiation of hematopoietic progenitor cells, erythroids, and megakaryocytes/platelets, respectively, also activate a JNK signaling cascade. In-gel kinase assay as well as in vitro kinase assay clearly showed that IL-3, Epo, and Tpo rapidly and transiently activated both JNK1 and JNK2 in IL-3–, Epo-, or Tpo-dependent mouse hematopoietic progenitor cells. However, immunoblot analysis and in vitro kinase assay showed that neither phosphorylation nor activation of SEK1/MKK4 was induced by IL-3, Epo, or Tpo stimulation. Therefore, we concluded that the JNK signaling cascade plays an important role not only in stress responses and proinflammatory cytokine actions but also in hematopoietic cytokine actions and that hematopoietic cytokines may activate the JNKs through a kinase other than SEK1/MKK4, as previously suggested for stress-activated cells.
RECENT STUDIES identified a novel subgroup of the mitogen-activated protein kinase (MAPK) superfamily in addition to classical MAPK (ERK1 and ERK2) in vertebrate cells. ERKs are protein-serine/threonine kinases that are rapidly activated by a variety of cell growth and differentiation stimuli1-3 and play a central role in mitogenic signaling.4 The subgroup is called c-Jun amino-terminal kinase (JNK) or stress-activated protein kinase (SAPK) and includes 46-kD JNK1 and 55-kD JNK2 isoforms.5,6 Activation of ERK and JNK requires dual phosphorylation at Thr and Tyr residues,7 but the phosphorylation motif present in JNK (Thr-Pro-Tyr) is different from that of ERK (Thr-Glu-Tyr). Actually, the kinases that activate JNK and ERK are distinct.7 JNK is phosphorylated by a dual-specific kinase SAPK/ERK kinase-1 (SEK1) or MAP kinase kinase 4 (MKK4),8-10 and SEK1/MKK4 is phosphorylated at Ser and Thr residues by an upstream kinase MAPK/ERK kinase kinase 1 (MEKK1).8,9 ERK is phosphorylated by MAPK kinase (MEK), MEK is activated by Raf-1,2 and Raf-1 is activated by Ras.11
The SEK-JNK cascade is primarily activated by various environmental stresses (UV radiation, heat shock, x-ray radiation, osmotic shock, hydrogen peroxide, and protein synthesis inhibitors) and by the proinflammatory cytokines tumor necrosis factor-α (TNFα) and interleukin-1 (IL-1).6,9,12-17 It can also be weakly activated by such mitogenic factors as epidermal growth factor and phorbol esters and by T-cell activation signaling.8 18 The exact mechanism of how the SEK-JNK cascade integrates with other signaling pathways to achieve specific response to different stimuli remains to be elucidated.
The hematopoietic cytokines, interleukins, and colony stimulating-factors are known to regulate hematopoietic cell growth and differentiation. These cytokine receptor-mediated signaling pathways have been extensively studied by a number of groups, and activation of Ras/Raf-1/MEK/ERK cascade by various hematopoietic cytokines has been evidenced.19-24 However, whether the Ras/MEKK1/SEK1/JNK pathway can be activated by these cytokines has not been determined. Therefore, we examined the possible activation of SEK1/MKK4 and JNKs by IL-3, erythropoietin (Epo), and thrombopoietin (Tpo), which are hematopoietic cytokines regulating the growth and differentiation of hematopoietic progenitor cells, erythroids, and megakaryocytes/platelets, respectively. Using FD-EPO cells and FD-TPO cells, which are Epo- and Tpo-dependent cell lines derived from IL-3–dependent mouse hematopoietic progenitor FDC-P2 cells, respectively, we measured the activities of JNK1, JNK2, and SEK1/MKK4 after IL-3, Epo, and Tpo stimulation. We found that IL-3, Epo, and Tpo induced activation of JNK1 and JNK2 but not SEK1/MKK4 and thus concluded that this cascade represents an important signaling pathway that mediates the actions of hematopoietic cytokines as well and that hematopoietic cytokines activate JNKs through a kinase other than SEK1/MKK4, as previously suggested for stress-activated cells.25
MATERIALS AND METHODS
Cytokines and antibodies.Antibodies against mouse JNK1 and JNK2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antibody against Thr223-phosphorylated SEK1/MKK4 was purchased from New England Biolabs (Beverley, MA). Human Epo (2.6 × 105 U/mg) was a gift from Kirin Brewery (Tokyo, Japan). Mouse IL-3 (1 × 106 U/mg) was obtained from Genzyme (Cambridge, MA). Mouse Tpo was purified from COS-7 cell secreting recombinant mouse Tpo.26
Cell culture.Epo-dependent FD-EPO cells and Tpo-dependent FD-TPO cells were established by culturing IL-3–dependent FDC-P2 cells in the presence of 0.5 U/mL of human Epo or of 50 U/mL of mouse Tpo in RPMI 1640 medium supplemented with 10% fetal calf serum but without IL-3 for over 2 weeks. After limiting dilution, the Epo- or Tpo-dependent clonal cell line was established. FDC-P2 cells were cultured with 500 U/mL of mouse IL-3.
Immunoprecipitation.Cells were starved in RPMI 1640 medium containing 0.4% fetal calf serum, 0.125 μg/mL of transferrin, and 0.01% bovine serum albumin without Tpo, Epo, or IL-3 for 12 hours and restimulated with or without 50 U/mL of Tpo, 0.5 U/mL of Epo, 500 U/mL of IL-3, or 500 mmol/L sorbitol for up to 30, 60, or 120 minutes. The stimulated and unstimulated cells were immediately lysed in a lysis buffer: 50 mmol/L Tris-HCl, pH 7.5, 0.5% Nonidet P-40, 150 mmol/L NaCl, 100 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 2 mmol/L Pefabloc, 10 ng/mL leupeptin, and 10 ng/mL aprotinin. Insoluble material was then removed by centrifugation and the cleared cell lysate was incubated with a specific antibody at 4°C for 2 hours. The immunocomplexes were then bound to protein A-Sepharose at 4°C for 1 hour. The beads were washed five times with lysis buffer containing 0.1% Nonidet P-40.
Plasmids and expression of glutathione-S-transferase (GST) fusion proteins.GST-c-Jun or JNK1 fusion protein expression vector, pGEX2T-c-Jun or -JNK1, was constructed by inserting a mouse c-jun cDNA fragment encoding amino acids 1-79 or full-length mouse JNK1 into pGEX2T (Pharmacia, Uppsala, Sweden). The plasmids were transfected into NM522. The bacteria grew at OD650 = 0.7 and were incubated with 0.1 mmol/L isopropyl β-D-thiogalactopyranoside for 1 additional hour. Cells suspended in a buffer containing phosphate-buffered saline, 2% Triton X-100, 1% Tween 20, and 10 mmol/L dithiothreitol (DTT) were lysed by sonication. The GST fusion proteins in the lysates were purified by glutathione agarose chromatography as described previously.27 The amounts of purified fusion proteins were estimated by the method of Bradford.28
In vitro protein kinase assay.Immunoprecipitates with anti-JNK1 antibody or anti-JNK2 antibody were mixed with 3 μg of purified GST-c-Jun, 20 μmol/L ATP, and 5 μCi of [γ-32P]ATP in 30 μL of kinase buffer (20 mmol/L HEPES, pH 7.6, 20 mmol/L MgCl2 , 0.1 mmol/L sodium orthovanadate, and 2 mmol/L DTT) and incubated at 30°C for 20 minutes. The reactions were terminated by mixing with Laemmli sample buffer and boiling. The samples were resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiographed. Similarly, immunoprecipitates with anti-phospho–specific SEK1/MKK4 antibody were incubated with 3 μg of purified GST-JNK1, 50 μmol/L ATP, and 10 μCi of [γ-32P]ATP in 30 μL of kinase buffer (20 mmol/L HEPES, pH 7.6, 15 mmol/L MgCl2 , 5 mmol/L MnCl2 , 0.1 mmol/L sodium orthovanadate, and 2 mmol/L DTT) and incubated at 30°C for 40 minutes. The samples were resolved by 10% SDS-PAGE and autoradiographed.
In-gel kinase assay.In-gel kinase assays were performed by the modified method of Hibi et al.5 Immunoprecipitates with anti-JNK1 or anti-JNK2 antibody were resolved on a 10% SDS-polyacrylamide gel, which was polymerized in the absence or presence of 50 μg/mL GST-c-Jun. After electrophoresis, the gel was washed twice for 30 minutes with 100 mL of 20% 2-propanol in 50 mmol/L HEPES, pH 7.6, to remove SDS. It was then washed twice for 30 minutes with 100 mL of buffer A (50 mmol/L HEPES, pH 7.6, 5 mmol/L β-mercaptoethanol) and then incubated in 200 mL of 6 mol/L guanidine in buffer A at 25°C for 1 hour. After washing several times for 1 hour each with 100 mL of buffer A containing 0.05% Tween 20 at 4°C, the gel was incubated in kinase buffer containing 50 μmol/L ATP and 0.25 μCi/mL of [γ-32P]ATP at 30°C for 1 hour. Finally, it was washed several times with 100 mL of 5% trichloroacetic acid and 1% sodium pyrophosphate at 25°C, followed by drying and autoradiography.
Immunoblotting.Samples (150 μg) were fractionated in 10% SDS-polyacrylamide gels and electrotransferred to ECL membrane (Amersham, Buckinghamshire, UK). The membrane was blocked in 5% bovine serum albumin in 20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.5% Tween 20 (TBS-T) and incubated with anti-phospho–specific SEK1/MKK4 antibody for 2 hours. After washing 3 times with TBS-T, the membrane was incubated with antirabbit IgG-conjugated horseradish peroxidase antibody, and the antibody complexes were visualized by an ECL system (Amersham).
RESULTS
In vitro kinase assay showed that Tpo, Epo, and IL-3 activate both JNK1 and JNK2.We examined possible JNK activation with IL-3, Epo, or Tpo in IL-3–dependent FDC-P2 cells, Epo-dependent FD-EPO cells, and Tpo-dependent FD-TPO cells. These cell lines express endogenous receptors for IL-3, Epo, and Tpo, respectively, and respond to each cytokine in a dose-dependent manner. The JNKs were immunoprecipitated with anti-JNK1– or anti-JNK2–specific antibody at various time points after IL-3, Epo, or Tpo stimulation, and protein kinase activity in the immunoprecipitates was measured in the presence of [γ-32P]ATP and the purified GST-c-Jun fusion protein (molecular weight, 35 kD) as a substrate.
It was clear that all three cytokines (IL-3, Epo, and Tpo) rapidly and transiently activated both JNK1 (Fig 1A) and JNK2 (Fig 1B). JNK activity was rarely seen in unstimulated cells, but a rapid and marked increase in JNK1 activity was seen within 5 minutes of treatment with IL-3, Epo, or Tpo (Fig 1A). This activity reached maximal level at 15 minutes and then declined to the basal level in all three cases (Fig 1A). The levels of JNK1 activity differed from cytokine to cytokine; it was highest in Tpo-stimulated cells and lowest in IL-3–treated cells.
IL-3, Epo, and Tpo activate both JNK1 and JNK2 in in vitro kinase assay. FDC-P2 cells (upper panel), FD-EPO cells (middle panel), and FD-TPO cells (lower panel) were stimulated with IL-3, Epo, and Tpo, respectively, for the indicated time up to 120 minutes. JNK1 (A) and JNK2 (B) activity was measured in the immunoprecipitates with anti-JNK1– and anti-JNK2–specific antibody and [γ-32P]ATP and GST-c-Jun as a substrate. Arrows indicate the phosphorylated GST-c-Jun (molecular weight, 35 kD).
IL-3, Epo, and Tpo activate both JNK1 and JNK2 in in vitro kinase assay. FDC-P2 cells (upper panel), FD-EPO cells (middle panel), and FD-TPO cells (lower panel) were stimulated with IL-3, Epo, and Tpo, respectively, for the indicated time up to 120 minutes. JNK1 (A) and JNK2 (B) activity was measured in the immunoprecipitates with anti-JNK1– and anti-JNK2–specific antibody and [γ-32P]ATP and GST-c-Jun as a substrate. Arrows indicate the phosphorylated GST-c-Jun (molecular weight, 35 kD).
Similarly, JNK2 activity was the highest 15 minutes after IL-3, Epo, or Tpo stimulation and returned to the basal level at 30 to 60 minutes (Fig 1B). Tpo activated JNK2 most efficiently, whereas IL-3 and Epo did so only moderately (Fig 1B).
As shown in Fig 1, the anti-JNK1 and anti-JNK2 antibodies used here, which are rabbit polyclonal antibodies against recombinant full-length human JNK1 or JNK2 expressed in Escherichia coli, specifically immunoprecipitated JNK1 and JNK2, respectively, and did not cross-react with each other.
In-gel kinase assay confirmed that IL-3, Epo, and Tpo specifically activate both JNK1 and JNK2.To confirm that the kinases phosphorylated GST-c-Jun in the immunoprecipitates with anti-JNK1 and anti-JNK2 antibody in vitro were, in fact, JNK1 and JNK2, we next performed in-gel kinase assay of anti-JNK1 and anti-JNK2 immunoprecipitates in SDS-PAGE gels containing GST-c-Jun. The immunoprecipitates with anti-JNK1 or anti-JNK2 antibody in the cell extracts with or without 15 minutes of cytokine treatment were separated by 10% SDS-PAGE containing GST-c-Jun, and incubated with [γ-32P]ATP after protein renaturation. As shown in Fig 2A, in the anti-JNK1 immunoprecipitates, the 32P-labeled GST-c-Jun was clearly observed at the exact molecular weight of JNK1 (46 kD) only after IL-3, Epo, or Tpo stimulation. No JNK2 or other nonspecific band was observed. As true in the in vitro kinase assay, JNK1 activity was highest in Tpo-stimulated cells and lowest in IL-3–treated cells (Fig 2A).
IL-3, Epo, and Tpo specifically activate both JNK1 and JNK2. FDC-P2 cells (left 2 lanes), FD-EPO cells (middle 2 lanes), and FD-TPO cells (right 2 lanes) were stimulated with IL-3, Epo, or Tpo, respectively, for 0 minutes (−) or 15 minutes (+). In-gel JNK assays were performed in the immunoprecipitates with anti-JNK1 (A) or anti-JNK2 (B) antibody. The immunoprecipitates were separated by SDS-polyacrylamide gels containing GST-c-Jun and incubated with [γ-32P]ATP after protein renaturation. The phosphorylated GST-c-Jun corresponding to the molecular weights of JNK1 (46 kD) and JNK2 (55 kD) are indicated by arrows.
IL-3, Epo, and Tpo specifically activate both JNK1 and JNK2. FDC-P2 cells (left 2 lanes), FD-EPO cells (middle 2 lanes), and FD-TPO cells (right 2 lanes) were stimulated with IL-3, Epo, or Tpo, respectively, for 0 minutes (−) or 15 minutes (+). In-gel JNK assays were performed in the immunoprecipitates with anti-JNK1 (A) or anti-JNK2 (B) antibody. The immunoprecipitates were separated by SDS-polyacrylamide gels containing GST-c-Jun and incubated with [γ-32P]ATP after protein renaturation. The phosphorylated GST-c-Jun corresponding to the molecular weights of JNK1 (46 kD) and JNK2 (55 kD) are indicated by arrows.
Similarly, in the anti-JNK2 immunoprecipitates, bands corresponding to the molecular weight of JNK2 (55 kD) were clearly detected in all three cytokine-stimulated cells (Fig 2B). JNK2 activity also was highest in Tpo-stimulated cells and lowest in IL-3–treated cells (Fig 2B). A nonspecific band (≈70 kD) was always seen in this case. Activity of neither JNK1 nor JNK2 was detected in the cell extracts of untreated cells (Fig 2A and B) or when GST-c-Jun was not included in the gels (data not shown). Therefore, we concluded that IL-3, Epo, and Tpo specifically activate both JNK1 and JNK2.
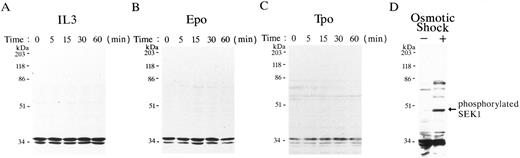
Phosphorylation of SEK1/MKK4 was not induced by either IL-3, Epo, or Tpo stimulation.It has been reported that SEK1/MKK4 phosphorylates and activates JNKs8,9; thus, we sought to learn whether SEK1/MKK4 is phosphorylated upon IL-3, Epo, and Tpo stimulation. Figure 3 shows the results of immunoblot analyses with anti-phospho–specific SEK1/MKK4 (Thr223) antibody at various time points after IL-3, Epo, or Tpo stimulation. This phospho-specific SEK1/MKK4 antibody detects SEK1/MKK4 only when phosphorylated at Thr223. Little phosphorylated SEK1/MKK4 (46 kD) was detected before or after stimulation with the hematopoietic cytokines (IL-3, Epo, and Tpo; Fig 3A, B, and C), whereas phosphorylation of SEK1/MKK4 was clearly induced by osmotic shock (Fig 3D).
Phosphorylation of SEK1/MKK4 was not induced by IL-3, Epo, or Tpo stimulation. FDC-P2 cells (A and D), FD-EPO cells (B), and FD-TPO cells (C) were stimulated with IL-3 (A), Epo (B), and Tpo (C), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) sorbitol (D) for 30 minutes. Total cell lysates were separated by SDS-PAGE and immunoblotted with anti-phospho–specific SEK1/MKK4 antibody. An arrow indicates the phosphorylated SEK1 (46 kD).
Phosphorylation of SEK1/MKK4 was not induced by IL-3, Epo, or Tpo stimulation. FDC-P2 cells (A and D), FD-EPO cells (B), and FD-TPO cells (C) were stimulated with IL-3 (A), Epo (B), and Tpo (C), respectively, for the indicated time up to 60 minutes or stimulated with (+) or without (−) sorbitol (D) for 30 minutes. Total cell lysates were separated by SDS-PAGE and immunoblotted with anti-phospho–specific SEK1/MKK4 antibody. An arrow indicates the phosphorylated SEK1 (46 kD).
No change in SEK1/MKK4 activity by IL-3, Epo, and Tpo stimulation.We next examined whether SEK1/MKK4 is activated by IL-3, Epo, or Tpo stimulation. The protein kinase activity in the immunoprecipitates with anti-phospho–specific SEK1/MKK4 antibody was measured in the presence of [γ-32P]ATP and GST-JNK1 (Fig 4). Weak SEK1/MKK4 activity was always detected before and after IL-3, Epo, and Tpo stimulation, and the level of this activity was not changed by any of the three hematopoietic cytokine treatments (Fig 4, lanes 1 through 6). In contrast, SEK1/MKK4 activity was clearly enhanced by osmotic shock (Fig 4, lanes 7 and 8). No phosphorylated GST-JNK1 was detected when either GST-JNK1 or the immunoprecipitate was absent from the reaction mix (data not shown). It was therefore concluded that neither IL-3, Epo, nor Tpo activated SEK1/MKK4.
IL-3, Epo, and Tpo did not activate SEK1/MKK4. FDC-P2 cells (lanes 1, 2, 7, and 8), FD-EPO cells (lanes 3 and 4), and FD-TPO cells (lanes 5 and 6) were stimulated with (+) or without (−) IL-3 (lanes 1 and 2), Epo (lanes 3 and 4), Tpo (lanes 5 and 6), and sorbitol (lanes 7 and 8), respectively. SEK1/MKK4 activity was measured in the immunoprecipitates with anti-phospho–specific SEK1/MKK4 antibody and [γ-32P]ATP and GST-JNK1. An arrow indicates the phosphorylated GST-JNK1.
IL-3, Epo, and Tpo did not activate SEK1/MKK4. FDC-P2 cells (lanes 1, 2, 7, and 8), FD-EPO cells (lanes 3 and 4), and FD-TPO cells (lanes 5 and 6) were stimulated with (+) or without (−) IL-3 (lanes 1 and 2), Epo (lanes 3 and 4), Tpo (lanes 5 and 6), and sorbitol (lanes 7 and 8), respectively. SEK1/MKK4 activity was measured in the immunoprecipitates with anti-phospho–specific SEK1/MKK4 antibody and [γ-32P]ATP and GST-JNK1. An arrow indicates the phosphorylated GST-JNK1.
Taken together, we concluded that hematopoietic cytokines, at least IL-3, Epo, and Tpo, clearly induce activation of both JNK1 and JNK2, which is activated primarily by a kinase other than SEK1/MKK4, and that the JNK signaling pathway plays an important role not only in the response to environmental stresses and proinflammatory cytokines, but also to hematopoietic cytokines.
DISCUSSION
We show here that hematopoietic cytokines, at least IL-3, Epo, and Tpo, clearly activate the JNK signaling pathway, which has heretofore been believed to be activated only by the environmental stresses of UV radiation, heat shock, and osmotic shock, or proinflammatory cytokines such as TNFα and IL-1.6,9,12-17 To our knowledge, this is the first report that hematopoietic cytokines, whose receptors belong to the type I cytokine superfamily, induce activation of the JNK pathway. Because it has been described that hematopoietic cytokines activate the Ras/Raf-1/MEK/ERK pathway,19-24 it is likely that at least these three hematopoietic cytokines activate both JNK cascade and ERK cascade.
We observed that both JNK1 and JNK2 are clearly activated, but contrary to our expectations, neither induction of SEK1/MKK4 phosphorylation nor activation of SEK1/MKK4 was detected after IL-3, Epo, and Tpo stimulation. Therefore, the activity of JNK1 and JNK2 appeared not to be correlated with that of SEK1/MKK4. Furthermore, we found that Tpo activated both JNK1 and JNK2 most efficiently, Epo moderately activated them, and IL-3 only modestly stimulated them. However, the SEK1/MKK4 activity was very weak and was not changed by any of the three hematopoietic cytokine treatments. This fact also strengthens our conclusion that SEK1/MKK4 may in part but not primarily activate JNKs in the hematopoietic cytokine signaling pathway. It has also been reported that epidermal growth factor induced only a slight increase in SEK1/MKK4 phosphorylation, whereas JNKs were significantly activated.29 Furthermore, it has been postulated that there is another member of MKK involved in JNK activation in stress-activated cells.25 Although we cannot eliminate the possibility that SEK1/MKK4 partly activates JNK1 and/or JNK2 in these hematopoietic cytokine stimulated cells, it is most likely that a kinase other than SEK1/MKK4 is mainly involved in the activation of JNK by factors such as epidermal growth factor and hematopoietic cytokines. The characterization of this unidentified MKK remains to be resolved, and the activation mechanism of JNK must be identified in more detail.
It is possible that other interleukins and colony-stimulating factors may also activate the JNK cascade; this point should be clarified soon. The targets of this cytokine-induced JNK signaling pathway need identification. Furthermore, what kind of role the JNK signaling cascade plays among hematopoietic cytokine actions, ie, cell differentiation, proliferation, tissue-specific functions, and inhibition or stimulation of apoptosis and/or cell survival, also has to be clarified.
ACKNOWLEDGMENT
The authors thank Dr M. Karin (University of California at San Diego, San Diego, CA) for GST-JNK1, Drs M. Hibi (Osaka University, Osaka, Japan) and T. Satoh (Tokyo Institute of Technology, Tokyo, Japan) for their helpful discussions, Kirin Brewery for Epo, and C. Hisano and K. Okutomi for their technical assistance.
Supported in part by a Grant-in-Aid from the Mitsubishi Foundation and a Special Grant for Promotion of Research from The Institute of Physical and Chemical Research (RIKEN).
Address reprint requests to Kazuo Todokoro, PhD, Tsukuba Life Science Center, The Institute of Physical and Chemical Research (RIKEN), 3-1, Koyadai, Tsukuba, Ibaraki 305, Japan.

![Fig. 1. IL-3, Epo, and Tpo activate both JNK1 and JNK2 in in vitro kinase assay. FDC-P2 cells (upper panel), FD-EPO cells (middle panel), and FD-TPO cells (lower panel) were stimulated with IL-3, Epo, and Tpo, respectively, for the indicated time up to 120 minutes. JNK1 (A) and JNK2 (B) activity was measured in the immunoprecipitates with anti-JNK1– and anti-JNK2–specific antibody and [γ-32P]ATP and GST-c-Jun as a substrate. Arrows indicate the phosphorylated GST-c-Jun (molecular weight, 35 kD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2664/4/m_bl_0055f1.jpeg?Expires=1763546282&Signature=HK-9g02Hb-x3-CQXFLOaC3BzmGr93XUL9X0l3XPOVMOlnNuMb~-nMoJN2wSV3hmokzu6oEF8mPg2TR45Ynr4Wbor2FunzvhvNCXKy~ggaJ5cYLNT-0qSdPvdT0NY5KPOvWCfXVhx5pA0ymWYIwuBCNBVZxOzkNfp7oL8KYtm2~qE1Zn4HnOsKHI8DxzrHn~pOtEZG4FUNtGpr9Wt3PglxgFrQTthQoFevfzpu6M3NZBdinvEhz5bYbIVVqiPvoCJlW1kNj~ghuuGOEPo2p~5bJ79qCJchhc6GcM4XsWyzvBOTA~W4Qy4JHF8SKfWOGPx-PdJw-7GvAHwBhDiG2SZvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. IL-3, Epo, and Tpo specifically activate both JNK1 and JNK2. FDC-P2 cells (left 2 lanes), FD-EPO cells (middle 2 lanes), and FD-TPO cells (right 2 lanes) were stimulated with IL-3, Epo, or Tpo, respectively, for 0 minutes (−) or 15 minutes (+). In-gel JNK assays were performed in the immunoprecipitates with anti-JNK1 (A) or anti-JNK2 (B) antibody. The immunoprecipitates were separated by SDS-polyacrylamide gels containing GST-c-Jun and incubated with [γ-32P]ATP after protein renaturation. The phosphorylated GST-c-Jun corresponding to the molecular weights of JNK1 (46 kD) and JNK2 (55 kD) are indicated by arrows.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2664/4/m_bl_0055f2.jpeg?Expires=1763546282&Signature=mI1ED7X1AiiiFHcSnFGgPhxAhPmV2-ZoOn4M7OJkUR1uv8uAPWVcfPvwt21NCHASl7A6P7hFTOTArsr~9JmLvlQdviZERXfwT5I6XRYr5Qam~Xet958YhaY5ZAwiQqZjbEAzR5xoFXT7dN3j9WzROq8wC7xMw2vqyNLe4ifJd5Sz5CN8JGxUlk2cFaheCsdBwJhF3aGwAttzj-DS1ZYIMLyRVdqhBv5wrEyVpI3JPS0aveLAvLOiyvNjriOyd04mptJJGy2U2fnKaPsAxs~tM1wplZ5lQTQlpcI-MFI~Tjrrf5iCENGyXgdJQAXvHWAeuKEWzU7AcZrO5a-0rezaeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. IL-3, Epo, and Tpo did not activate SEK1/MKK4. FDC-P2 cells (lanes 1, 2, 7, and 8), FD-EPO cells (lanes 3 and 4), and FD-TPO cells (lanes 5 and 6) were stimulated with (+) or without (−) IL-3 (lanes 1 and 2), Epo (lanes 3 and 4), Tpo (lanes 5 and 6), and sorbitol (lanes 7 and 8), respectively. SEK1/MKK4 activity was measured in the immunoprecipitates with anti-phospho–specific SEK1/MKK4 antibody and [γ-32P]ATP and GST-JNK1. An arrow indicates the phosphorylated GST-JNK1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2664/4/m_bl_0055f4.jpeg?Expires=1763546282&Signature=0tzV-an0TClgsy01r6qqgXdA1czIVmp9pD6vwvJyzdSrLp14diWwXPklu76rmB-tmlRKp7OrwwzR-sUNMFit27gk9FM9hBGHGQ3qT6bM1wNuSfPZkj~DxUXyDDeqvg4DCwZcFh0YEGPDCYtOukJs1DBYiIME8F0YF~Fj431grrK8W9Up6X3tc37f0DUpCqe6hHHCGqAg9ifZrVpK8tgkDHiVez9x31oyrp9uuSThAy9DS40u-dm5LG6AaA2nEXnQCrueitydBtRNwnNpcoRRRDosqs2PoG~oZSuwKc8GOQlIFvTzeiFVRi~ej1doVP9qgKTtNm244ZTgGZxhCRdXpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal