Abstract
In genetic hemochromatosis (GH), excess iron is deposited in parenchymal cells, whereas little iron is found in reticuloendothelial (RE) cells until the later stages of the disease. As iron absorption is inversely related to RE cells stores, a failure of RE to retain iron has been proposed as the basic defect in GH. In RE cells of GH subjects, we examined the activity of iron regulatory protein (IRP), a reliable indicator of the elusive regulatory labile iron pool, which modulates cellular iron homeostasis through control of ferritin (Ft) and transferrin receptor gene expression. RNA-bandshift assays showed a significant increase in IRP activity in monocytes from 16 patients with untreated GH compared with 28 control subjects (1.5-fold) and five patients with secondary hemochromatosis (SH) with similar iron burden (fourfold). In 17 phlebotomy-treated GH patients, IRP activity did not differ from that of control subjects. In both GH and SH monocyte-macrophages, Ft content increased by twofold and the L subunit-rich isoferritin profile was unchanged as compared with controls. IRP activity was still upregulated in vitro in monocyte-derived macrophages of GH subjects but, following manipulations of iron levels, was modulated normally. Therefore, the sustained activity of monocyte IRP found in vivo in monocytes of GH patients is not due to an inherent defect of its control, but is rather the expression of a critical abnormality of iron metabolism, eg, a paradoxical contraction of the regulatory iron pool. By preventing Ft mRNA translation, high IRP activity in monocytes may represent a molecular mechanism contributing to the inadequate Ft accumulation and insufficient RE iron storage in GH.
GENETIC HEMOCHROMATOSIS (GH) is a very common autosomal recessive disease of iron metabolism. Although the location of the gene on chromosome 6 in linkage with the HLA locus is well established,1 the gene product has not yet been identified. Recently, mutations in a sequence encoding a major histocompatibility complex (MHC) class I-like protein have been reported in a large majority of GH patients, making this gene, HLA-H, a very strong candidate for the GH gene.2 However, the function of the HLA-H gene product is still unknown. Despite intensive investigations by various groups over the past decades, the cellular location of the defect is still controversial. In fact, the liver, duodenum, and reticuloendothelial (RE) system have all been proposed as the primary sites of the metabolic abnormality.3
The RE system, in particular, plays a central role in iron metabolism, as it is responsible, through processing hemoglobin-iron from senescent erythrocytes, for iron supply to peripheral tissues, including the bone marrow.4 One peculiarity of GH is that excess iron is deposited mainly in parenchymal cells with relatively low iron stores in RE cells until late in the disease.5,6 As intestinal iron absorption is inversely related to RE cell iron deposits, an iron-handling defect of RE cells might be responsible for both excess iron deposition in parenchymal cells and lack of feed-back regulation of duodenal iron uptake.7 In GH, several studies on different aspects of RE cell iron metabolism have been performed using a variety of technical approaches,8-18 but conclusive evidence for a specific defect is still lacking.
Recently, considerable progress has been made in the molecular characterization of cellular iron metabolism. The metabolic status of iron in the cell is reflected in a regulatory pool of low molecular weight iron, the labile iron pool. As a consequence, the iron regulatory proteins (IRPs), the cytoplasmic sensors of the labile iron pool, are now regarded as the main regulators of cellular iron homeostasis.19-21 In addition to IRP-1 (formerly referred to as IRE-BP, FRP, IRF or IRP), a second IRP (IRP-2) has been characterized in rodent22,23 and human cells.24 IRPs control cellular iron storage and uptake by binding to specific RNA motifs called iron responsive elements (IRE) in untranslated regions of mRNAs for the H and L subunits of ferritin (Ft), which sequesters iron, and of transferrin receptor (TfR), which mediates uptake of the metal. IRP binding prevents translation of Ft mRNAs and increases TfR mRNA stability. When iron in the labile pool is scarce, IRPs bind to IRE and coordinately inhibit Ft synthesis and increase that of TfR, thus providing the cell with readily available iron. Conversely, in conditions of iron abundance, IRPs do not bind IRE and iron sequestration exceeds iron uptake. In iron replete cells, IRP-1 bears a 4Fe-4S cluster and represents the cytoplasmic homologue of mitochondrial aconitase; reversible disassembly of the cluster converts IRP-1 to its RNA-binding form. This posttranslational interconversion between the two forms constitutes the basis for the regulation of IRP-1 activity by iron. IRP-2 binds to IRE with an affinity and a specificity similar to those of IRP-1, but lacks aconitase activity and is not activated by reducing agents.25 Furthermore, its response to iron follows different pathways: de novo synthesis after iron starvation and degradation by the proteasome following iron repletion.26-28 Moreover, it has recently been demonstrated that molecules other than iron such as nitric oxide produced during inflammation29-31 and reactive oxygen species generated under conditions of oxidative stress31-34 can also modulate activity of IRPs.
We reasoned that, whatever the biochemical lesion of GH might be, assaying IRP activity in circulating monocytes and monocyte-derived macrophages was the only way to gain precise insights into the status of the regulatory labile iron pool and, as a consequence, the molecular mechanisms responsible for the suggested impairment of iron accumulation in RE cells of patients with GH.
MATERIALS AND METHODS
Reagents
RPMI 1640 and (α-32P) uridine triphosphate (UTP) were purchased from ICN Biomedicals (Opera MI, Italy), Ficoll-Paque and Percoll from Pharmacia Biotech (Cologno Monzese MI, Italy), desferrioxamine and ferric ammonium citrate from Sigma Chemical Co (Milano, Italy), Dynabeads M-450 and antihuman CD14 antibody from Unipath (Garbagnate MI, Italy), Enzymun-test for serum ferritin immunoassay from Boehringer Mannheim (Milano, Italy), and Magic-Fer radioimmunoassay kit for ferritin from Ciba Corning (Cassina de' Pecchi MI, Italy).
Subjects
Sixty-six subjects were studied. Informed consent was obtained from all subjects, and the protocol was approved by the Ethics Committees of the Universities of Milano and Modena.
GH patients.Of the 33 patients with GH (23 men and 10 women, age range, 25 to 69 years), 16 were untreated and 17 were on a phlebotomy program. Of the latter group, nine were fully iron depleted at the time of the study. Nine GH patients were investigated before and during phlebotomy treatment. The diagnosis of GH was established according to standard criteria as previously reported.35 Twenty-one patients (63%) had an A3, B7, and/or B14 HLA haplotype. Removal of excess body iron was defined by normalization of serum iron and Ft levels and, in selected cases, by normal findings at repeat liver biopsy.
Patients with secondary hemochromatosis (SH).We also studied 5 patients with SH (3 men and 2 women, age range, 26 to 58 years); this group included 2 patients with sporadic porphyria cutanea tarda (PCT), 1 subject with thalassemia intermedia, 1 with sideroblastic anemia, and 1 with siderosis due to transfusion therapy for treatment of myelofibrosis-related anemia. Sporadic PCT was confirmed as previously described.35 The possible presence of GH alleles in PCT patients36 was considered, but MHC haplotype analysis and evaluation of extent and distribution of iron in the liver did not suggest the presence of heterozygote or homozygote GH. None of the SH subjects was undergoing phlebotomy therapy at the time of the study.
Control subjects.Twenty-eight healthy blood donors (25 men and 3 women, age range, 24 to 67 years) with no abnormalities of serum iron indexes and no clinical history of disorders of iron metabolism served as controls. All of the subjects, except 7 GH patients, were unrelated.
Biochemical Evaluation
Serum iron, total iron binding capacity, transferrin saturation index, and HLA typing were determined by standard techniques as previously reported.35 Serum Ft was measured by an enzyme immunoassay. Hepatic iron stores were evaluated and graded microscopically (0-4) by two independent observers and chemically by atomic absorption spectrophotometry as described previously.35
Isolation of Monocytes
Monocytes were purified as described by Colotta et al.37 Briefly, buffy coats prepared from heparinized venous blood were diluted with saline, layered on Ficoll-Paque, and centrifuged at 440g for 30 minutes. Mononuclear cells were then washed twice with saline and resuspended in RPMI 1640 medium containing 2 mmol/L glutamine, antibiotics and 10% fetal calf serum, adjusted to 285 mOsm. A total of 5 mL of the cellular suspension was layered over an equal volume of a solution composed of RPMI 1640 medium (54%) and 285 mOsm Percoll (46%) and centrifuged at 550g for 30 minutes. Lymphocytes at the bottom of the tube were resuspended in saline, pelletted in aliquots, and stored at −80°C. Monocytes banding at the interface were collected, washed twice with saline, and examined under the microscope to check yield, purity, and viability. A total of 10 to 15 × 106 cells/100 mL blood were recovered consisting of 95% to 98% monocytes, as shown by analysis of specific markers.37 Cell viability, measured by trypan blue dye exclusion test, was greater than 90%. The cells were either pelletted and stored at −80°C in aliquots or cultured (see below). Monocytes of patients with anemia and transfusional siderosis were separated from 50 mL of blood by the use of magnetic beads coated with antihuman CD14 antibody.38 Purity and viability of monocytes were similar to those reported above.
Culture of Monocytes
Monocytes were resuspended in RPMI 1640 medium containing 20% autologous or heat-inactivated human serum and cultured in 5% CO2 at 37°C.39 On different culture days, both adherent and nonadherent cells were harvested, pelletted, and stored at −80°C. As previously observed,39 both cell populations terminally differentiated to macrophages. Iron overload was obtained by culturing cells continuously in the presence of 50 μg/mL ferric ammonium citrate and iron deprivation by culture in the presence of 50 μmol/L desferrioxamine for 16 hours.
In Vitro RNA Transcription
The pSPT-fer plasmid containing the IRE of the human ferritin H chain40 was linearized with BamHI and transcribed in vitro with T7 RNA polymerase in the presence of 100 μCi of (α-32P) UTP (800 Ci/mmol). Probe-specific activity was determined by trichloroacetic acid precipitation of the reaction products and was routinely 1 × 106 dpm/ng.
RNA-Protein Gel Retardation Assay
Cells were lysed in the buffer described by Leibold and Munro,41 the lysate was centrifuged at 16,000g for 5 minutes, and the supernatant was used for RNA-protein band-shift assays. Samples containing 2 μg protein, as determined using the Bio Rad protein assay kit, were incubated with a molar excess of IRE probe, digested with RNase T1, and treated with heparin as described previously.42 After separation on 6% nondenaturing polyacrylamide gels, RNA-protein complexes were visualized by autoradiography. For quantitation of IRP activity, radioactivity of bands excised from dried gels was determined by liquid scintillation counting and was converted to pmol/mg protein taking into account the specific activity and molecular mass (15.7 kD) of the probe and assuming a 1:1 molar ratio between IRP and RNA.43
Determination of Ft Content
The intracellular concentration of Ft was determined in the cytoplasmic extracts used for bandshift assays using a radioimmunoassay kit (Magic-Fer; Ciba Corning, Cassina de Pecchi, Milano, Italy) based on an antihuman liver Ft antibody. The subunit composition of Ft was measured by immunoassays based on specific anti-H and anti-L Ft subunit monoclonal antibodies.44
Statistical Analysis
The values shown in Table 1 are expressed as means ± standard deviation (SD). Significant differences were evaluated with Student's t-test using the Stat View 4.0 program (Abacus Concept Inc, Berkeley, CA).
IRP Activity and Ft Content in Monocytes in Relation to Iron Status
| . | No. . | Serum Iron . | Transferrin Saturation . | Serum Ft . | Monocyte Ft . | IRP Activity . |
|---|---|---|---|---|---|---|
| . | . | μg/dL . | % . | μg/L . | ng/mg Protein . | pmol/mg Protein . |
| Controls | 28 | 88 ± 29 | 19 ± 8 | 31 ± 32 | 611 ± 427 | 0.62 ± 0.18 |
| GH untreated | 16 | 173 ± 56* | 80 ± 25† | 2,249 ± 1,967† | 1,261 ± 763* | 0.95 ± 0.15† |
| GH treated | 17 | 133 ± 68* | 50 ± 30† | 251 ± 229† | 637 ± 312 | 0.63 ± 0.19 |
| SH | 5 | 154 ± 46* | 59 ± 18† | 1,446 ± 1,039† | 1,204 ± 433* | 0.15 ± 0.04† |
| . | No. . | Serum Iron . | Transferrin Saturation . | Serum Ft . | Monocyte Ft . | IRP Activity . |
|---|---|---|---|---|---|---|
| . | . | μg/dL . | % . | μg/L . | ng/mg Protein . | pmol/mg Protein . |
| Controls | 28 | 88 ± 29 | 19 ± 8 | 31 ± 32 | 611 ± 427 | 0.62 ± 0.18 |
| GH untreated | 16 | 173 ± 56* | 80 ± 25† | 2,249 ± 1,967† | 1,261 ± 763* | 0.95 ± 0.15† |
| GH treated | 17 | 133 ± 68* | 50 ± 30† | 251 ± 229† | 637 ± 312 | 0.63 ± 0.19 |
| SH | 5 | 154 ± 46* | 59 ± 18† | 1,446 ± 1,039† | 1,204 ± 433* | 0.15 ± 0.04† |
Analysis of serum iron indexes was performed as described in Materials and Methods. Monocyte Ft was measured by a radioimmunoassay based on an antihuman liver Ft antibody. IRP activity was determined by liquid scintillation counting of regions of the gels corresponding to the IRE/IRP complexes as reported in Materials and Methods. All values represent mean ± SD.
P < .05 v controls.
P < .001.
RESULTS
IRP Activity in Circulating Monocytes
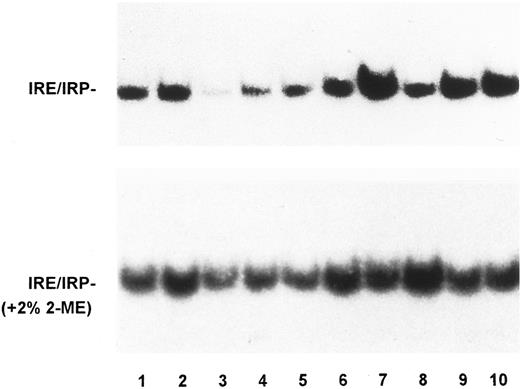
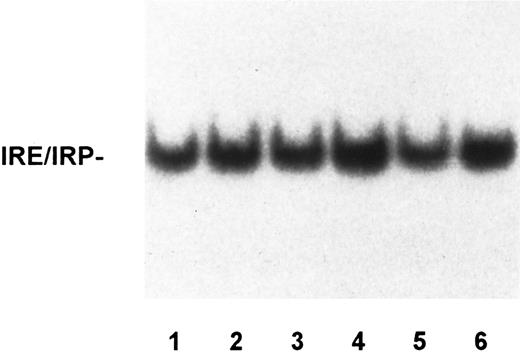
To assess whether alterations of the molecular control of iron metabolism are present in RE cells from GH subjects, we examined IRP activity in monocytes from a large series of patients with untreated or treated GH in comparison to that of control subjects and patients with SH. A representative bandshift assay of IRP activity is shown in Fig 1 and quantitation of IRP activity in monocytes of all the subjects examined is reported in Table 1. Human IRPs are of similar charge and cannot be distinguished by bandshift assay, thus precluding analysis of the individual activities of the two forms. In this report, we use the term IRP activity to mean a combination of the two IRPs, although IRP-1 is probably the major contributor to the detected activity. Although some variability of IRP activity was found within all the groups examined, evaluation of a large number of subjects showed that untreated GH patients presented the highest amount of active IRP with a significant difference from control subjects (Table 1). IRP activity after phlebotomy returned to that seen in controls. Interestingly, in subjects with secondary iron overload with a tissue iron burden comparable to that of GH patients (Table 1), IRP binding activity was significantly decreased. Incubation of the extracts in the presence of 2% 2-mercaptoethanol to measure total cellular IRP increased the binding activity in all of the samples and almost blunted the differences between the various subjects (Fig 1). The remaining differences in total IRP activity are possibly accounted for by the contribution of IRP-2, which is not sensitive to 2-mercaptoethanol. Regarding total IRP activity, similar results were found throughout all the experiments performed in both monocytes and monocyte-derived macrophages (see below). With the limitations due to the influence of IRP-2 on quantitation of total activity (see above), the percentage of spontaneous IRP activity was 18% in normal subjects. As internal control to the experiments, lymphocyte IRP activity was also measured in selected cases, and a typical example is shown in Fig 2. No significant differences of IRP activity were found in lymphocytes from individual patients with GH when compared with IRP activity of lymphocytes from control subjects.
IRP activity in monocytes of controls, GH and SH patients. Autoradiogram of a representative RNA-bandshift assay. Lysates of monocytes from controls (lanes 1 and 2), patients with SH due to sporadic PTC (lane 3), and unrelated GH patients treated (lanes 4 to 6) and untreated (lanes 7 to 10), were incubated with a 32P-labeled riboprobe spanning the IRE of the ferritin H chain in the absence (top panel) or presence (bottom panel) of 2% 2-mercaptoethanol. RNA-protein complexes were separated on nondenaturing acrylamide gels as described in Materials and Methods. The exposure time of the autoradiogram shown in the top panel was twice longer. IRP activity was higher in untreated GH patients than in controls and SH patients, whereas no significant difference was found between treated GH patients and controls.
IRP activity in monocytes of controls, GH and SH patients. Autoradiogram of a representative RNA-bandshift assay. Lysates of monocytes from controls (lanes 1 and 2), patients with SH due to sporadic PTC (lane 3), and unrelated GH patients treated (lanes 4 to 6) and untreated (lanes 7 to 10), were incubated with a 32P-labeled riboprobe spanning the IRE of the ferritin H chain in the absence (top panel) or presence (bottom panel) of 2% 2-mercaptoethanol. RNA-protein complexes were separated on nondenaturing acrylamide gels as described in Materials and Methods. The exposure time of the autoradiogram shown in the top panel was twice longer. IRP activity was higher in untreated GH patients than in controls and SH patients, whereas no significant difference was found between treated GH patients and controls.
IRP activity in lymphocytes of controls and GH patients. Lysates of lymphocytes from controls (lanes 1 to 3) and unrelated GH patients, untreated (lane 4) or treated (lanes 5 and 6), were assayed for IRP activity as described in the legend to Fig 1. IRP activity was not significantly different in the various samples.
IRP activity in lymphocytes of controls and GH patients. Lysates of lymphocytes from controls (lanes 1 to 3) and unrelated GH patients, untreated (lane 4) or treated (lanes 5 and 6), were assayed for IRP activity as described in the legend to Fig 1. IRP activity was not significantly different in the various samples.
We also had the opportunity to measure IRP activity in monocytes of nine GH patients before and during phlebotomy treatment. A representative gelshift is shown in Fig 3, together with the corresponding values of serum and monocyte Ft for each subject. We consistently found a decrease of IRP activity in parallel with a reduction of body iron stores in all subjects investigated, regardless of the initial level of RNA-binding activity.
Effect of phlebotomy on IRP activity in monocytes of GH patients. Monocytes from four unrelated GH patients were isolated before (a) and during (b) phlebotomy treatment and lysates were assayed for IRP activity as described in the legend to Fig 1. The bottom panels report values of serum and monocyte ferritin of the individual subjects. The former were obtained at the time of blood sampling for buffy-coat preparation, the latter were determined in the extract used for bandshift analysis using a radioimmunoassay kit as described in Materials and Methods. Phlebotomy caused a decrease of IRP activity in parallel with reductions of both serum and monocyte ferritin content.
Effect of phlebotomy on IRP activity in monocytes of GH patients. Monocytes from four unrelated GH patients were isolated before (a) and during (b) phlebotomy treatment and lysates were assayed for IRP activity as described in the legend to Fig 1. The bottom panels report values of serum and monocyte ferritin of the individual subjects. The former were obtained at the time of blood sampling for buffy-coat preparation, the latter were determined in the extract used for bandshift analysis using a radioimmunoassay kit as described in Materials and Methods. Phlebotomy caused a decrease of IRP activity in parallel with reductions of both serum and monocyte ferritin content.
Ft Content in Circulating Monocytes
To investigate whether high IRP activity in monocytes is associated with low Ft accumulation, as predicted by the model of IRP-regulated cellular iron metabolism, we measured intracellular Ft content. Table 1 shows that Ft concentration in monocytes of untreated GH patients was twofold higher than in control subjects and returned to normal levels after iron depletion (see also Fig 3, bottom panel). A similar increase in monocyte Ft was found in patients with SH. Determination of the relative content of H and L Ft subunits using specific monoclonal antibodies44 showed a predominant expression of L subunits (L/H ratio 1.6), with the L/H composition not significantly different in the groups studied.
IRP Activity in Monocyte-Derived Macrophages
As tissutal RE cells might represent the site of the metabolic defect in GH,15,45 monocytes from control subjects, patients with treated or untreated GH and with SH were cultured and allowed to mature to macrophages for several days, as reported.37,39 In agreement with a previous study,46 IRP activity of cells from controls and from patients with GH or SH increased with monocyte differentiation (Fig 4). In RE cells of untreated GH patients, IRP activity increased to a lesser extent, but still remained at a higher level than that of cells of other subjects.
IRP activity in monocytes of controls, GH, and SH patients during in vitro maturation to macrophages. Monocytes from 10 controls (▧), 2 patients with SH caused by sporadic PTC and β-thalassemia intermedia (), 5 untreated (▪), and 6 treated unrelated GH patients (□) were allowed to differentiate in vitro. Lysates were prepared from cells freshly isolated (day 0) or cultured for 3 and 6 days and IRP activity was measured by RNA-bandshift assay as described in the legend to Fig 1. Values were calculated as described in Materials and Methods and are expressed as means with SD. The inset shows a representative bandshift analysis of samples from a control (A) and an untreated GH patient (B) at the various time points. IRP activity increased with differentiation to macrophages in all the populations examined, maintaining the differences observed in freshly isolated monocytes.
IRP activity in monocytes of controls, GH, and SH patients during in vitro maturation to macrophages. Monocytes from 10 controls (▧), 2 patients with SH caused by sporadic PTC and β-thalassemia intermedia (), 5 untreated (▪), and 6 treated unrelated GH patients (□) were allowed to differentiate in vitro. Lysates were prepared from cells freshly isolated (day 0) or cultured for 3 and 6 days and IRP activity was measured by RNA-bandshift assay as described in the legend to Fig 1. Values were calculated as described in Materials and Methods and are expressed as means with SD. The inset shows a representative bandshift analysis of samples from a control (A) and an untreated GH patient (B) at the various time points. IRP activity increased with differentiation to macrophages in all the populations examined, maintaining the differences observed in freshly isolated monocytes.
To evaluate the effect of iron manipulation on IRP activity in in vitro–differentiated macrophages, cells were exposed to culture conditions of iron loading (ie, presence of ferric ammonium citrate) or iron starvation (ie, presence of desferrioxamine). IRP activity was downmodulated by the iron salt and upregulated by the iron chelator at all stages of differentiation in control monocytes (Fig 5). The same pattern of response to iron manipulation in vitro was found in GH monocytes. The decrease of IRP activity in iron-supplemented cultures, although moderate compared with that reported in other cell types,43 47 was observed consistently and found to depend on the amount of added iron (data not shown). Intracellular Ft content was modulated by the addition of iron and desferrioxamine (data not shown), thus indicating that the treatments effectively altered intracellular iron levels.
Effect of modulation of iron availability on IRP activity in monocytes of controls and GH patients maturing to macrophages in vitro. Monocytes from 5 controls, 5 untreated, and 4 treated unrelated GH patients were grown for different days in unsupplemented medium (♦) or in the presence of either 50 μg/mL ferric ammonium citrate (▪) or 50 mmol/L desferrioxamine (•). Iron loading was achieved by continuous culture in the presence of the iron salt and iron deprivation by adding desferrioxamine 16 hours before cell harvest. Lysates were prepared from cells freshly isolated (day 0) or cultured for 3 and 6 days, and IRP activity was measured by RNA-bandshift assay as described in the legend to Fig 1. Values were calculated as described in Materials and Methods and are expressed as means with SD. IRP activity was repressed by iron loading and upregulated by iron chelation at all stages of differentiation in RE cells of both controls and GH patients.
Effect of modulation of iron availability on IRP activity in monocytes of controls and GH patients maturing to macrophages in vitro. Monocytes from 5 controls, 5 untreated, and 4 treated unrelated GH patients were grown for different days in unsupplemented medium (♦) or in the presence of either 50 μg/mL ferric ammonium citrate (▪) or 50 mmol/L desferrioxamine (•). Iron loading was achieved by continuous culture in the presence of the iron salt and iron deprivation by adding desferrioxamine 16 hours before cell harvest. Lysates were prepared from cells freshly isolated (day 0) or cultured for 3 and 6 days, and IRP activity was measured by RNA-bandshift assay as described in the legend to Fig 1. Values were calculated as described in Materials and Methods and are expressed as means with SD. IRP activity was repressed by iron loading and upregulated by iron chelation at all stages of differentiation in RE cells of both controls and GH patients.
DISCUSSION
The basic defect of GH is still elusive. The role of the product of the newly described candidate gene for GH in the derangement of iron metabolism in GH is still unknown.2 Using biochemical and molecular biology approaches, it has been shown that expression of the major iron-related genes in GH is normal in the liver,48-50 whereas duodenal abnormalities have been reported, including reduced accumulation of Ft and upregulation of TfR.51-53 Low accumulation of Ft is not due to a defective control of Ft synthesis, but to low expression of the corresponding mRNA and sustained activity of IRP.35,54 Thus, if the duodenal labile iron pool in GH is reduced, as IRP activity indicates, excessive transfer/release of iron to the bloodstream may represent the underlying cause.55 The cytoplasmic proteins from the whole duodenal tissue specimen used in a previous study35 derive from a variety of cell types, including both absorptive and nonabsorptive cells. Among the latter, macrophages of the lamina propria are especially important for iron metabolism, as they may be implicated in the control of iron absorption.16 In the absence of a convenient experimental system for studying human intestinal macrophages, circulating monocytes, the precursors of tissue macrophages, or monocyte-derived macrophages, can be reliably used as a suitable model for studying generalized disorders of the RE system.8 10
In the present study, a consistent increase of IRP activity was found in monocytes of untreated GH subjects with respect to normal subjects. This upregulation seems to be cell-specific, as it is absent in other circulating blood cells such as lymphocytes and is likely to be present in tissutal macrophages, as suggested by experiments with monocyte-derived macrophages allowed to differentiate in vitro. Moreover, this abnormality seems characteristic of the genetic form of hemochromatosis. In fact, IRP activity is severely reduced in monocytes from patients with SH, despite the iron-rich environment surrounding circulating monocytes in both conditions. The reduction of the differences among the various groups of subjects when total IRP activity was measured indicates that the modulation of IRP activity, rather than its amount, is defective in GH, according to the current model of IRP-1 regulation.19-21 The small differences remaining after treatment with 2-mercaptoethanol possibly reflect the activity of IRP-2, which is not sensitive to reductive activation.22 23
IRP-mediated upregulation of TfR expression by iron has been reported in normal monocytes, thus implying the existence of a positive feedback specific for cells assigned to iron storage.39,46 Therefore, it has been proposed that TfR and Ft regulation in monocytes is unidirectional as opposed to the bidirectional regulation observed in other cell types.46,56 Thus, according to this model, increased IRP activity and Ft content in monocytes of GH patients would not be surprising. However, in agreement with a previous study,57 we found increased IRP activity and decreased Ft accumulation in desferrioxamine-treated macrophages in vitro, as well as downmodulation of IRP activity and increased Ft content after in vitro iron-loading, indicating that a bidirectional control of IRP activity and Ft synthesis exists also in the RE cells. Moreover, the downregulation of IRP activity observed in monocytes of patients with SH shows that an IRP-mediated regulation of iron metabolism no different from that of other cells is present in RE cells also in vivo. We have no clear explanations for the discrepancy between the present findings and those reported by Testa et al.46 However, our conclusions are based on the results of a more extensive manipulation of iron availability (ie, upmodulation and downmodulation of iron levels using iron citrate and desferrioxamine, respectively) and on the analysis of a larger number of subjects with normal or abnormal iron balance.
As reported here, GH monocytes respond to manipulation of iron availability in vitro as expected on the basis of the current model of IRP regulation. Moreover, the increase of IRP activity following differentiation/maturation of cultured GH monocytes was similar to that described in monocytes of normal subjects46; thus, in vitro experiments seem to indicate that regulation of IRP activity per se is normal in GH monocytes. On the other hand, an abnormal upregulation of IRP clearly exists in vivo where a cellular defect uncouples body iron stores and IRP activity. The presence of such an abnormality is reinforced by the finding that in treated GH patients changes of IRP (ie, decreased activity) and Ft (ie, decreased accumulation as compared with pretreatment levels) still go in the same direction.
Normally, the labile iron pool is in equilibrium with intracellular Ft-iron deposits. Enhanced IRP activity in GH monocytes, which is indicative of a reduced free iron pool, would predict a reduced content of storage iron (ie, Ft), however, we found increased Ft levels in monocytes of untreated GH patients, in agreement with previous reports.8,9,12 Considering the massive expansion of iron stores in other organs such as the liver,49 one would expect a greater increase of Ft. However, this twofold increase may represent a significant rise of cellular iron deposits, as circulating monocytes are not an important iron storage compartment. In fact, an increase of similar extent was able to downregulate IRP activity in SH monocytes. On the contrary, in monocytes of GH patients, elevated IRP activity was associated with relatively elevated Ft content.
The high IRP activity in GH monocytes seems to suggest that, in vivo, a cellular defect impedes the usual inverse relation between iron stores and IRP activity. Theoretically, this could be due to false signalling to IRP, eg, even in the presence of relatively high levels of iron in the labile pool, other factors29-34 could possibly activate IRP, leading to inadequate Ft synthesis and insufficient incorporation of the metal in RE cellular stores. However, iron overload usually prevails over the variations induced by other stimuli.42 More likely, a reduction of the labile iron pool could be present in GH monocytes. Plausible causes for such an abnormal reduction of the iron pool include a defect of iron uptake and an abnormal release of iron. The first possibility seems to be challenged by previous studies showing that iron uptake,8,10 as well as TfR expression,11 17 were not impaired in GH monocytes. Moreover, our in vitro data clearly show that iron is able to enter the cells and modulate both IRP activity and Ft content.
Therefore, a likely cause of the low iron pool in GH monocytes could be inappropriate release of iron. Indeed, Fillet et al14 have demonstrated that the early release of hemoglobin iron to plasma is abnormally increased in GH macrophages. The mechanisms underlying the release of iron from cells remain largely obscure. An integral membrane protein that transports cations across membranes, such as the copper transporting ATPase involved in the pathogenesis of Wilson and Menkes diseases,58 might be responsible for cellular iron extrusion. Moreover, ceruloplasmin deficiency has been shown to induce iron accumulation in macrophages of copper-deficient swine, possibly due to deficient ferroxidase activity.59 In this context, enhanced activity of an iron transporter involved in the movement of ferrous iron out of RE cells and/or of a copper-dependent oxidase converting the metal to the ferric form for combination with transferrin might be responsible for enhanced release of iron. This could account for a reduction of the iron pool and the increased IRP activity.
In conclusion, our finding of sustained IRP activity in monocytes of GH subjects, and hence a low level of iron in the labile pool, demonstrates a specific metabolic abnormality of the iron regulatory pool in RE cells in this widespread genetic disorder. This defect, by hindering Ft mRNA translation, could lead to inadequate Ft accumulation and insufficient RE iron storage in GH. In turn, the lack of iron in bone marrow and/or intestinal RE cells would prevent feedback regulation of iron absorption, and thus be responsible for the continuous iron uptake and transfer from the gut that leads to body iron overload in GH.
ACKNOWLEDGMENT
We are grateful to S. Levi for determination of ferritin content, F. Colotta and N. Polentarutti for help and advice in setting-up the procedure for monocyte isolation and culture, L. Kuhn for the generous gift of the pSPTFer plasmid, and F. Ravagnani for providing buffy coats of control subjects.
Supported by grants from Ministero Università e Ricerca Scientifica e Tecnologica and from IRCCS Ospedale Maggiore, Milano, Italy.
Address reprint requests to Dr Gaetano Cairo, Centro di Studio sulla Patologia Cellulare CNR, via Mangiagalli 31, 20133 Milano, Italy.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal