Abstract
The SJL/J mouse strain has a high spontaneous incidence of a B-cell neoplasm, reticulum cell neoplasm type B (RCN B). In addition, following irradiation, 10% to 30% of these mice develop acute myelomonocytic leukemia (radiation-induced acute myeloid leukemia [RI-AML]), an incidence that can be increased to 50% by treatment of the mice with corticosteroids after irradiation. The role played by the mononuclear phagocyte growth factor, colony-stimulating factor-1 (CSF-1), in the development of RI-AML in SJL/J mice was investigated. Mice dying of RI-AML, but not those dying of RCN B or without disease, possessed elevated concentrations of circulating CSF-1. In addition, in mice developing RI-AML with a more prolonged latency, circulating CSF-1 concentrations were increased before overt expression of RI-AML. First-passage tumors from 14 different RI-AMLs all contained high concentrations of CSF-1, and six of six different first- or second-passage tumors expressed the CSF-1 receptor (CSF-1R). Furthermore, in vitro colony formation by first- or second-passage tumor cells from 20 of 20 different RI-AMLs was blocked by neutralizing anti–CSF-1 antibody, and four of four of these tumors were inhibited by anti–CSF-1R antibody. The results of these antibody neutralization studies, coupled with the observation of elevated circulating CSF-1 in mice developing RI-AML, show an autocrine role for CSF-1 in RI-AML development in SJL/J mice. Southern blot analysis of tumor DNA from six of six of these tumors failed to reveal any rearrangements in the genes for CSF-1 or the CSF-1R. Studies in humans have shown that patients with AML possess elevated levels of circulating CSF-1 and that AML cells can express CSF-1 and the CSF-1R. Thus, RI-AML in the SJL/J mouse appears to be a useful model for human AML.
THE SJL/J MOUSE STRAIN, established in the early 1960s by Murphy,1 has a high spontaneous incidence of reticulum cell neoplasms with a mean latency of 400 days. These multicellular types of tumors were designated by Dunn2 as reticulum cell neoplasms type B (RCN B). Similar types of tumors developing spontaneously in old BALB/c mice were classified as lymphoid cell neoplasms derived from follicular center cells. The B-cell nature of these neoplasms was further suggested on the basis of immunomorphologic classification.3 SJL/J mice, as well as the RF, CBA, and C3H strains, were also found to be moderately susceptible (10% to 30%) to radiation-induced acute myeloid leukemia (RI-AML) following a single exposure to 1.5 to 3.5 Gy.4-7 A general characteristic of these leukemias is a deletion in one allele of chromosome 2.8-11
Exposure of SJL/J mice to a single dose of 3- to 3.5-Gy whole-body irradiation induced RI-AML in 10% to 30% of the treated mice with a mean latency of 1 year. Administration of corticosteroids early or late after irradiation promoted RI-AML development (up to 50%) and reduced latency.12 Continuous administration of corticosteroids to SJL/J mice without previous exposure to radiation did not trigger RI-AML development. Treatment of young SJL/J mice with corticosteroids actually significantly retarded the spontaneous development of RCN B.13 Since high levels of T cells having 20α-hydroxysteroid dehydrogenase activity were shown to populate RCN B,14 their elimination by corticosteroids may reduce the proliferative capacity of the neoplastic cells present among these multicellular tumor types.
The occurrence of multiphase events in RI-AML development, involving radiation-induced initiation of potential leukemic cells but often requiring an additional contribution of promoting factors for tumor progression, was suggested following a series of in vivo studies.15 Potential leukemic cells were found among bone marrow cells of overtly nonleukemic SJL/J mice exposed to radiation (tested at 3 to 4 months following radiation, long before overt RI-AML occurs); bone marrow fractions enriched in spleen colony-forming units16 had optimal leukemogenic potential, yielding a high AML incidence of marrow donor origin in these recipients. Transfer of bone marrow or similar bone marrow fractions from nontreated SJL/J mice into susceptible recipients did not result in RI-AML development. However, the fraction enriched in lymphoid cells yielded, as expected, a high incidence of RCN B. The occurrence of radiation-induced potential leukemic cells during the preleukemic phase was also indicated by cytogenetic studies. The characteristic deletion in one copy of chromosome 2 observed in all RI-AMLs developing in SJL/J mice (never observed in RCN B that develops in some irradiated SJL/J mice) was already apparent at the preleukemic stage. Cells bearing the deletion in chromosome 2 were observed in the bone marrow of 80% to 100% of SJL/J mice 4 months after exposure to radiation (with or without further treatment with corticosteroids), long before overt RI-AML developed in some of these treated mice.10,11 Thus, radiation results in the initiation of potential RI-AML cells (multipotential hematopoietic stem or progenitor cells) with a predisposition to subsequent neoplastic progression. Further in vivo treatment of irradiated SJL/J mice with different hematopoietic growth factors resulted in a striking modulatory effect on leukemia incidence and latency. Early administration of different growth factors (starting 14 days after radiation and dexamethasone treatment) showed that colony-stimulating factor-1 (CSF-1) increased AML incidence (to 75%) and reduced its latency, whereas similar treatments with granulocyte-macrophage CSF or granulocyte CSF had no promoting effect. However, growth factors administered 140 days after the initial leukemogenic treatment showed that granulocyte-macrophage CSF and, to a lesser degree, granulocyte CSF increased AML incidence, whereas CSF-1 had no effect.15 Although the promoting mechanism of corticosteroids in RI-AML has not been elucidated, it seems that the high doses administered to mice could affect the long-term integrity and/or function of cells involved in the production of hematopoietic growth factors. Indeed, we recently observed a significant increase in CSF-1 production in irradiated SJL/J mice and a further increase following treatment with dexamethasone.17 A coleukemogenic effect of CSF-1 preparations, administered as substitutes for dexamethasone following exposure to radiation, suggested that CSF-1 possessed a promoting effect in RI-AML development.17 The coleukemogenic effect of CSF-1 preparations was also observed when administered shortly after treatment with both radiation and dexamethasone.15 These results suggested that CSF-1 production might be involved in RI-AML development. Studies concerned with humoral and cell-surface interactions during radiation leukemogenesis in vitro have indicated that a factor with the biologic and antigenic properties of CSF-1 is released from irradiated bone marrow stromal cell lines and induces the in vitro expansion of hematopoietic precursor cells.18 19
In the present study, we investigated the role of CSF-1 in the development of RI-AML in the SJL/J mouse. The results indicate that CSF-1 is involved in RI-AML development in a way that may parallel its involvement in several human neoplastic diseases, including leukemia.
MATERIALS AND METHODS
RI-AML induction.Female SJL/J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Three-month-old mice were exposed to 3 Gy total-body irradiation, followed 1 to 3 hours later by subcutaneous inoculation with 0.5 mg dexamethasone (Teva, Petach Tikva, Israel). For irradiation, mice were placed in a curved Lucite tube at a focal distance of 75 cm from a 60Co gammabeam 150 source (Atomic Energy Canada, Ottawa, Ontario) with an exposure rate of 0.66 r/min. Tumor development was monitored every second week by palpation. The success rate for detection of RI-AML by palpation was approximately 90%. Thus, mice developing RI-AML or RCN B had usually been exsanguinated before autopsy. Autopsy invariably showed enlargement of the spleen and mesenteric, intrathoracic, and peripheral lymph nodes, and often enlargement of the liver. An increased percentage of blast cells was observed only in marrow smears (not in peripheral blood). In every instance, the diagnosis of RI-AML or RCN B was confirmed by histologic examination and by staining cells with RB6-8C5 monoclonal antibody (kindly provided by R.L. Coffman, DNAX, Palo Alto, CA), which specifically recognizes antigenic determinants on the surface of murine myeloid cells that are absent on RCN B cells.
CSF-1 extraction and assay.CSF-1 was extracted from tumors by homogenization and heating of the homogenized extracts at 56°C for 30 minutes, as previously described for the extraction of CSF-1 from mouse tissues.20 CSF-1 concentrations in tumor extracts and sera were measured by a radioimmunoassay (RIA) that detects the biologically active growth factor at concentrations of 120 pg/mL and higher and has interassay and intraassay variations of approximately 10%.21,22 The RIA is based on the competition by CSF-1 for the interaction between 125I-labeled purified mouse L-cell CSF-1 glycoprotein with a rabbit polyclonal antibody to purified L-cell CSF-121 and is more sensitive than the conventional CSF-1 bioassay based on bone marrow colony formation.22 RIAs were performed in duplicate on 20-μL samples. The concentration of CSF-1 in picograms per milliliter was determined with reference to a standard curve prepared using a stable, partially purified L-cell CSF-1 preparation. For comparison of the data with previous reports using this RIA that reported concentrations in units per milliliter, 1 U is exactly equivalent to 12 pg. As an added precaution, serum samples of known CSF-1 concentration were included in each RIA to check standardization.
Flow cytometry.Fresh samples of RI-AMLs grown in vivo for one or two passages were minced in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline containing 1% fetal calf serum and 0.05% sodium azide). To remove possible cytophilic Ig from the cells, a brief low-pH wash was used. One milliliter of RPMI medium at pH 3.0 was added to a cell pellet, which was immediately resuspended and neutralized by addition of 10 mL FACS buffer at pH 7.5. The cells were washed twice and adjusted to 20 × 106 cells/mL. Cells (100 μL) were incubated with 20 μL 1:10 dilution of a rat anti–CSF-1R antibody23 (clone 604 B52 E11, kindly provided by Dr G.L. Gilmore and Dr R. Shadduck, Pittsburgh, PA) for 30 minutes at 4°C. The cells were then washed twice with 3 mL FACS buffer, and 20 mL fluorescein isothiocyanate (FITC)-conjugated goat anti–rat Ig (H + L) (ultracentrifuged by airfuge to remove aggregates; Caltage Laboratories, San Francisco, CA) was added to the pellet for an additional 30-minute incubation at 4°C. For each RI-AML tested, a control tube with FITC-labeled secondary antibody was added. Thereafter, samples were washed twice and analyzed on a FACScan flow cytometer.
Abrogation of in vitro growth of RI-AML cells.RI-AML cells (2 × 105/mL, 0.5 mL) were plated in duplicate by mixing with 1.5 mL plating medium (Iscove's modified Dulbecco's medium containing 0.9% methylcellulose and 20% fetal calf serum in a 35-mm tissue culture dish).24 Normal goat serum, goat anti–mouse CSF-1 antiserum,22 or goat anti–mouse CSF-1R monoclonal antibody25 (25 to 50 μL) were added to individual dishes at final concentrations of 1:50 and 1:100. To some plates, 60 ng/mL human recombinant CSF-1 (a gift from Chiron Corp, Emeryville, CA) was added with or without goat anti–human CSF-1 antiserum22 (final concentration, 1:50 and 1:100). For some RI-AMLs, the final number of cells per dish was reduced due to poor recovery of tumor cells. For these, equal numbers of cells were plated for the different groups within each experiment. The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 . Selected colonies were plucked from the cultures and spread over glass slides, and the cellular composition was determined microscopically using Wright's stain.
The inhibitory effects of anti–CSF-1 and anti–CSF-1R antibodies were also tested on normal murine bone marrow cells at the same concentrations with or without the addition of CSF-1. Both antibodies inhibited mouse CSF-1–stimulated colony formation at the concentrations used for the RI-AML experiments, and the effect of the anti–CSF-1 antibody could be abrogated when excess mouse CSF-1 was added to the cultures.
RESULTS
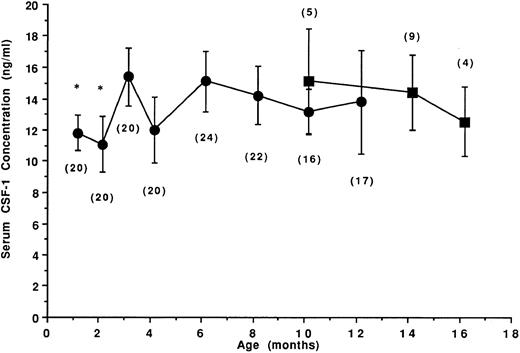
Age-related CSF-1 concentrations in sera of untreated SJL/J mice.Twenty-four female SJL/J mice were bled repeatedly at the ages of 1, 2, 3, 4, 6, 8, 10, and 12 months, and the levels of CSF-1 in individual serum samples were determined. To accurately measure circulating concentrations of CSF-1, serum samples were subjected to a mouse CSF-1–specific RIA. In biologic samples, this RIA detects only biologically active CSF-1,21,26 including both glycoprotein and proteoglycan27 forms. The mean CSF-1 concentrations in the sera of 1- to 12-month-old normal untreated female SJL/J mice and in 10- to 16-month-old mice that spontaneously developed a B-cell neoplasm (RCN B) are shown in Fig 1. In the sera of 1- and 2-month-old SJL/J mice, mean CSF-1 levels were less than 12 ng/mL (11.5 and 10.8 ng/mL, respectively). At 3 months, a significant increase in CSF-1 levels was noted (mean, 15.1 ng/mL), but serum CSF-1 levels thereafter were not significantly different from the levels in 3-month-old mice. In mice developing RCN B, the levels were not significantly different from those of disease-free mice.
Age-related circulating CSF-1 levels in untreated SJL/J mice. Female SJL/J mice were bled repeatedly at the ages of 1, 2, 3, 4, 6, 8, 10, and 12 months and the concentration of CSF-1 in the sera was determined by RIA (•). Up to age 8 months, CSF-1 levels reflect the mean ± SD serum CSF-1 concentrations from 20 to 24 disease-free mice. Commencing at 10 months, approximately one third of the mice spontaneously developed a B-cell neoplasm (RCN B, ▪). Values in parentheses indicate the number of mice at each time point. *CSF-1 levels significantly different (Student's t-test, P < .05) from the levels at 3 months of age.
Age-related circulating CSF-1 levels in untreated SJL/J mice. Female SJL/J mice were bled repeatedly at the ages of 1, 2, 3, 4, 6, 8, 10, and 12 months and the concentration of CSF-1 in the sera was determined by RIA (•). Up to age 8 months, CSF-1 levels reflect the mean ± SD serum CSF-1 concentrations from 20 to 24 disease-free mice. Commencing at 10 months, approximately one third of the mice spontaneously developed a B-cell neoplasm (RCN B, ▪). Values in parentheses indicate the number of mice at each time point. *CSF-1 levels significantly different (Student's t-test, P < .05) from the levels at 3 months of age.
Because of the possible involvement of circulating CSF-1 in the development of RI-AML and the increase in circulating CSF-1 observed within the first 3 months of life, the age-related susceptibility to RI-AML in SJL/J mice was tested by exposing 1-, 2-, or 3-month-old SJL/J mice to leukemogenic treatment involving exposure to a single dose of 3-Gy total-body irradiation followed by a single injection of dexamethasone (0.5 mg subcutaneously) within 1 to 3 hours postirradiation. The results indicate that the susceptibility to RI-AML increased with age. Mice treated at 1 and 2 months of age, which also had significantly lower serum CSF-1 concentrations than older mice, had a significantly lower incidence (3 of 17, or 17%, and 5 of 17, 30%) of RI-AML than mice treated at 3 months of age (14 of 21, 66%). The average latency period was not significantly different in the age groups tested (284 ± 40, 255 ± 22, and 269 ± 45 days, respectively; data not shown).
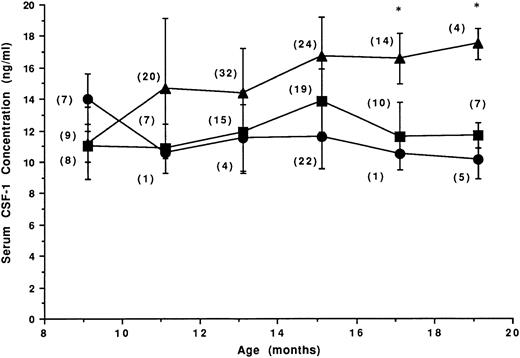
Circulating CSF-1 levels in mice at time of death following RI-AML leukemogenic treatment.Two hundred twenty 3-month-old female SJL/J mice were exposed to the leukemogenic treatment and palpated every second week to follow tumor development. Of these mice, 103 developed RI-AML, 76 developed RCN B, and 41 died tumor-free. Serum CSF-1 levels at the time of death in mice that developed RI-AML or RCN B compared with those that did not develop any lesion are illustrated in Fig 2. In contrast to mice that did not receive leukemogenic treatment (Fig 1), the mean circulating CSF-1 concentration in treated mice that did not develop either RI-AML or RCN B was usually lower than 12 ng/mL (Fig 2). CSF-1 levels in mice developing RCN B were similar to those observed in sera of the tumor-negative mice. RI-AMLs with a short (6-month) latency (nine mice at 9 months of age) had a low CSF-1 concentration similar to the levels observed in the other two groups. With the prolongation of RI-AML latency, a significant increase in CSF-1 levels in the sera of mice with RI-AML was observed. The data reflect an association between elevated circulating CSF-1 and RI-AML that is specific for RI-AML (not seen in RCN B mice). The increase in the circulating CSF-1 concentration in mice dying of RI-AML at 17 and 19 months of age was equivalent to the increase in circulating CSF-1 in pregnant mice in which the uterine concentration of CSF-1 has increased by 1,000-fold.20
CSF-1 levels at death in mice receiving leukemogenic treatment. Mice (N = 220) were treated at 3 months of age with 3 Gy and dexamethasone (0.5 mg subcutaneously); 47% developed RI-AML (▴), 35% developed RCN B (▪), and 18% died tumor-free (•). The number of mice at each time point is shown in parentheses. *Circulating CSF-1 levels in RI-AML mice that were significantly different from the corresponding levels in tumor-free mice (Student's t-test, P < .005).
CSF-1 levels at death in mice receiving leukemogenic treatment. Mice (N = 220) were treated at 3 months of age with 3 Gy and dexamethasone (0.5 mg subcutaneously); 47% developed RI-AML (▴), 35% developed RCN B (▪), and 18% died tumor-free (•). The number of mice at each time point is shown in parentheses. *Circulating CSF-1 levels in RI-AML mice that were significantly different from the corresponding levels in tumor-free mice (Student's t-test, P < .005).
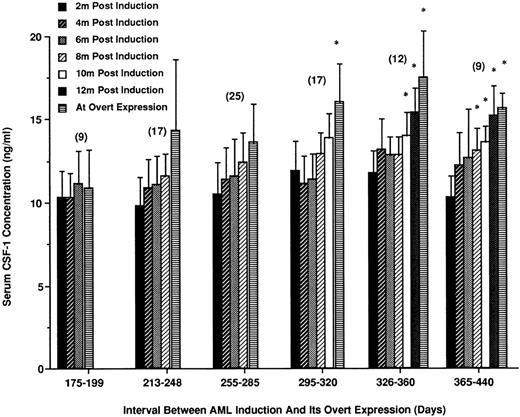
CSF-1 levels increase before overt expression of RI-AML.To determine whether the elevation of circulating CSF-1 occurred gradually during the latent period or more abruptly before overt expression of RI-AML, female SJL/J mice exposed to the leukemogenic treatment were individually bled every 2 months, starting 2 months after leukemogenic treatment. Of these mice, 89 eventually developed RI-AML. On the basis of age at death, these mice were divided arbitrarily into six separate latency periods. The number of mice per age group and CSF-1 levels leading up to and at death are summarized in Fig 3.
Increases in CSF-1 levels in the period between leukemogenic induction and development of RI-AML. Following leukemogenic treatment, mice were individually bled at 2-month intervals for 12 months until the appearance of overt leukemia. Mice that developed RI-AML were separated into six groups depending on their latency period. Mean CSF-1 levels at death and for the 2-month intervals before death are shown for each latency period. The number of mice within each latency period is indicated in parentheses. *CSF-1 concentrations that are significantly different from 2-month posttreatment levels (Student's t-test, P < .05).
Increases in CSF-1 levels in the period between leukemogenic induction and development of RI-AML. Following leukemogenic treatment, mice were individually bled at 2-month intervals for 12 months until the appearance of overt leukemia. Mice that developed RI-AML were separated into six groups depending on their latency period. Mean CSF-1 levels at death and for the 2-month intervals before death are shown for each latency period. The number of mice within each latency period is indicated in parentheses. *CSF-1 concentrations that are significantly different from 2-month posttreatment levels (Student's t-test, P < .05).
CSF-1 levels in sera of mice that developed RI-AML within a short latency period (175 to 210 days, ∼10% of mice developing RI-AML) were comparatively low. In mice with a more prolonged latency (255 to 285 days to 365 to 440 days), elevated levels of CSF-1 were observed before the development of overt leukemia. In general, in mice developing RI-AML within 200 days of the leukemogenic treatment, the circulating levels were less than 12 ng/mL. However, from 260 days posttreatment, there was an increase in mean CSF-1 levels that was often apparent 2 to 4 months before the mice developed overt RI-AML (increases significantly different for the two groups of longest latency).
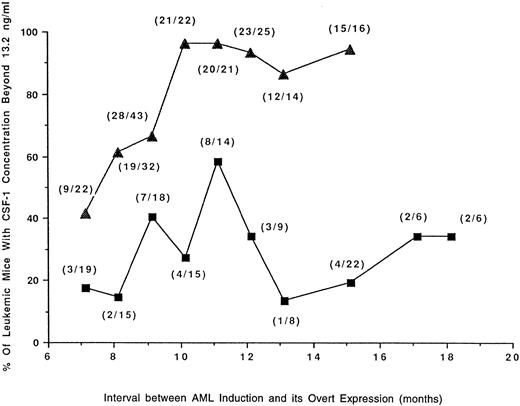
Mice developing RI-AML with a longer latency period have higher concentrations of circulating CSF-1.The mean circulating CSF-1 concentration in mice subjected to leukemogenic treatment that failed to develop any neoplasm (Fig 2) was significantly lower than in normal SJL/J mice (Fig 1); (11 ng/mL v 15.0 ng/mL). CSF-1 in at least two thirds of these tumor-free mice was less than 13.2 ng/mL. Using this figure as a cutoff and the data from the experiments described in Figs 2 and 3, the percentage of tumor-bearing mice with CSF-1 concentrations higher than 13.2 ng/mL was plotted against the latency period (Fig 4). The data demonstrate that RI-AML mice with a prolonged latency have higher levels of CSF-1 at death. These results contrast with those for RCN B, where the proportion of mice with higher CSF-1 levels initially increases but subsequently declines with increasing latency.
Proportion of mice with RI-AML or RCN B possessing high levels of CSF-1 in relation to latency. Data are from the experiments depicted in Figs 2 and 3. The percentage of mice bearing tumors (RI-AML, ▴; RCN B, ▪) that have circulating CSF-1 concentrations <13.2 ng/mL are plotted against latency periods that have been arbitrarily classed. The total number of mice contributing to each data point is shown in parentheses. By Fisher's exact test, mice with AML had a significantly greater chance of having CSF-1 concentrations <13.2 ng/mL than mice with RCN B (P ≤ .0099 at 8, 10, 11, 12, 13, and 15 months of latency). In mice with AML, there was a direct correlation between age (or latency) and CSF-1 concentration <13.2 ng/mL (r = .7930, P = .0189), whereas in mice with RCN B there was no significant correlation (r = .1570, P = .6649).
Proportion of mice with RI-AML or RCN B possessing high levels of CSF-1 in relation to latency. Data are from the experiments depicted in Figs 2 and 3. The percentage of mice bearing tumors (RI-AML, ▴; RCN B, ▪) that have circulating CSF-1 concentrations <13.2 ng/mL are plotted against latency periods that have been arbitrarily classed. The total number of mice contributing to each data point is shown in parentheses. By Fisher's exact test, mice with AML had a significantly greater chance of having CSF-1 concentrations <13.2 ng/mL than mice with RCN B (P ≤ .0099 at 8, 10, 11, 12, 13, and 15 months of latency). In mice with AML, there was a direct correlation between age (or latency) and CSF-1 concentration <13.2 ng/mL (r = .7930, P = .0189), whereas in mice with RCN B there was no significant correlation (r = .1570, P = .6649).
CSF-1 expression in first-passage RI-AML.To investigate the possibility that the elevated levels of CSF-1 reflected production of the growth factor by RI-AML cells, extracts of tumors derived from independently arising RI-AMLs were assayed for CSF-1. For this purpose, the original RI-AMLs were injected intramuscularly to obtain first-passage tumor cells largely free of other hematopoietic cells. The results of assays performed on 14 such tumors are presented in Table 1. All tumor extracts contained CSF-1 at levels similar to those described previously for tissues containing high levels in normal mice, using the same assay. The CSF-1 concentration in RI-AML tumors ranged from 35 to 178 pg/mg tissue (Table 1). The three normal tissues expressing the highest levels of CSF-1 were spleen (26 pg/mg), lung (29 pg/mg), and submaxillary gland (104 pg/mg).20 These observations raised the possibility that RI-AMLs themselves synthesize CSF-1. Regression analysis of the data presented in Table 1 also indicates that there is a direct correlation between RI-AML latency and tumor CSF-1 concentration.
CSF-1 Expression in First-Passage RI-AML
| Tumor Designation . | Tumor CSF-1 Concentration (pg/mg)* . | Latency (d) . |
|---|---|---|
| 17A-980 | 35.2 | 254 |
| 17B-188 | 36.6 | 206 |
| 17A-I-10 | 40.8 | 205 |
| 17A-954 | 53.6 | 304 |
| 17A-I-8 | 54.0 | 275 |
| 17A-918 | 54.0 | 278 |
| 17B-100 | 55.2 | 238 |
| 17B-69 | 66.2 | 281 |
| 17A-865 | 67.2 | 213 |
| 17B-108 | 76.8 | 310 |
| 17B-149 | 82.2 | 274 |
| 17A-876 | 84.0 | 325 |
| 17B-186 | 109.0 | 299 |
| 17A-I-31 | 178.4 | 454 |
| Tumor Designation . | Tumor CSF-1 Concentration (pg/mg)* . | Latency (d) . |
|---|---|---|
| 17A-980 | 35.2 | 254 |
| 17B-188 | 36.6 | 206 |
| 17A-I-10 | 40.8 | 205 |
| 17A-954 | 53.6 | 304 |
| 17A-I-8 | 54.0 | 275 |
| 17A-918 | 54.0 | 278 |
| 17B-100 | 55.2 | 238 |
| 17B-69 | 66.2 | 281 |
| 17A-865 | 67.2 | 213 |
| 17B-108 | 76.8 | 310 |
| 17B-149 | 82.2 | 274 |
| 17A-876 | 84.0 | 325 |
| 17B-186 | 109.0 | 299 |
| 17A-I-31 | 178.4 | 454 |
There is a direct correlation between RI-AML latency and tumor CSF-1 concentration (r = .8664, P < .0001).
Determined in duplicate by CSF-1 RIA.
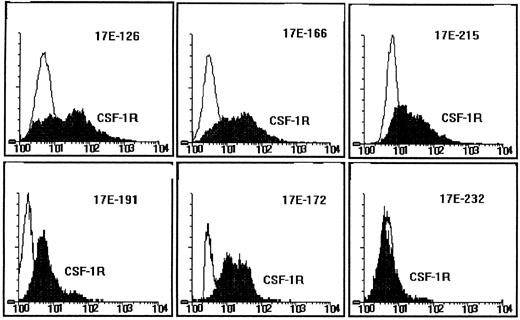
Expression of the CSF-1R on RI-AML cells.To determine whether RI-AMLs have the potential to respond to CSF-1 through the CSF-1R, expression of the CSF-1R was examined immunologically by flow cytometry. First- or second-passage intramuscular RI-AML tumors were analyzed using a rat monoclonal antibody directed to the extracellular domain of the mouse CSF-1R (clone 604 B52 E11). In five of six different tumors tested (designated 17E-126, -166, -172, -191, and -215), the CSF-1R was expressed markedly, but in one tumor (17E-232) it was present at a low level (Fig 5). In addition, CSF-1R mRNA was detected by Northern blot analysis in three of three tumors tested, including 17E-232 (data not shown). Thus, the CSF-1R was expressed in all six RI-AMLs tested.
CSF-1R expression on RI-AML cells. Fluorescence microfluorometry analysis of six first- or second-passage RI-AML tumors. Cell-surface CSF-1R expression was detected with a rat monoclonal antibody to the mouse CSF-1R and FITC goat anti–rat IgG (H + L). Ordinate: cell number; abscissa, intensity of fluorescence.
CSF-1R expression on RI-AML cells. Fluorescence microfluorometry analysis of six first- or second-passage RI-AML tumors. Cell-surface CSF-1R expression was detected with a rat monoclonal antibody to the mouse CSF-1R and FITC goat anti–rat IgG (H + L). Ordinate: cell number; abscissa, intensity of fluorescence.
Abrogation of in vitro growth of RI-AML cells by neutralizing anti–CSF-1 and anti–CSF-1R antibodies.To determine whether CSF-1 has an autocrine role in the development of RI-AML, we examined the ability of neutralizing antibodies raised against purified CSF-1 and purified CSF-1R to inhibit the colonial growth of RI-AML cells from first or second in vivo intramuscular passages of primary RI-AMLs. RI-AML colonies were established in methylcellulose cultures, and colonies were counted at 3 to 7 days. A colony was defined as a cluster of greater than 40 cells and consisted of granulocytes, monocyte-macrophages, or both. Colonies appeared to be derived exclusively from RI-AML cells rather than from contaminating hematopoietic cells, since erythroid colonies, which developed under the same conditions when normal marrow was plated with erythropoietin, failed to develop in RI-AML cultures in the presence of erythropoietin and RI-AML colonies generally had a more compact appearance than colonies of normal myeloid cells. The effects of anti–CSF-1 and anti–CSF-1R antisera on colony formation by 20 different RI-AML tumors derived from 20 different primary RI-AMLs were investigated. Results for six of these are summarized in Table 2. The colony-forming efficiency of these RI-AML cell suspensions ranged from 0.35% to 1.3%. Two of the RI-AMLs subjected to this analysis (17E-166 and 17E-172), were among those shown to express the CSF-1R by fluorescence microfluorometry analysis (Fig 5). For all six tumors, the formation of RI-AML colonies was blocked by inclusion of anti–CSF-1 antiserum at a concentration that blocked CSF-1–induced macrophage colony formation by normal bone marrow cells. To control for any toxic effects of the antiserum, human recombinant CSF-1, which is not neutralized by the anti–mouse CSF-1 antiserum, was included in cultures of three of the RI-AMLs. In all cultures receiving human CSF-1, plating efficiencies at least returned to the values observed in the absence of any antiserum (Table 2). Of 20 RI-AML tumors analyzed, colony formation by all 20 was neutralized by the anti–CSF-1 antiserum (data not shown). A goat anti–mouse CSF-1R antiserum, shown previously to block the action of CSF-1 in stimulating normal bone marrow colony formation,20 had a similar inhibitory effect on colony formation by four RI-AML tumors (Table 2). In other experiments, neutralizing monoclonal antibodies to interleukin-6, interleukin-1α, and interleukin-1β failed to inhibit colony formation (data not shown). These data indicate that CSF-1 plays an autocrine role in RI-AML growth in vitro.
Abrogation of In Vitro RI-AML Cell Colony Formation by Neutralizing Anti–CSF-1 and Anti–CSF-1R
| Tumor Designation . | Additions to Culture . | |||||
|---|---|---|---|---|---|---|
| . | None . | Normal Goat Serum (25 to 50 μL/dish) . | Goat Anti–Mouse CSF-1 Antiserum (50 μL/dish) . | Goat Anti–Mouse CSF-1 Antiserum + . | Mouse CSF-1 . | Goat Anti–Mouse CSF-1R Antiserum (25 μL) . |
| . | . | . | . | Human CSF-1 (60 ng/mL) . | (18 ng/mL) . | . |
| 17A-I-8 pII | 1,265 ± 310 | 1,096 ± 265 | 7 ± 4 | ND | ND | ND |
| 17A-I-31 pII | 1,386 ± 412 | 1,410 ± 307 | 0 | ND | ND | ND |
| 17E-172 pI | 535 ± 164 | 487 ± 261 | 20 ± 6 | 623 ± 202 | 764 ± 198 | 28 ± 9 |
| 17E-76 pI | 285 ± 88 | ND | 0 | ND | 783 ± 238 | 0 |
| 17E-248 pI | 294 ± 102 | ND | 22 ± 8 | 912 ± 365 | 822 ± 238 | 27 ± 17 |
| 17E-166 pI | 344 ± 108 | 322 ± 78 | 57 ± 21 | 680 ± 206 | 650 ± 202 | 50 ± 27 |
| Tumor Designation . | Additions to Culture . | |||||
|---|---|---|---|---|---|---|
| . | None . | Normal Goat Serum (25 to 50 μL/dish) . | Goat Anti–Mouse CSF-1 Antiserum (50 μL/dish) . | Goat Anti–Mouse CSF-1 Antiserum + . | Mouse CSF-1 . | Goat Anti–Mouse CSF-1R Antiserum (25 μL) . |
| . | . | . | . | Human CSF-1 (60 ng/mL) . | (18 ng/mL) . | . |
| 17A-I-8 pII | 1,265 ± 310 | 1,096 ± 265 | 7 ± 4 | ND | ND | ND |
| 17A-I-31 pII | 1,386 ± 412 | 1,410 ± 307 | 0 | ND | ND | ND |
| 17E-172 pI | 535 ± 164 | 487 ± 261 | 20 ± 6 | 623 ± 202 | 764 ± 198 | 28 ± 9 |
| 17E-76 pI | 285 ± 88 | ND | 0 | ND | 783 ± 238 | 0 |
| 17E-248 pI | 294 ± 102 | ND | 22 ± 8 | 912 ± 365 | 822 ± 238 | 27 ± 17 |
| 17E-166 pI | 344 ± 108 | 322 ± 78 | 57 ± 21 | 680 ± 206 | 650 ± 202 | 50 ± 27 |
Abbreviation: ND, not determined.
The differences between colony numbers on dishes incubated with normal goat serum and those incubated with goat anti–CSF-1 or goat anti–CSF-1R antisera are significant (P ≤ .05 by Student's t-test).
Absence of detectable rearrangements in CSF-1 and CSF-1R genes in RI-AMLs by Southern blot analysis.The existence of autocrine regulation in RI-AML must reflect inappropriate coexpression of the CSF-1 and CSF-1R genes in RI-AML cells. One mechanism by which this may occur is via alterations in the CSF-1 and CSF-1R genes themselves. To determine whether any gross rearrangement within these genes was responsible, DNA from six RI-AML tumors was subjected to Southern analysis, and the resulting blots were probed with CSF-1 and CSF-1R DNA. No significant rearrangements in either gene could be detected in any of the tumors (data not shown).
DISCUSSION
The circulating CSF-1 concentrations of untreated adult SJL/J mice, including those that spontaneously developed RCN B, were within the normal range of other strains of mice (eg, 15.1 ± 1.3 ng/mL v 15.2 ± 1.9 ng/mL for C3H/HeJ mice20 ). Following leukemogenic treatment, circulating CSF-1 concentrations in SJL/J mice that failed to develop any neoplasm were significantly decreased. However, in mice that received leukemogenic treatment, CSF-1 concentrations were significantly elevated in those that developed RI-AML with prolonged latency compared with those that developed RCN B or died without neoplasia. Furthermore, in mice developing RI-AML with prolonged latency, there was a significant increase in the serum CSF-1 concentration 2 to 4 months before the development of overt leukemia. These results indicate that significant elevations in circulating CSF-1 are specifically associated with the development of RI-AML and that they are prognostic for leukemia development. The increase in circulating CSF-1 concentration in these mice was comparable to the increase in circulating CSF-1 observed in pregnant mice at a time when the uterine CSF-1 concentration is elevated 1,000-fold due to steroid hormone–regulated uterine synthesis.20 Similar elevations of circulating CSF-1 have been observed in AML in man.28 In addition to the high RI-AML concentration of CSF-1, we have also shown that CSF-1 is present in the medium conditioned by five independently arising RI-AML cell lines (N.H.G. and E.R.S., unpublished observations, 1996), which, together with the data indicating autocrine regulation by CSF-1, prove that RI-AML cells synthesize significant amounts of the growth factor. Thus, the elevated circulating CSF-1 in mice with RI-AML may be due to production of CSF-1 by the leukemic cells themselves.
Analysis of the RI-AML cells, grown as intramuscular tumors to reduce contamination due to other hematopoietic cells, showed that the tumors themselves contained high concentrations of CSF-1 and that the tumor CSF-1 concentration was significantly increased in RI-AMLs with longer latencies. Cells from such tumors were shown to express the CSF-1R and grew as colonies when cultured in methylcellulose medium in the absence of exogenous CSF-1. Inclusion of specific neutralizing antibodies to either CSF-1 or the CSF-1R blocked colony formation by tumor cells, demonstrating autocrine regulation of RI-AML cell proliferation by CSF-1 in vitro. Recent studies indicate that despite the high circulating concentration of CSF-1 in normal mice, local production and/or selective sequestration of growth factor is necessary for the regulation of CSF-1R–expressing cells in several tissues.29 Thus, the antibody-neutralization data are consistent with the possibility that the autocrine regulation of RI-AML cells by CSF-1 in vivo could play an important role in RI-AML development.
The autonomous growth of RI-AML cells is a result of their coexpression of CSF-1 and the CSF-1R. The CSF-1R is normally expressed by myeloid cells, whereas CSF-1 is rarely expressed in myeloid cells, and only then in response to other agents. Thus, it is possible that it is the expression of CSF-1 rather than its receptor that is the dysregulating event leading to the autocrine regulation of these leukemic cells. However, one of two integration sites for the replication-competent Friend murine leukemia virus, used in approximately 20% of in vivo primary myeloid leukemias, has been shown to be at the 5′ end of the CSF-1R gene. Proviral integration in this region results in the high expression of CSF-1R mRNA of normal size, suggesting that inappropriate early expression of the CSF-1R may also be an early event in leukemia development.30 Our studies do not address the question of when the inappropriate expression of CSF-1 takes place and how it might contribute to the development of disease. It is possible that it takes place either before or after the immortalizing event. Establishment of the autocrine state could lead to uncontrolled proliferation of a clone of cells, increasing the opportunity for a second, immortalizing event. Alternatively, a slowly expanding immortalized clone may gain the selective advantage of an increased proliferative rate due to the secondary acquisition of autocrine regulation. Indeed, the simplest interpretation of the data indicating that RI-AMLs of longer latency express higher concentrations of CSF-1 would favor the latter. As indicated earlier, transplantation studies show that there are potential leukemic cells among bone marrow cells of nonleukemic SJL/J mice only 3 to 4 months following irradiation.15 Furthermore, a partial deletion in one copy of chromosome 2, a constant feature of RI-AMLs in this model,10 is present in the bone marrow of 80% to 100% of SJL/J mice at this time.10,11 Events involved in the immortalization and/or dysregulation of CSF-1 or CSF-1R expression could be associated with these changes. Our failure to demonstrate any gross rearrangement of either the CSF-1 or CSF-1R genes indicates that the mechanism of activation of their expression is not one involving the insertion of a retrovirus or intracisternal A particle-like sequence into their genes.31 32 However, it is possible that an independent genetic event, such as the chromosome 2 deletion, could result in an alteration of the regulation of CSF-1 gene expression and inappropriate CSF-1 production, or of the regulation of CSF-1R gene expression and inappropriate expression of the CSF-1R early in development. One remarkable aspect of this model is the extremely high frequency at which both the chromosome 2 deletion and autocrine regulation by CSF-1 are observed, raising the possibility that they may be directly connected in some way in the etiology of the disease.
The effect of age on susceptibility to RI-AML induction and the effect of glucocorticoids and CSF-1 on RI-AML induction should be considered in relation to the regulation of the mononuclear phagocytic system. The observation that RI-AML induction rates increased with age at leukemogenic treatment up to 3 months of age may simply reflect a progressive increase in radiation target size during this period. In the mouse, the first 2 to 3 months of postnatal life comprise the period during which the adult mononuclear phagocytic system is established.29 As there is considerable expansion of the system, it is likely that there is an increased chance of appropriate radiation-induced genetic change as the numbers of hematopoietic precursor cell targets increase, until 3 months of age, when they attain adult values.33 The coleukemogenic effect of glucocorticoids on RI-AML development appears complex. Previous studies have indicated that administration of cortisone to mice induces a decrease in the circulating CSF concentration and in the concentration of bone marrow colony forming cells, which return to normal at 7 days.34 Unfortunately, no subsequent time points were obtained in that study. However, in other experiments, 17 days after cotreatment of SJL/J mice with irradiation and glucocorticoids, circulating CSF-1 was found to be significantly elevated by comparison with irradiated control mice.17 These studies of the short-term effects of glucocorticoids together suggest an initial decrease in circulating CSF-1 followed by an elevation to above normal levels. Such an elevation could lead to expansion of a genetically altered potential leukemic cell population. In contrast, we find that the long-term effect of irradiation and glucocorticoids in SJL/J mice that fail to develop RI-AML is that CSF-1 concentrations appear to be slightly depressed.
Because glucocorticoids are also known to inhibit the proliferation of mononuclear phagocytic cells,35,36 it would seem that additional studies are required to examine the effects of glucocorticoids on circulating CSF-1 and the mononuclear phagocytic system. Additional studies of the postulated coleukemogenic effect of CSF-1 would also seem warranted, since in the original studies17 effects were seen with very small amounts of crude mouse or purified human CSF-1 and the human CSF-1 can be antigenic in mice (E.R. Stanley, unpublished observations, 1990).
Studies in humans suggest that CSF-1 might play a similar autocrine role in several neoplastic diseases, including AML. Kacinski, Stanley, and colleagues have demonstrated that circulating CSF-1 concentrations are elevated in patients with ovarian and endometrial cancer, that CSF-1 and the CSF-1R are expressed in ovarian, endometrial, and breast carcinoma cells, and that elevated circulating CSF-1 might be a useful marker of disease status in these neoplasias (reviewed in ref 37). Elevated levels of circulating CSF-1 are an indicator of poor prognosis in ovarian,38 endometrial,39,40 and breast41 cancer. These studies suggest that CSF-1 can be involved in the development and/or progression of neoplasias of the female reproductive system, in which we have shown that CSF-1 normally plays an important regulatory role.42 CSF-1 and the CSF-1R are expressed in leukemia and lymphoma cell lines,43-45 and we have shown that circulating CSF-1 is elevated in patients with myelodysplastic syndrome, AML, chronic myeloid leukemia (CML), non-Hodgkin's leukemia (NHL), Hodgkin's disease (HD), multiple myeloma (MM), and chronic lymphocytic leukemia (CLL).44 High CSF-1 levels were correlated with disease activity (NHL and HD) or the degree of tumor burden (MM, AML, and CLL), but not with total peripheral blood monocyte or neutrophil counts. Although the circulating CSF-1 concentrations of significant proportions of patients with lymphoid neoplasias were within the normal range, all CSF-1 levels for each of the 15 AML and 7 CML patients with active disease were above the normal range. In the case of the leukemias, we favored the possibility of production of CSF-1 by the leukemic cells themselves as part of an autocrine or paracrine regulation by CSF-1, because of the reported expression of CSF-1 and the CSF-1R in leukemic cells.46-49 The involvement of CSF-1 in RI-AML in the SJL/J mouse is similar to its involvement in the human neoplastic diseases discussed, suggesting that it is a useful model system for studying the role of CSF-1 in neoplasia.
ACKNOWLEDGMENT
We thank Reza Zadeh for technical assistance.
Supported by Grant No. 2628 from the Ministry of Health, Chief Scientists Office, Jerusalem, Israel (N.H.-G.), National Institutes of Health Grant No. CA 32551 (E.R.S.), and the Albert Einstein College of Medicine Cancer Center Core Grant No. P30-CA 13330.
Address reprint requests to E. Richard Stanley, PhD, Department of Developmental and Molecular Biology, Albert Einstein College of Medicine, 1300 Morris Park Ave, New York, NY 10461.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal