Abstract
T-cell development requires a series of discrete selection and activation signals delivered to maturing progenitors in the thymic cortex and medulla. We have previously shown the constitutive activity of the integrin, α4β1 (VLA4), on a unique subpopulation of immature cortical thymocytes and proposed a role for integrin-mediated adhesion in positive selection by cortical epithelium. In the present report we show that thymic epithelial cell lines express vascular cell adhesion molecule-1 (VCAM-1) a high-affinity ligand for α4β1, and that VCAM-1 mediates thymocyte binding to these lines. Immunohistochemistry and confocal microscopy show that VCAM-1 is selectively expressed in situ by thymic epithelium in the cortex and corticomedullary junction, two locations at which VCAM-1 could determine the interaction between immature thymocytes and selecting elements on epithelial cells. In parallel, we confirmed that fibronectin (FN), the alternative ligand for α4β1, is expressed predominantly in the medulla. These results suggest that VCAM-1 is an adhesive ligand in the thymic cortex for the activated form of α4β1 constitutively expressed during development by immature double positive thymocytes. The structural segregation of the alternative ligand, FN, to the medulla suggests that medullary FN may regulate the migration, development, and export of more mature thymocytes.
THE NORMAL maturation of T lymphocytes requires the delivery of a complex series of signals orchestrated in the thymus. This maturation pathway is characterized by incremental stages of progenitor cell development and is correlated phenotypically with the expression of distinct cell surface markers.1-7 The central roles of the T-cell receptor and the costimulatory molecules, CD4 and CD8, are well documented. However, thymic T-cell development also requires other mechanisms mediating the selective adhesion of thymocytes to specific cellular elements in the thymic microenvironments (eg, epithelial and dendritic cells) and a regulated migration of developing thymocytes from cortex to medulla to the peripheral lymphoid system. Our hypothesis is that the dual mechanisms of adhesion and migration are responsible for physically placing cells in specific thymic microenvironments at given stages in development and thus, regulate the nature and timing of signals required for T-cell selection.
Integrins are a candidate group of cell surface molecules capable of regulating thymocyte interactions with epithelial and stromal cell elements during development. Integrins are a large supergene family of heterodimeric adhesion molecules known to mediate selective adhesion, activation, and migration of mature T cells.8 We have previously shown that a constitutively active form of α4β1 is expressed by a unique subset of immature double positive thymocytes and mediates firm adhesion of these cells in vitro to either fibronectin (FN) or vascular cell adhesion molecule-1 (VCAM-1).9 However, α4β1 binding to either substrate cannot mediate proliferation of this immature subset. In contrast, more mature thymocytes expressing the phenotype of cells found in the medulla express increased levels of a second FN receptor, α5β1 (VLA 5), which does not bind to VCAM-1. α5β1 Mediates activation and proliferation of these cells when cross-linking of the TCR/CD3 complex is combined with adhesion to FN.9 These data are most compatible with a model in which α4β1 is constitutively activated at an early stage of thymocyte development in the cortex, binds immature thymocytes to cortical epithelial cells, and stabilizes the interaction of these early T-cell progenitors with major histocompatibility complex (MHC) molecules presenting self peptides to facilitate selection.
One important question raised by our model is the nature of the physiologic ligand (FN or VCAM-1), which interacts with the activated α4β1 of the immature double positive cells in the cortex. FN is a structural element in the thymus10 although its level of expression was recently shown to be higher in the medulla as compared to the cortex.11 There is also evidence that FN is expressed by murine thymic epithelial cell lines (TECs) and that it may play a role in thymocyte development in vitro.12,13 On the other hand, several groups have shown that VCAM-1 is expressed by human bone marrow (BM) stromal cells and may regulate B-cell progenitor development, stromal-dependent erythropoiesis, and hematopoietic progenitor release from the BM compartment.14-18 Thus, it is possible that VCAM-1 may play a similar role in the thymus. Finally, there is growing interest in the clinical use of novel peptide analogs and engineered molecules to selectively inhibit α4β1-adhesion pathways in various disease states such as autoimmunity, organ transplant rejection, cancer, and atherosclerosis.19-22 Therefore, understanding the functions of α4β1 and its two ligands, VCAM-1 and FN, in cell development will be crucial to the design, interpretation, and safety of these clinical trials.
Here we present the first evidence that primary human TEC lines express VCAM-1, upregulate VCAM-1 expression with cytokines, and bind thymocytes via α4β1. Furthermore, combining immunohistochemical analyses with scanning laser confocal microscopy of tissue sections, we establish that VCAM-1 is selectively expressed by thymic epithelium in both the cortex and corticomedullary junction of human thymus, two locations at which VCAM-1 might be involved in the selection of immature double positive thymocytes. In parallel, we confirm that the majority of FN is expressed in the medulla. Collectively, these results suggest that VCAM-1 is an adhesive ligand on the epithelial cells of the thymic cortex for the activated form of α4β1 expressed by immature double positive thymocytes. The structural expression of FN predominantly in the medulla may play a role in directing the migration of successfully selected cortical thymocytes to this compartment. Moreover, our data suggest a common theme for hematopoietic progenitor cell development in the BM and thymic compartments involving the expression of VCAM-1 by critical stromal cell elements in specific microenvironments and regulated signaling via the integrin, α4β1, presented by developing progenitor cells.
MATERIALS AND METHODS
Antibodies and cytokines.The following monoclonal antibodies (MoAbs) were used: HP2/1 anti-α4β1 (Immunotech Inc, Westbrook, ME); P4C2 anti-α4β1 (generous gift of Dr M. Elices, Cytel, La Jolla, CA); MAB13 anti-β1 (generous gift of Drs K. Yamada and S. Akiyama, National Institutes of Health, Bethesda, MD); 1B10 antihuman fibroblast (Sigma, St Louis, MO); LM609 anti-αvβ3 (generous gift of Dr D. Cheresh, The Scripps Research Institute, La Jolla, CA); 15F11 anti-αvβ5 (generous gift of Drs I. Stuiver, The Scripps Research Institute, La Jolla, CA, and J. Smith, Burnham Institute, La Jolla, CA); 2.2.9 anti-von Willebrand's factor (generous gift of Dr Z. Ruggeri, The Scripps Research Institute, La Jolla, CA); GC4 anti-N-cadherin (Sigma); 84H10 anti-ICAM-1 (Immunotech); 7.1 antihuman FN (ATCC, Rockville, MD); 333 anti-FN (Drs Yamada and Akiyama); 25.3.1 anti-αL (Immunotech); 1G11 (Immunotech), P3H12 and 4B9 (Dr Elices) anti-VCAM-1 antibodies; Bear 1 anti-αMβ2 (CD11b, Mac-1) (Immunotech); B9.11 fluorescein isothiocyanate (FITC)-labeled antibody control (Immunotech); mouse IgG1 and IgG2 isotype controls (Immunotech); 18.5 IgG2 isotype control (ATCC). The antihuman pan-cytokeratin antibodies used were: KL1 (reacts with cytokeratins 1, 2, 5-8, 11, 14, 16-18) (Immunotech) and PCK-26 (cytokeratins 5, 6, and 8) (Sigma). A rabbit polyclonal anti-rat FN cross-reactive with human FN was purchased from GIBCO-BRL (Gaithersburg, MD) and a monoclonal anti-FN antibody, FN-15, from Sigma. Streptavidin (SA)-lissamine rhodamine (LRSC) and affinity-purified donkey antirabbit LRSC were obtained from Jackson Laboratories (West Grove, PA), rabbit antimouse rhodamine from DAKO (Glostrup, Denmark), and affinity-purified goat F(ab′)2 antimouse phycoerythrin from Tago Immunologicals (Burlingame, CA). The following recombinant human cytokine preparations were obtained from Genzyme (Cambridge, MA): interferon-γ (IFN-γ) (2.5 × 107 U/mg), interleukin-1α (IL-1α) (1 × 108 U/mg), IL-4 (1 × 107 U/mg), and tumor necrosis factor-α (TNF-α) (4.55 × 108 U/mg).
TEC.TEC lines were established using a defined serum-free growth media (WAJC 404A; Kyokuto Pharmaceuticals, Tokyo, Japan) supplemented with dexamethasone (10 nmol/L, Sigma), transferrin (10 μg/mL, Sigma), epidermal growth factor (10 ng/mL, Boehringer Mannheim, Indianapolis, IN), and insulin (10 μg/mL, Sigma).23 Thymic tissue was finely minced and digested with collagenase P (Boehringer, 10 mg/mL) for 1 hour at 37°C. After washing in Ca++/Mg++ free phosphate-buffered saline (PBS) the partially digested tissue was placed in 0.05% Trypsin/ 0.53 mmol/L EDTA (GIBCO-BRL) for 30 minutes at 37°C, washed and plated in the supplemented WAJC growth media. For the first 48 hours the growth media was further supplemented with 2% iron-enriched calf serum (Sigma) after which all subsequent culture was done serum-free. The media was changed twice weekly and the cells passed when confluent by gently dislodging the adherent monolayer with trypsin/EDTA as described above. This method gave us phenotypically uniform TECs expressing high levels of the epithelial marker cytokeratin in the characteristic filamentous network pattern.
Cell separation and adhesion assays.Fresh thymus fragments were obtained during elective cardiac surgery on children (aged 1 month to 5 years) for repair of congenital cardiac malformations. Single cell suspensions of thymocytes were prepared by physical disruption of tissue over a 60-mesh stainless steel screen, separation on Ficoll-Paque (Pharmacia LKB, Piscataway, NJ), and washing into a serum-free culture media (AIM V; GIBCO-BRL) at 4°C.
For adhesion to TEC monolayers, the thymocytes were labeled overnight with 35S-Cysteine/Methionine (DuPont NEN, Boston, MA) in cysteine/methionine-free RPMI 1640 (ICN, Costa Mesa, CA) supplemented with L-glutamine (GIBCO-BRL) and 10% dialyzed fetal calf serum (FCS; GIBCO-BRL). TECs were grown as adherent monolayers to near confluence in 12-well culture plates (Falcon, Becton Dickinson Labware, Lincoln Park, NJ). Where indicated rIL-4 (1,000 U/mL) was added to the culture overnight. The monolayers were washed with Hanks' balanced salt solution (HBSS) and any exposed plastic blocked with 1% heat denatured bovine serum albumin (BSA) for 1 hour at 37°C. The blocked monolayers were washed into AIM V media, 2 × 106 35S-labeled thymocytes/well were added and the plates were spun at 100g for 3 minutes, incubated for 30 minutes at 37°C, and then washed 4 times with HBSS to remove nonadherent cells. Purified preparations of adhesion-blocking MoAbs were added at the initiation of the assays at a concentration of 20 μg/mL. The adhered thymocytes and the epithelial monolayer were obtained with 200 μL of 0.05% Trypsin/ 0.53 mmol/L EDTA (GIBCO-BRL) and counted in 1 mL of liquid scintillation fluor (OptiPhase HiSafe 3; Wallac, Turku, Finland) in 24-well plastic plates using a Wallac Model 1450 MicroBeta scintillation counter.
Confocal microscopy.Fresh cut segments of thymus were embedded in Tissue-Tek OCT compound (Miles Inc, Elkhart, IN), frozen immediately in liquid nitrogen, and stored at −80°C. Ten micrometer thick sections were cut at −20°C with a Cryocut 1800 (Leica, Deerfield, IL), fixed with freshly prepared 2% paraformaldehyde/PBS pH 7.4, and permeabilized with 0.1% Triton X-100 (Fisher Scientific, Pittsburgh, PA) for 10 minutes at 20°C. Sections were blocked in 50 mmol/L glycine/PBS for 5 minutes followed by incubation in 1% BSA, 5% normal goat serum in PBS overnight at 4°C. Sections were then incubated in the presence of unconjugated mouse antihuman VCAM-1 (25 μg/mL; 1G11) for 30 minutes at 20°C, washed, and incubated with a biotinylated secondary antibody, goat antimouse F(ab′)2 , followed by SA-LRSC. Control sections were also stained with a mouse IgG1k antibody shown to have no cross-reactivity with human tissue in multiple experiments including thymus and pancreas tissue as well as blocked with normal goat serum followed by the biotinylated goat antimouse secondary antibody. The unbound sites of the antimouse secondary antibody were blocked with purified polyclonal mouse IgG (10 μg/slide, Sigma) and a directly FITC-conjugated anticytokeratin antibody (1:100 dilution; KL-1) was added for 30 minutes at 20°C. Sections were mounted under glass coverslips with a fluorescent antifade solution (SloFade; Molecular Probes, Eugene, OR). The sections were examined with a BioRad MRC600 single argon/krypton laser scanning and confocal system (Hercules, CA).24 For analysis of cytokeratin expression by cultured TEC monolayers using confocal microscopy the TECs were grown on glass coverslips, fixed in absolute alcohol, and blocked with 5% mouse serum and heat aggregated 1% BSA. Cytokeratin was stained using either PKC-26 or KL-1 directly labeled with FITC.
Cytofluorometric analysis.Flow cytometry was performed on a FACScan (Becton Dickinson) and analyzed with Lysis II software. Adherent TEC lines were detached by incubation in EDTA (Versene 1:5,000; GIBCO-BRL) for 10 minutes at 37°C and washed at 4°C in PBS (without Ca++/Mg++) supplemented with 1% BSA/0.1% NaAzide. Cells were suspended for staining and analysis in sorter buffer with a 1:2 dilution of EDTA. MoAbs (1 to 2 μg/1 × 106 cells) were added in a 50-μL volume, incubated at 4°C for 1 hour, washed, and stained with a secondary antibody, affinity-purified goat F(ab′)2 antimouse PE (Tago Immunologicals) at a 1:300 dilution. The voltage gains for the FACScan detectors were set with the negative controls using antibodies that were species and isotype matched to the experimental reagents.
Polymerase chain reaction (PCR).TECs were removed from monolayer cultures as described above and mRNA was prepared with the Invitrogen Micro-Fast Track kit (San Diego, CA). Briefly, cells were lysed in a 2% sodium dodecyl sulfate (SDS), 200 mmol/L Tris pH 7.5 buffer and the supernatant collected after high-speed centrifugation. mRNA was purified by binding to oligo dT cellulose in 500 mmol/L NaCl, 10 mmol/L Tris-Cl pH 7.5 and was eluted with a low salt buffer, 250 mmol/L NaCl, in centrifugal spin columns and stored in 100% EtOH at −80°C. cDNA was synthesized from 0.5 μg mRNA with 200 U of Superscript II Maloney Murine Leukemia Virus Reverse Transcriptase (GIBCO-BRL). 0.3 μg cDNA was amplified by PCR with 1.25 U Taq DNA polymerase, 10 mmol/L Tris-HCl pH 8.3, 2.0 mmol/L MgCl2 , 50 mmol/L KCl, and 200 μmol/L each of the deoxynucleotide triphosphates (Boehringer Mannheim). The VCAM-1 primers were synthesized by Operon (Almeda, CA) according to published sequences25: sense (5′ CAAGTCTACATATCACCCAAG 3′) and antisense (5′ GGAACCTT-GCAGCTTACAGTGACAGAGCTCCC 3′). The β-actin control primers were purchased from Clontech (Palo Alto, CA). To determine the linear range for amplification the thermal cycler (Model 480, Perkins-Elmer, Norwalk, CT) was set for 15, 20, 25, and 30 cycles: 94° (1 minute), 55° (1 minute), and 72° (2.5 minutes)/cycle. Ten microliters of each PCR product was analyzed by 1.5% agarose (BioRad) gel electrophoresis, visualized with ethidium bromide, and the images captured with an EagleEye II Still Video System (Stratagene, La Jolla, CA). Densitometry was performed by transferring the digital image file to National Institutes of Health image (v1.52, Bethesda, MD). Integrated Density was computed using the following formula: Integrated Density = N* (Mean − Background); where N is number of pixels in the selection, and Background is the modal gray value (most common pixel value) after smoothing the histogram.
Immunoprecipitation.Confluent monolayers of TECs or human umbilical vein endothelial cells (HUVECs) were cultured overnight in 1,000 U/mL human rIL-4 (Genzyme) (TECs) or 10 ng/mL TNF-α (Genzyme) (HUVECs). The monolayers were dissociated by incubation in Ca++/Mg++-free PBS containing 0.02% EDTA, washed, and labeled with biotin by incubation with 100 μg/mL of NHC-LC biotin (Pierce, Rockford, IL) for 30 minutes at room temperature. Labeled cells were lysed in a 1% Triton X-100 solution containing 20 mmol/L Tris (pH 7.2), 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 20 ng/mL aprotinin (Sigma), 20 ng/mL leupeptin (Sigma) for 30 minutes at 4°C. Immunoprecipitation of VCAM-1 from precleared lysates was done with a combination of 1G11 and 4B9 MoAbs overnight at 4°C followed by absorption to protein G-sepharose beads (GIBCO-BRL) for 1 hour at room temperature. Control lysates were incubated with a mouse IgG1 isotype matched antibody. The sepharose beads were washed sequentially in 0.1% Triton X-100/ 50 mmol/L HEPES (pH 7.2) buffer containing either 1 mol/L NaCl, 150 mmol/L NaCl, or no NaCl. After boiling the washed beads for 10 minutes in reducing SDS sample buffer the proteins along with appropriate molecular weight standards were separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE) in a 7% gel and transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA). Detection of the biotinylated proteins was performed using streptavidin-alkaline phosphatase and a chemiluminescent detection system (Tropix, Bedford, MA).
Immunohistochemistry.Frozen thymic sections (10 μmol/L) were fixed for 2 minutes in absolute ethanol (−20°C) and blocked for 1 hour at room temperature in PBS/5% BSA/goat IgG (20 μg/mL). Sections were then incubated for 1 hour with either anti-VCAM-1 antibodies (1G11 and 4B9), anti-FN MoAb (clone FN-15), or an isotype-matched IgG1 control antibody. After washing the stained sections were incubated for 20 minutes with a biotin-conjugated goat antimouse IgG Fab2 fragment (Caltag, South San Francisco, CA) followed by a 15-minute incubation with streptavidin-peroxidase (Pierce). After development of the peroxidase-mediated color reaction by addition of the diamino benzidine substrate, sections were counterstained with Contrast Blue (Kirkegaard and Perry, Gaithersburg, MD) and mounted in Gel-Mount (Biomedia, Foster City, CA). Images were obtained at 125× magnification on a Olympus microscope (Tokyo, Japan).
RESULTS
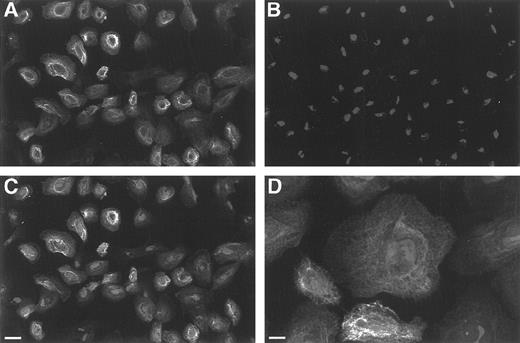
Characterization of human TEC.It was important to establish that the majority of thymic cells in the adherent cell lines we developed in the serum-free growth media were of epithelial origin. A representative line was stained (Fig 1A) with an antihuman pan-cytokeratin antibody (PCK-26) and counterstained with propidium iodide to identify all nuclei (Fig 1B). When the two are superimposed (Fig 1C) essentially all the cells in the field are revealed to be cytokeratin positive. In addition, the cells display the characteristic filamentous cytokeratin structure of epithelium (Fig 1D) and spread concentrically as large cells in an adherent monolayer when analyzed by differential interference contrast (DIC) microscopy. The percentage of cytokeratin positive cells was >90% during culture in all the TEC-cell lines examined in this study.
Scanning laser confocal microscopy of TECs in monolayer cultures. (A) Depicts a relatively low power view of staining with the directly FITC-labeled pan-cytokeratin antibody (PKC-26) showing that the majority of TEC cells in the monolayers are cytokeratin positive. (B) Represents the same field stained with propidium iodide to reveal the cell nuclei and (C) is the merged image showing that essentially all the cells are cytokeratin positive (bar = 11.3 μm). (D) Is a higher power image of TECs showing the characteristic filamentous structure of cytokeratin expressed by epithelial cells (bar = 7.2 μm).
Scanning laser confocal microscopy of TECs in monolayer cultures. (A) Depicts a relatively low power view of staining with the directly FITC-labeled pan-cytokeratin antibody (PKC-26) showing that the majority of TEC cells in the monolayers are cytokeratin positive. (B) Represents the same field stained with propidium iodide to reveal the cell nuclei and (C) is the merged image showing that essentially all the cells are cytokeratin positive (bar = 11.3 μm). (D) Is a higher power image of TECs showing the characteristic filamentous structure of cytokeratin expressed by epithelial cells (bar = 7.2 μm).
Flow cytometry studies demonstrated that the TECs express N-cadherin and αvβ5 (epithelial cell antigens). They were also positive for CD40, CD58 (LFA3), and expressed substantial MHC class I and relatively low levels of MHC class II. By immunohistochemistry the cell lines were negative for the expression of von Willebrand factor showing that they are not endothelial cell-derived. They are also CD11b (Mac 1)-negative, which combined with the limited MHC class II expression indicates that they are not substantially contaminated by cells of the macrophage/dendritic lineage. The cell lines did not express the human thymic fibroblast antigen recognized by the MoAb, 1B10. This antibody has been used to eliminate thymic fibroblasts by complement-mediated lysis and thus enrich for human thymic epithelial cells in culture.26 A similar microscopic morphology, cytokeratin structure, CD40, CD58, and relative MHC Class I and II molecule expression has been described for human thymic epithelial cell clones developed in D-valine MEM and FCS.27
Human TECs express VCAM-1 in culture.All the TEC lines including one developed from fetal thymus expressed VCAM-1 constitutively when analyzed by flow cytometry with MoAb 1G11 (Fig 2A). The results were confirmed with two additional antihuman VCAM-1 antibodies (P3H12 and 4B9, data not shown). In addition, all lines were also positive for intracellular adhesion molecule-1 (ICAM-1) (Fig 2B), which is a cellular ligand for the integrin, αLβ2 (LFA-1). Similar results were obtained with 6 TEC lines produced in either RPMI or d-Valine/MEM with 20% FCS indicating that the constitutive expression of VCAM-1 by TEC was not a unique property of TECs cultured in the serum-free growth media.
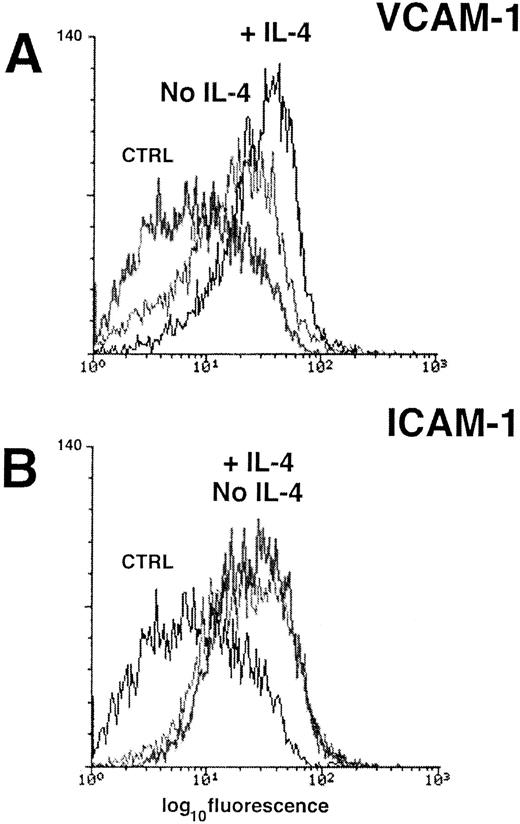
Analysis of VCAM-1 (A) and ICAM-1 (B) expression on cultured human TECs by flow cytometry. Pretreatment of the TECs with rIL-4 overnight (1,000 U/mL) consistently increased the constitutive expression of VCAM-1, but had no effect on the level of ICAM-1 expression. The results of staining with an isotype-matched control antibody is shown in both panels for comparison (CTRL).
Analysis of VCAM-1 (A) and ICAM-1 (B) expression on cultured human TECs by flow cytometry. Pretreatment of the TECs with rIL-4 overnight (1,000 U/mL) consistently increased the constitutive expression of VCAM-1, but had no effect on the level of ICAM-1 expression. The results of staining with an isotype-matched control antibody is shown in both panels for comparison (CTRL).
VCAM-1 expression is upregulated on TECs by cytokines.Most endothelial surfaces in vivo and endothelial cell monolayers in culture express little or no VCAM-1 in the absence of stimulation by inflammatory cytokines such as IL-1α, IL-4, and TNF-α.22 28-30 In contrast, we have shown that our TEC lines express VCAM-1 in culture even in a serum-free growth media. To determine whether an exogenous cytokine could increase the expression of VCAM-1 on TEC, we incubated subconfluent cultures of TECs overnight with various concentrations of the recombinant cytokine IL-4 (eg, 2,000 to 10 U/mL). IL-4 induced upregulation of VCAM-1 expression (Fig 2A) but had no effect on ICAM-1 expression by the same TECs (Fig 2B). The median log channel fluorescence of the negative control was 7.8, VCAM-1/No IL-4 was 16.6 and VCAM-1/+IL-4 was 30.5. The median log channel fluorescence of the ICAM-1 histograms (±IL-4) in Fig 2B were 22 and 24.6, respectively.
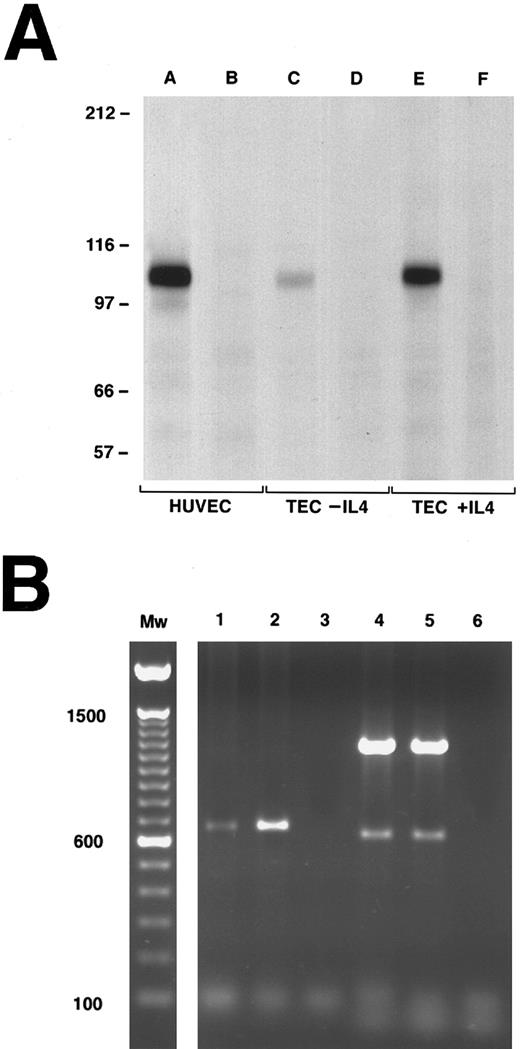
To further characterize the expression of VCAM-1 on TECs, the monoclonal anti-VCAM-1 antibodies (4B9 and 1G11) were used in immunoprecipitation studies of resting and IL-4–stimulated TEC (Fig 3A). TNF-α–stimulated HUVEC were used as the positive control. A single major band with an apparent molecular weight of ∼110 kd, consistent with VCAM-1,31 32 was immunoprecipitated from all three cell populations (Fig 3A; lanes A, C, and E v negative controls; lanes B, D, and F). Each lane was loaded with the immunoprecipitates from an equal number of cells. It is apparent that IL-4 increases the amount of VCAM-1 protein expressed per TEC, which is consistent with the flow cytometry profiles.
Detection of VCAM-1 expression on cultured human TECs by immunoprecipitation (A) and PCR (B). (A) Immunoprecipitation of VCAM-1 from detergent lysates of biotin-labeled TECs (with or without rIL-4 pretreatment) or HUVEC (with TNF pretreatment). Immunoprecipitations with a control MoAb are shown in lanes B, D, and F. Immunoprecipitations with a combination of anti-VCAM-1 MoAbs (4B9 and 1G11) are shown in lanes A, C, and E. The constitutive expression of VCAM-1 (TEC-IL-4) is compared to the expression after overnight culture in IL-4 (TEC + IL-4). The apparent Mw of the major band (∼110 kd) in lanes A, C, and E is consistent with VCAM-1. (B) PCR analysis of VCAM-1 expression. Lanes 1 (unstimulated TEC) and 2 (IL-4 stimulated TEC) show a single major band at an apparent Mw of 650 kb consistent with the 7-domain form of VCAM-1. Lanes 4 and 5 are the actin controls for the same cells. Lanes 3 and 6 are the negative water controls.
Detection of VCAM-1 expression on cultured human TECs by immunoprecipitation (A) and PCR (B). (A) Immunoprecipitation of VCAM-1 from detergent lysates of biotin-labeled TECs (with or without rIL-4 pretreatment) or HUVEC (with TNF pretreatment). Immunoprecipitations with a control MoAb are shown in lanes B, D, and F. Immunoprecipitations with a combination of anti-VCAM-1 MoAbs (4B9 and 1G11) are shown in lanes A, C, and E. The constitutive expression of VCAM-1 (TEC-IL-4) is compared to the expression after overnight culture in IL-4 (TEC + IL-4). The apparent Mw of the major band (∼110 kd) in lanes A, C, and E is consistent with VCAM-1. (B) PCR analysis of VCAM-1 expression. Lanes 1 (unstimulated TEC) and 2 (IL-4 stimulated TEC) show a single major band at an apparent Mw of 650 kb consistent with the 7-domain form of VCAM-1. Lanes 4 and 5 are the actin controls for the same cells. Lanes 3 and 6 are the negative water controls.
TECs express the seven Ig domain repeat form of VCAM-1. Cytokine-activated human endothelial cells have been shown to express several forms of VCAM-1 based on alternative splicing including a predominant form with seven Ig-like repeat domains.25,33 We synthesized a single set of PCR primers designed to hybridize with sequences common to both the six and seven domain forms of human VCAM-1.25,34 The primers were chosen so that the the amplified PCR product spans the alternative splice site for addition of the fourth domain that creates the seven domain VCAM-1 from the six domain form.33 Figure 3B is representative of one of three experiments in which the linear range of amplification for these VCAM-1 primers and the β-actin controls was predetermined so that the results shown are semiquantitative. The reverse-transcribed cDNA amplified for 25 cycles in lane 1 is from unstimulated TECs and in lane 2 from TECs stimulated overnight with IL-4. The single band in lanes 1 and 2 at ∼650 kb represents the expression of the seven Ig domain form of VCAM-1.25 Lanes 4 and 5 represent the β-actin controls for these same preparations (major band 1,200 kb, minor band 600 kb) and lanes 3 and 6 are negative water controls. The VCAM-1 band in lane 2 (+ IL-4) is approximately 8 times the intensity of the matching band in lane 1 (No IL-4) (integrated density: 5.6 v 42.0). In contrast, the bands amplified with the actin control primers (lanes 4 and 5) have essentially equivalent integrated densities (major: 520 v 567; minor: 19.8 v 15) indicating that the amount of starting cDNA amplified in each PCR was equal and the increased intensity of the VCAM-1 product in lane 2 represents a real increase in mRNA transcript levels after IL-4 treatment.
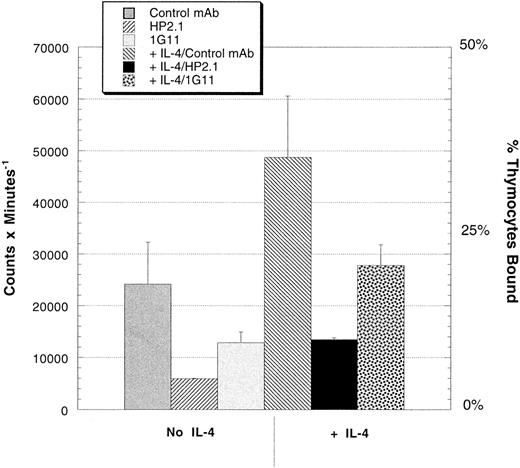
Thymocytes use α4β1 to adhere to TEC monolayers.The capacity of the TEC expressed VCAM-1 to bind thymocytes was analyzed by culturing 35S-labeled thymocytes for 30 minutes with confluent TEC monolayers in the presence or absence of monoclonal anti-α4 (HP2.1) or anti-VCAM-1 (1G11). Figure 4 shows that the binding of thymocytes is enhanced by overnight treatment of the TEC with rIL-4 to approximately 40% of the thymocytes plated and inhibited by both the HP2.1 and 1G11 antibodies. As shown in Table 1, in two additional experiments there is a 30% increase in thymocyte adhesion after rIL-4 in one experiment and no increase after rIL-4 in the other. Note that IL-4 upregulates the expression of VCAM-1 by TECs without increasing ICAM-1 expression (Fig 2), which could mediate thymocyte adhesion via another integrin, αLβ2 (LFA-1).35 Moreover, there was no inhibition of adhesion demonstrated with any combination of blocking anti-ICAM-1 and αLβ2 antibodies (eg, control MoAb; 25,593 counts per minute [cpm] v anti-αLβ1 + anti-ICAM-1; 27,000 cpm).
Adhesion of thymocytes to TEC monolayers with or without the pretreatment of the monolayers with rIL-4 (1,000 U/mL overnight). Freshly prepared thymocytes were metabolically labeled with 35S and plated onto confluent TEC monolayers (untreated or rIL-4 pretreated) in serum-free culture media for 30 minutes at 37°C in the presence of either a control MoAb, an anti-α4 antibody (HP2.1), or an anti-VCAM-1 MoAb (1G11). This experiment is representative of three experiments depicted in Table 1.
Adhesion of thymocytes to TEC monolayers with or without the pretreatment of the monolayers with rIL-4 (1,000 U/mL overnight). Freshly prepared thymocytes were metabolically labeled with 35S and plated onto confluent TEC monolayers (untreated or rIL-4 pretreated) in serum-free culture media for 30 minutes at 37°C in the presence of either a control MoAb, an anti-α4 antibody (HP2.1), or an anti-VCAM-1 MoAb (1G11). This experiment is representative of three experiments depicted in Table 1.
Effect of IL-4 on Thymocyte Adhesion to TEC Monolayers
| . | . | Experiment #1 . | Experiment #2 . | Experiment #3* . |
|---|---|---|---|---|
| No IL-4 | Control | 21,403 | 16,532 ± 727 | 24,151 ± 5,765 |
| + anti-VLA4‡ | 4,163 | 12,038 ± 465 | 5,926 ± 51 | |
| + anti-VCAM-1ρ | 19,758 | 13,948 ± 799 | 12,852 ± 1,451 | |
| + IL-4† | Control | 29,867 | 17,035 ± 2,588 | 48,755 ± 8,384 |
| + anti-VLA4‡ | 4,749 | 7,930 ± 1,231 | 13,502 ± 212 | |
| + anti-VCAM-1ρ | 17,297 | 6,692 ± 2,191 | 27,817 ± 2,842 | |
| + both | 4,750 | 8,837 ± 2,903 | ND |
| . | . | Experiment #1 . | Experiment #2 . | Experiment #3* . |
|---|---|---|---|---|
| No IL-4 | Control | 21,403 | 16,532 ± 727 | 24,151 ± 5,765 |
| + anti-VLA4‡ | 4,163 | 12,038 ± 465 | 5,926 ± 51 | |
| + anti-VCAM-1ρ | 19,758 | 13,948 ± 799 | 12,852 ± 1,451 | |
| + IL-4† | Control | 29,867 | 17,035 ± 2,588 | 48,755 ± 8,384 |
| + anti-VLA4‡ | 4,749 | 7,930 ± 1,231 | 13,502 ± 212 | |
| + anti-VCAM-1ρ | 17,297 | 6,692 ± 2,191 | 27,817 ± 2,842 | |
| + both | 4,750 | 8,837 ± 2,903 | ND |
Table 1 depicts the results of three consecutive experiments. Inhibition was greater with the HP2.1 anti-α4 antibody as compared to anti-VCAM-1 in two of the three which suggests the possibility that FN is a second ligand for α4β1 at the TEC cell surface. Experiments with a combination of two anti-FN antibodies (7.1 and 333), which can block the adhesion mediated by the integrin, α5β1, did not show inhibition of adhesion (eg, control MoAb 11,193 cpm v anti-FN 12,421 cpm). The epitope for the 333 antibody has been mapped just outside the RGDS site on FN and effectively blocks the RGDS-dependent binding of α5β1.36 This is important because human thymocytes express both FN receptors, α4β1 and α5β1. Unfortunately, we cannot formally prove that α4β1 is binding the CS1 sequence of FN on TEC cells. We are not aware of a CS1-specific blocking antibody nor an anti-α4β1 MoAb that selectively inhibits either VCAM-1 or FN binding. CS1-specific peptide analogs and a recombinant VCAM-1 fusion protein block α4β1-mediated adhesion to either VCAM-1 or FN. Nonetheless, the primary point is that approximately 50% of thymocyte adhesion to TECs is inhibited by anti-VCAM-1 MoAbs and adhesion is upregulated by IL-4, which increases VCAM-1 expression.
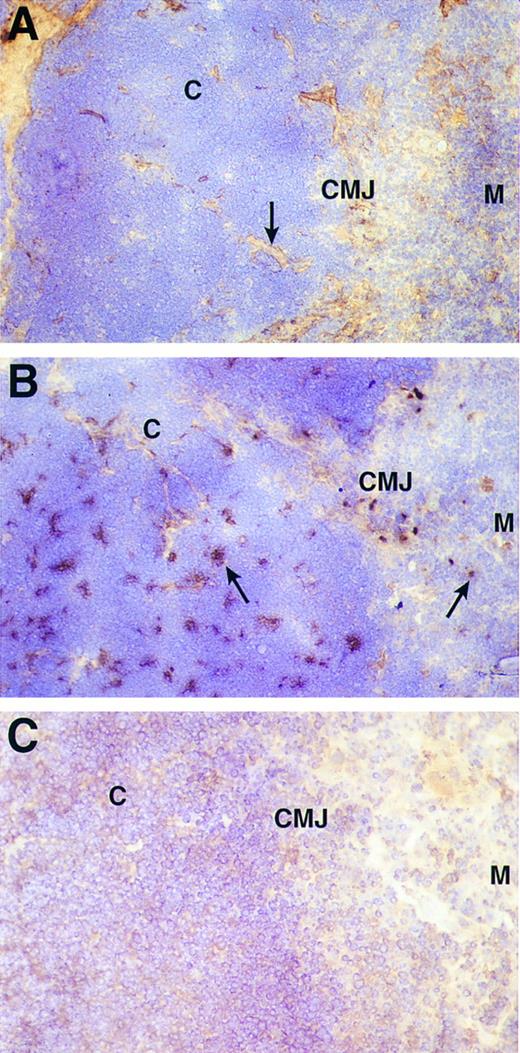
VCAM-1 is expressed by thymic epithelium in the cortex and corticomedullary junction of the human thymus.To determine the localization of VCAM-1 expression in the thymus, fresh frozen sections of thymic tissue were stained with anti-FN (Fig 5A), anti-VCAM-1 (Fig 5B) and a control MoAb (Fig 5C). The majority of FN staining was located in the medulla (M) and at the corticomedullary junction (CMJ) with the remainder tracing the connective tissue septa (5A; arrow) crossing the cortex (C) to the capsule. FN is also present in the Hassal's corpuscles of the medulla. In contrast, VCAM-1 staining was extensively distributed in the cortex in irregularly shaped islands of cells (5B; left arrow) that in some sections include the corticomedullary junction. On the other hand, only isolated VCAM-1 positive cells are seen in the medulla (5B; right arrow).
Immunohistochemical detection of FN (A) and VCAM-1 (B) in human thymus. Frozen sections of human thymus were labeled with anti-FN MoAb (FN-15) or a mixture of anti-VCAM-1 MoAbs (1G11 and 4B9) and the results developed by immunoperoxidase staining. (C) Is the staining with a negative control antibody. The cortex (C), corticomedullary junction (CMJ), and medulla (M) are marked in each panel. In (A), the majority of FN staining is apparent in the medulla and connective tissue septa crossing the cortex to the subcapsular region (arrow). In contrast, (B) shows that the majority of VCAM-1 staining is evident in irregularly shaped islands of cells in the cortex and corticomedullary junction (left arrow). Nonetheless, a number of isolated VCAM-1 positive cells are also seen in the medulla (right arrow).
Immunohistochemical detection of FN (A) and VCAM-1 (B) in human thymus. Frozen sections of human thymus were labeled with anti-FN MoAb (FN-15) or a mixture of anti-VCAM-1 MoAbs (1G11 and 4B9) and the results developed by immunoperoxidase staining. (C) Is the staining with a negative control antibody. The cortex (C), corticomedullary junction (CMJ), and medulla (M) are marked in each panel. In (A), the majority of FN staining is apparent in the medulla and connective tissue septa crossing the cortex to the subcapsular region (arrow). In contrast, (B) shows that the majority of VCAM-1 staining is evident in irregularly shaped islands of cells in the cortex and corticomedullary junction (left arrow). Nonetheless, a number of isolated VCAM-1 positive cells are also seen in the medulla (right arrow).
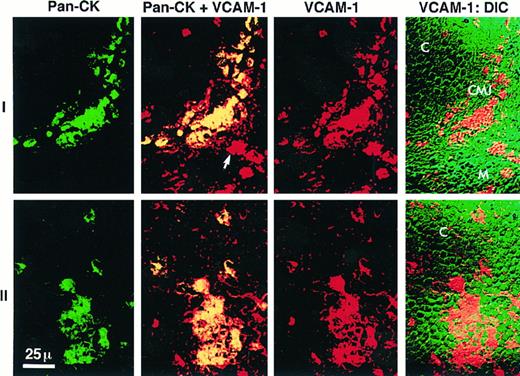
To confirm that VCAM-1 expression in the cortex was expressed on epithelial cells, another series of tissue sections was analyzed by scanning laser confocal microscopy. These sections were stained for two parameter imaging with antibodies against human epithelial cytokeratins (KL-1) and VCAM-1 (1G11 or P3H12). Scanning confocal microscopy has the major advantage of permitting a true immunolocalization of staining for two markers that is not possible with immunohistochemistry. This is accomplished by superimposing the images collected simultaneously on a single section in a series of confocal image planes using two separate fluorescence detectors. Figure 6 depicts representative fields from the analysis of three different thymic donors which were prepared, stained, and examined separately. Thymic epithelial cytokeratin was identified with a directly FITC-labeled antibody (shown in green) and VCAM-1 was identified by indirect staining with a secondary antibody labeled with LRSC (shown in red). Negative control antibodies were used in each experiment to set the detector gain such that the background reveals minimal nonspecific staining. Images constructed by DIC microscopy were used to determine the location of the cortex and medulla while revealing the relationship of positive VCAM-1 staining to cellular detail lost in the superimposed color images (VCAM-1:DIC). Serial images constructed by confocal z-plane analysis demonstrated true colocalization of staining for both epithelial cytokeratins and VCAM-1 and excluded the possibility that VCAM-1 might be expressed on a separate cell physically superimposed over a cytokeratin positive epithelial cell in the tissue section. Figure 6 (series I) shows results representative of the CMJ. The DIC frame of this series (VCAM-1:DIC) identifies the VCAM-1 expression in the CMJ as a semilunar shaped structure at the intersection of the densely packed, relatively homogenous cortex (C) in the upper left portion of the field with the heterogenous medullary pattern (M) to the lower right. Series II is representative of the cortex. The colocalization of cytokeratin and VCAM-1 expression is shown in both series I and II by showing the two separate images of pan-cytokeratin staining (green) and VCAM-1 staining (red) flanking the computer-merged images (Pan-CK + VCAM-1). In the merged images the colocalization of cytokeratin and VCAM-1 staining is marked by yellow (green + red). In the last panel of each series we merged the VCAM-1 image (red) with the DIC image to show the relationship of the VCAM-1 staining to the thymic morphology revealed by DIC.
Laser scanning confocal microscopy of cytokeratin and VCAM-1 expression in the human thymus. Frozen sections of human thymus were labeled with a directly-FITC labeled pan-cytokeratin MoAb (KL-1) shown in green and an anti-VCAM-1 MoAb (1G11) indirectly stained with a LRSC-labeled secondary antibody shown in red. The last panel of each series is the differential interference contrast image (DIC), which allows the localization of staining in the thymus based on morphological criteria. Series I depicts a representative field at the corticomedullary junction (CMJ) identified at the intersection of the cortex (C) and medulla (M). Series II depicts the cortex. The first image in each series is the pan-cytokeratin staining (Pan-CK, green). The second image is the computer-merged view of both pan-cytokeratin and VCAM-1 staining in which the yellow color represents colocalization of both antibodies (yellow = green + red). The third image of each series is the VCAM-1 staining (VCAM-1, red). The majority of cytokeratin staining in the CMJ and C is colocalized with VCAM-1 consistent with the expression of VCAM-1 by TECs. The fourth image is the DIC view merged with the VCAM-1 staining in red. In the second image of series 1 (Pan-CK + VCAM-1) an arrow marks a VCAM-1 positive cell in the medulla that is pan-cytokeratin negative.
Laser scanning confocal microscopy of cytokeratin and VCAM-1 expression in the human thymus. Frozen sections of human thymus were labeled with a directly-FITC labeled pan-cytokeratin MoAb (KL-1) shown in green and an anti-VCAM-1 MoAb (1G11) indirectly stained with a LRSC-labeled secondary antibody shown in red. The last panel of each series is the differential interference contrast image (DIC), which allows the localization of staining in the thymus based on morphological criteria. Series I depicts a representative field at the corticomedullary junction (CMJ) identified at the intersection of the cortex (C) and medulla (M). Series II depicts the cortex. The first image in each series is the pan-cytokeratin staining (Pan-CK, green). The second image is the computer-merged view of both pan-cytokeratin and VCAM-1 staining in which the yellow color represents colocalization of both antibodies (yellow = green + red). The third image of each series is the VCAM-1 staining (VCAM-1, red). The majority of cytokeratin staining in the CMJ and C is colocalized with VCAM-1 consistent with the expression of VCAM-1 by TECs. The fourth image is the DIC view merged with the VCAM-1 staining in red. In the second image of series 1 (Pan-CK + VCAM-1) an arrow marks a VCAM-1 positive cell in the medulla that is pan-cytokeratin negative.
Figure 6 (series I) also shows the presence of VCAM-1 staining on a small number of isolated cytokeratin-negative cells (merged image; arrow) in the medulla. This result confirms the immunohistochemical staining (Fig 5B), indicates that the medullary cells are not epithelial and suggests that VCAM-1 is also expressed by thymic dendritic cells, which are located primarily in the medulla.37 VCAM-1 expression has been demonstrated on dendritic cells in the germinal centers of peripheral lymphoid tissue.38 However, as VCAM-1 is expressed by stromal elements in the BM,17,18,39 it is also possible that VCAM-1 is expressed on medullary thymic stromal cells. We also cannot exclude the possibility that these cytokeratin-negative cells in the medulla might represent a population of epithelial cells not marked by our pan-cytokeratin antibody as medullary epithelial cell heterogeneity for cytokeratin expression has been previously described.40 41
DISCUSSION
The goal of the present study was to characterize the expression of VCAM-1 in the thymus. We initially determined VCAM-1 expression on human thymic epithelial cell lines. TECs in primary cultures constitutively express VCAM-1 and upregulate expression after exposure to IL-4. Freshly prepared and unactivated thymocytes can bind to TEC monolayers and MoAb inhibition studies show that binding can be inhibited by anti-α4β1 and anti-VCAM-1 MoAbs. The significance of the expression of VCAM-1 on the TEC lines is underscored by demonstrating that VCAM-1 is also detectable on human thymic epithelium in situ as shown by both immunohistochemistry and scanning laser confocal microscopy of frozen thymic tissue sections. VCAM-1 was predominantly detected on epithelial cells in the cortex and at the corticomedullary junction, whereas FN was predominantly found in the medulla and septa. These data suggest that the VCAM-1 on the surface of cortical thymic epithelium is an adhesive ligand in vivo for the constitutively active form of α4β1 expressed by immature cortical thymocytes.9 The selective expression of FN in the medulla suggests that there may be a functional significance in the cortical/medullary organization of the thymus which involves the differential expression of these two ligands for α4β1.
It is always difficult to conclude that the TEC lines derived from a complex tissue such as thymus are truly representative of the resident epithelial cell populations. Nevertheless, the serum-free growth media we used allowed us to develop phenotypically stable epithelial monolayers, which expressed VCAM-1 and which were capable of mediating thymocyte adhesion in vitro via this ligand. We have not as yet characterized our TEC lines with antibodies reported to be specific for human cortical and medullary epithelium.27,40,42 However, the immunohistochemistry and confocal microscopy results (Figs 5 and 6) show that VCAM-1 expression is confined to epithelial cells resident in the cortex and corticomedullary junction. In fact the phenotype of our TEC lines is identical to that published for human cortical epithelial cell clones with the major exception that the cloned lines did not express VCAM-1 by flow cytometry.27 A second paper grew the TECs that migrate spontaneously out of intact fragments of thymus in a serum enriched media with a mouse 3T3 fibroblast feeder layer and after complement-mediated lysis of human thymic fibroblast contaminants with the MoAb, 1B10.20 Only low levels of VCAM-1 staining were shown after exposure to INF-γ. In contrast, we have studied primary TEC lines developed from enzyme digested tissue after relatively short periods of culture in serum-free conditions supplemented with dexamethasone, insulin, transferrin, and epidermal growth factor. It is possible that the cortical epithelial clones described in the first paper lose their capacity to express VCAM-1 during the several months required for cloning. It is also possible that TECs migrating out of thymic fragments onto serum-derived extracellular matrix proteins bound to plastic are different than those developed with our enzyme digestion method. We also culture these TECs in serum-free media where they grow as an adherent monolayer by making and organizing their own matrix. Alternatively, there may be some growth factor signal provided in our primary TEC cultures that is lost after cloning or missing from the complex mixture generated by extended coculture with xenogeneic mouse fibroblast “feeder” cells used in the second paper. Thus, it is important to emphasize that differences in molecule expression reported for TECs developed and cultured with very different protocols must be interpreted carefully. This underscores the importance of the correlations with VCAM-1 expression on frozen sections of thymus, which we have depicted in Figs 5 and 6.
VCAM-1 is a member of the Ig supergene family and can be expressed in multiple forms secondary to alternative splicing of mRNA transcripts.31,33 The predominant form of VCAM-1 expressed on the cell surface of cytokine-activated endothelial cells has seven Ig-like repeat domains.25 An eight domain form33 and an alternatively spliced six domain form in which domain 4 has been deleted25,34 have also been reported. Either domain 1 or 4 can mediate the binding of the integrin, α4β1.34,43 Although the N-terminal domain 1 is dominant, cell adhesion is more efficient when both domains 1 and 4 are presented.43 Although the TEC lines expressed the seven domain form of VCAM-1 by PCR analysis, the TECs were only studied following stimulation with IL-4. As the six domain form expressed by endothelial cells was observed following induction with either IL-1α or TNF-α,25 we cannot exclude the possibility that the six domain form might be preferentially increased by cytokines other than IL-4 or even a combination of cytokines.44 There is also evidence for expression of an alternatively spliced three domain form (domains 1-3) of VCAM-1 in LPS-treated murine lung tissue.45 This form of VCAM-1 is further truncated by alternative splicing at the C-terminal tail to create a phosphatidylinositol-linked molecule, which is also functional as an adhesive ligand at the cell surface. Although no evidence of mRNA for this three domain form has been found in human endothelial cells,45 our choice of PCR primers would not have allowed detection of this form of VCAM-1 in the TECs.
Thymocyte adhesion to the TEC monolayers is mediated by the interaction of α4β1 with VCAM-1 as both anti-α4 and anti-VCAM-1 produced substantial inhibition of binding. However, somewhat greater inhibition of binding was seen with anti-α4 than anti-VCAM-1 antibodies. The anti-α4 antibody, HP2/1, binds the B1 region of α4 and can potently inhibit both VCAM-1 and FN adhesion.46,47 Although the binding site of 1G11 has not been determined, 4B9 binds to VCAM-1 at a site located between domains 1 and 4, which results in inhibition of α4β1 binding to both domains.43 Nevertheless, studies of α4β1 in mediating leukocyte adhesion to endothelial cells have raised the possibility of the existence of a third ligand for α4β1.43 Homotypic binding (eg, α4 → α4) has also been described for lymphocytes.46 α4β1 Was not expressed on TECs in one study,20 but was positive on cortical thymic epithelial clones in a second study.27 Therefore, we do not wish to exclude the possibility that a component of thymocyte binding to the TECs is mediated by alternatives to α4β1/VCAM-1 including the reasonable possibility of α4β1 binding to the CS1 site on FN reported to be expressed by TECs.
The differential expression of VCAM-1 by cortical epithelium suggests that VCAM-1 is a ligand for α4β1 in the cortex. A role for VCAM-1 in progenitor cell development in the thymic cortex is directly analogous to that described for VCAM-1 in the BM where stromal cell expression of VCAM-1 appears to regulate both lymphopoiesis and erythropoiesis.14,18,39,48 These events in the BM are also influenced by cytokines such as IL-1, IL-3, IL-4, granulocyte-macrophage colony-stimulating factor, and the KIT ligand.15,49,50 In fact, the release of mature cells from the BM compartment may also be regulated by α4β1-mediated adhesion since treatment of nonhuman primates with anti-α4β1 antibody resulted in peripheralization of hematopoietic precursors16 and stem cell factor can cause disengagement of ligand binding mediated by α4β1 and α5β1.51 As the more mature CD3hi single positive thymocytes use α5β1 to mediate adhesion and proliferation on FN in concert with TCR/CD3 cross-linking,52 it is possible that the FN in the thymic medulla is involved in regulating the development and exit of more mature thymocytes. Thus, the selective localization of VCAM-1 to the cortex and FN to the medulla may play an important role in regulating the participation of each ligand in thymocyte development. The organized expression of an α4β1 ligand has also been seen in studies of joint synovia obtained from patients with rheumatoid arthritis. The alternatively spliced binding site on FN, CS-1, was only expressed on the synovial endothelium where activated inflammatory cells accumulate and not in the extracellular matrix of the joint which is spared.53
The work presented here makes three important points. One, it is the first demonstration of VCAM-1 expression by epithelial cells in the thymus. The current literature has described VCAM-1 expressed by dendritic cells in the medulla and by a few arterioles in the cortex. Moreover, the fact that VCAM-1 is expressed on epithelial cells in a normal thymus is significant since the current rule is that VCAM-1 expression by other epithelial cells including endothelium requires inflammatory cytokine induction. Second, VCAM-1 expression is specifically limited to the cortical epithelium. The site-specific expression of this ligand has important implications for its role in supporting thymocyte development and the function of α4β1 in T-cell selection. In contrast, the expression of FN, the alternative α4β1 ligand, is predominantly in the medulla. The compartmentalization of these two ligands is a novel observation and important new evidence that thymic structure may be directly linked to specific stages in the thymocyte development program. It is also possible that thymic epithelial VCAM-1 is one signal regulating the migration of developing thymocytes from cortex to medulla following positive selection. This hypothesis is consistent with evidence that VCAM-1 can mediate lymphocyte migration after activation,54 that cross-linking of VCAM-1 by α4β1 augments CD3-dependent T-cell activation,55 and that VCAM-1 may also play a role in activation-induced apoptosis of CD4+ lymphocytes.56 Third, we show that thymocytes can adhere to thymic epithelial cell monolayers in vitro via the binding of α4β1 to VCAM-1. In the BM compartment VCAM-1 is expressed by a poorly characterized “reticular” cell and is involved in hematopoietic stem cell development. Therefore, our results advance the hypothesis that VCAM-1/α4β1 interactions represent a common theme in lymphohematopoietic stem cell development and introduces the novel concept that this mechanism is operative in both the thymic and BM compartments.
ACKNOWLEDGMENT
The authors thank Dr Mark Ellisman and colleagues at the University of California, San Diego, Microscopy and Imaging Resource for their expert assistance. We also thank Dr J. Lamberti and the nurses of the Children's Hospital Cardiac Surgery Team (San Diego, CA) for supplying the pediatric thymic tissue for these studies.
Supported in part by a Grant from the National Institutes of Health No. DK1820 (D.R.S.). Supported by the Barbara Botwinick Fellowship in Experimental and Molecular Immunology established by W. Robert and Marjorie Ramsdell and a grant from The Scripps Clinic and Research Foundation Department of Academic Affairs (#95-15) (L.C.).
Address reprint requests to Daniel R. Salomon, MD, Department of Molecular and Experimental Medicine-SBR5, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal