Abstract
The effects of platelet factor 4 (PF4) on the viability and chemosensitivity of normal hematopoietic cells and cancer cell lines were studied to determine the mechanisms whereby PF4 functions as either an inhibitor or a protector and to evaluate its clinical significance. Two other chemokines, interleukin-8 (IL-8) and neutrophil-activating peptide-2 (NAP-2), were also studied in comparison to PF4. Using a tetrazolium salt assay for cell viability, we observed that PF4 at 1 to 50 μg/mL supported the viability of normal human bone marrow cells. Approximately 45% of cells cultured for 48 hours survived, whereas 80% or more survived in the presence of PF4 5 μg/mL. PF4 also supported the viability of CD34+ cord blood (CB) cells and protected them from apoptosis induced by transforming growth factor β1 (TGFβ1) and cytotoxic drugs. Pretreatment of CD34+ cells by PF4, but not by TGFβ1, caused an increase in the number of megakaryocyte colonies after these cells were replated in secondary cultures. Flow cytometry analysis showed that when CD34+ cells were preincubated with PF4 or TGFβ1 for 12 days in hematopoietic growth factor–rich medium, an increased number of remaining CD34+ cells was observed only for PF4-treated cells. Furthermore, PF4 significantly reduced the chemosensitivity of bone marrow cells, as shown by its ability to increase the 50% inhibition concentration (IC50) of several cytotoxic agents. Like PF4, IL-8 and NAP-2 at 0.1, 0.6, and 1 μg/mL supported the survival of myeloid progenitors, including colony-forming units granulocyte, erythroblast, monocyte, megakaryocyte (CFU-GEMM), CFU-megakaryocyte (CFU-MK), CFU–granulocyte/macrophage (CFU-GM), and burst-forming units–erythroblast (BFU-E), and reduced their sensitivity to the toxicity of etoposide (ETP). Protamine sulfate at 1 to 100 μg/mL showed no such activity of PF4. Interestingly, the three chemokines failed to affect significantly the viability and chemosensitivity of three leukemic and two other tumor cell lines. Based on these results, we conclude for the first time that PF4 and IL-8 and NAP-2 support the survival of normal hematopoietic precursors and protect them from the toxicity of chemotherapeutic agents. Because such activities are unique to normal hematopoietic cells but not to the cancer cell lines evaluated, a potential clinical application of these molecules in the treatment of cancer is suggested.
PLATELET FACTOR 4 (PF4) belongs to a family of proinflammatory molecules called chemokines. This family has been divided into two subfamilies, CXC and CC chemokines, based on whether the first two cysteine residues in a conserved motif are adjacent to each other or separated by an intervening amino acid.1 CXC chemokines include PF4, β-thromboglobulin and its cleavage product neutrophil-activating protein-2 (NAP-2), interleukin-8 (IL-8), human proto-oncogene Gro/melanocyte growth-stimulating activity, and interferon-inducible protein 10. They share approximately 30% to 40% amino acid homology and have overlapping and some additive biologic activities in modulating inflammation, hemostasis, hematopoiesis, cell proliferation, angiogenesis, and glycosaminoglycan activity.1-3
Previous observations reported first by Gewirtz et al4,5 and subsequently by our group6-8 have shown that PF4 is a potent inhibitor of in vitro hematopoiesis, particularly megakaryocytopoiesis. In vivo in normal mice, administration of PF4 or its C-terminal peptides decreased the number of hematopoietic progenitor cells (colony-forming unit–megakaryocyte [CFU-MK] and CFU–granulocyte/macrophage [CFU-GM]), megakaryocytes, and platelets.9,10 However, in mice treated with PF4 followed by 5-fluorouracil (5-FU), an increased number of hematopoietic stem cells and progenitor cells including high proliferative potential colony-forming cell (HPP-CFC), CFU-MK, and CFU-GM was found compared with mice treated by 5-FU alone, suggesting that PF4 may protect hematopoietic stem cells and progenitor cells from the cytotoxicity of chemotherapeutic compounds.11 Furthermore, studies performed using cord blood (CB) CD34+ cells as target cells show that PF4 directly inhibits the development of megakaryocytes from CD34+ cells. Unlike transforming growth factor β1 (TGFβ1), PF4 exerts an inhibitory effect on the growth of target cells in a reversible manner that results in preservation of a more immature and 5-FU–resistant cell population.12
It is known that hematopoietic cells require certain hematopoietic growth factors (HGFs) for viability and survival and will die in the absence of these factors, and that TGFβ and chemotherapeutic agents inhibit cell viability and survival by inducing cell entry into a process of programed cell death, or apoptosis.13-19 To understand how PF4 can function as either an inhibitor and/or a protector, we have initiated several types of studies including cell viability, survival, and chemosensitivity. Because other CXC chemokines such as IL-8 and NAP-2 also have been shown to be able to inhibit megakaryocytopoiesis, we have compared their effects with that of PF4. Here, we provide for the first time evidence that PF4 supports the viability and survival of hematopoietic cells and reduces their chemosensitivity to the cytotoxicity of several chemotherapy agents. Like PF4, IL-8 and NAP-2 can also exert a chemoprotective effect on normal hematopoietic progenitors. Such an activity of the three chemokines is unique to normal hematopoietic cells and is not observed in the cancer cell lines evaluated.
MATERIALS AND METHODS
Cytokines and drugs.Recombinant human PF4 (Serbio, Gennevilliers, France) was prepared as previously described.15 Recombinant human TGFβ1 was supplied by R & D Systems (Minneapolis, MN). Recombinant human IL-8 (72–amino acid form) and NAP-2 were purchased from Petro Tech Inc (Rocky Hill, NJ). Recombinant human IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and erythropoietin (EPO) were provided by Beite-Kaito (Paris, France). Protamine sulfate was provided by Choay Laboratories (Paris, France). Aplastic anemia sera (AAS) used as the source of megakaryocyte growth-stimulating factors were collected from four patients with aplastic anemia, with informed consent. Etoposide ([ETP] produced by Sandoz, Basel, Switzerland) and 5-FU (la Roche, Neuilly-sur-Seine, France), daunorubicine (DNR), adriamycin, methotrexate, and vincristine were purchased from Shanghai Medical and Pharmaceutical Distribution (Shanghai, China).
Normal human bone marrow cell cultures.Normal human bone marrow samples were collected from nonhematologic patients under sterile conditions during chest surgery (procedure in accordance with local rules, with provision of informed consent from all donors). Heparin-anticoagulated bone marrow mononuclear cells were isolated by centrifugation in a Ficoll-Diatrizoate gradient (density 1.077; Eurobio, Les Ulis, France), and washed twice with RPMI 1640 medium (Eurobio). Mononuclear cells (2 × 106/mL) were then incubated overnight in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). Nonadherent mononuclear cells (NAMCs) were collected after incubation for 24 hours and then further cultured at 37°C and 5% CO2 at 105/100 μL/well in a 96-well plate in the presence or absence of PF4 or other chemokines. After incubation for 48 hours, chemotherapeutic drugs at concentrations of 10 ng/mL to 10 μg/mL were respectively added to the wells and the cells were cultured for an additional 24 hours.
CB cultures.The collection of umbilical CB and the isolation of NAMCs and CD34+ cells were performed as described elsewhere.12 In brief, ACD-anticoagulated CB was diluted 1:2 with alpha medium (Eurobio) and mononuclear cells were isolated by centrifugation in a Ficoll-Diatrizoate gradient. The cells were washed and then suspended in alpha medium supplemented with 10% FCS (Boehringer, Mannheim, Germany) and incubated in a petri dish at 37°C for 2 hours. NAMCs were then collected and washed. For the isolation of CD34+ cells, a direct positive selection method was used.12 Briefly, 5 to 10 × 107 NAMCs suspended in 5 mL 10% FCS-alpha medium were mixed with 0.5 mL Dynabeads CD34 (7 × 107 beads; Dynal, Oslo, Norway) and incubated at room temperature for 45 minutes on a gentle-tilt rotator. The cell-bead rosettes were collected by a Dynal magnetic separator and washed six times with 10% FCS-alpha medium. The cells were separated from the beads by incubation with 150 μL detachabeads (Dynal) at 4°C for 45 mins. After washing, the cells were resuspended in alpha medium containing 10% FCS until use.
To study the capacity to generate megakaryocyte colonies after incubation with PF4 or TGFβ1, 5 × 104 CD34+ CB cells, preincubated as before for 7 and 12 days, were replated into plasma clot culture at 3 × 103/well for an additional 12 days.
For chemosensitivity, CB NAMCs or CD34+ cells preincubated with or without PF4 for various days were incubated with 5-FU, ETP, or DNR for 1 additional day. The cells were washed twice and then replated in plasma clot culture for megakaryocyte colony assay or in a methylcellulose culture system for assay of other progenitors including CFU-GEMM, CFU-GM, and burst-forming units–erythroid (BFU-E).
Megakaryocyte progenitor assays.Megakaryocyte progenitors were assayed in a plasma clot system described previously,15 with slight modifications. Briefly, 3 × 103 CD34+ or 5 × 104 NAMC CB cells were incubated for 12 days in each well of the 24-well plate (in 0.25 mL/well) in alpha medium composed of 10% citrated bovine plasma (GIBCO, Paisley, UK), 10−4 mol/L 2-mercaptoethanol (Sigma, St Louis, MO), 1% deionized bovine serum albumin ([BSA] Fraction V; Sigma), 10% AAS, 5% phytohemagglutinin-stimulated human leukocyte conditioned medium, and 10% CaCl2 (0.34 mg/mL). Megakaryocyte colonies were identified by immunoperoxidase staining as described previously.6
Other progenitor assays in methylcellulose medium.The colony-forming unit–granulocyte, erythroblast, monocyte, megakaryocyte (CFU-GEMM), burst-forming unit–erythroblast (BFU-E), and CFU-GM were assayed by a methylcellulose culture system as described previously.12 Briefly, NAMCs were preincubated and washed, and 5 × 104 cells were seeded in triplicate in a 24-well plate (in 0.25 mL/well) in alpha medium containing 0.9% methylcellulose (Eurobio), 30% FCS, and 1% BSA and incubated for 14 days. The cultures were stimulated with a combination of IL-3 20 ng/mL, GM-CSF 10 ng/mL, and EPO 4 U/mL.
Human cancer cells.Three human leukemic cell lines, HEL, Dami, and HL-60, were used as target cells to test the effects of PF4 and other chemokines on viability and chemosensitivity. MKN-45 cells, a human stomach cancer cell line provided by Dr Motoyama of Niigata University (Japan), and SMMC-7721 cells, a human liver cancer cell line provided by Dr Dong of Changzhen Hospital (Shanghai, China), were also studied. Cells (generally 5 × 105/mL) were cultured for designed times in RPMI 1640 medium containing 10% FCS at 37°C and 5% CO2 in the presence or absence of PF4.
Determination of cell viability and apoptosis.The MTT (3(4,5-dimethylthiazol-2yl)2,5-diphenyl-tetrazolium bromide; Sigma) assay as previously described16 was used as a rapid test of the effect of PF4 on the viability and chemosensitivity of bone marrow cells to cytotoxic drugs. Briefly, after the PF4 and drug incubation, the cells were further incubated in situ with MTT solution (1 mg/mL) for 4 hours. The medium was removed from the wells. Fifty microliters 0.4N HCl-isopropanol was added directly to the wells. Ten minutes later, optical densities at 630 nm were measured using a 96-well multiscanner (Bio-Tek Instruments Inc, Winooski, VT).
The viability and apoptosis of CD34+ CB cells were determined as previously described.17 Briefly, CD34+ cells were preincubated with or without different compounds. Viable and dead cells were determined by counting the number of trypan-blue–excluding and –stained cells, respectively. The percentage of apoptotic cells was measured by counting 500 cells that were prepared by cytospin and then stained by May-Grünwald Giemsa. Apoptotic cells were identified by their typical morphology of fragmented nuclei and condensed chromatin.
Flow cytometry analysis.Flow cytometry analysis was performed with a FACScan cytometer (Becton Dickinson, Mountain View, CA) equipped with a 45-mW argon ion laser at 488 nm for excitation, as previously described.12 For CD34+ cell identification, the cells were examined after incubation with a phycoerythrin-conjugated monoclonal anti-CD34 antibody (HPCA-2; Becton Dickinson). For quantification of remaining CD34+ cells after incubation with PF4 or TGFβ1, CD34+ cells (5 × 103/well) were cultured in a 24-well plate with 250 μL IMDM containing 10% AAS, 10% FCS, 1% BSA, and 10−4 mol/L 2-mercaptoethanol for 12 days in the presence or absence of PF4 (5 μg/mL) or TGFβ1 (1 ng/mL). After washing, cells with remaining CD34 antigen were estimated by fluorescence intensity from cells gated based on the forward and side light-scatter properties of control cells. The amplification rate of the fluorescence detectors and the fluorescence compensations were adjusted with standard beads coupled with phycoerythrin or fluorescein isothiocyanate (Becton Dickinson).
Statistical analysis.The experiments were performed in triplicate, and the results are expressed as the mean ± SEM for data from separate experiments. The significance of differences between groups was determined by Student's t-test.
RESULTS
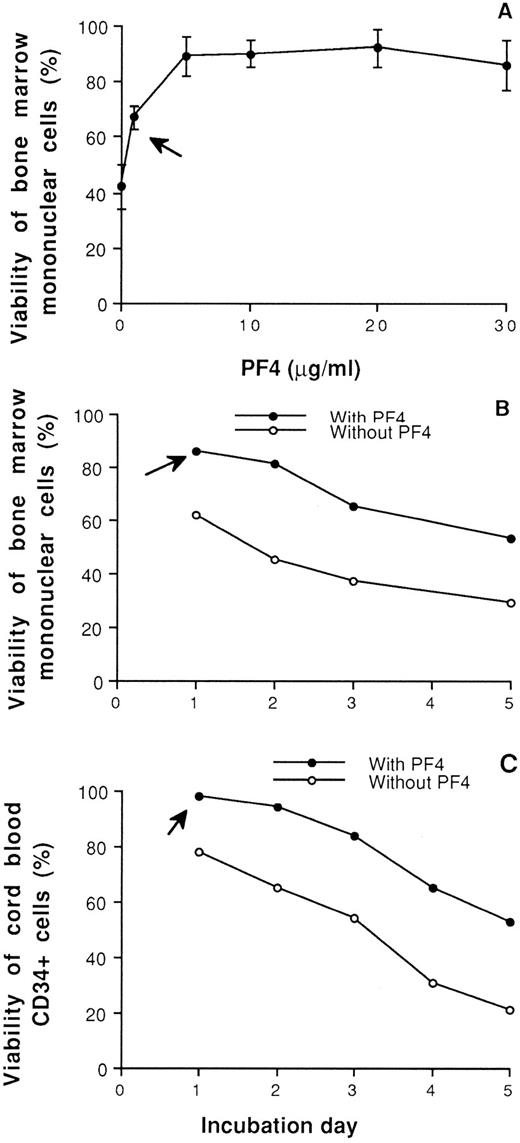
Effect on viability of normal hematopoietic cells.The effect of PF4 on the viability of normal human bone marrow cells and CB CD34+ cells is shown in Fig 1. When bone marrow cells were incubated with PF4 at various concentrations for 48 hours, a significantly increased viability or survival was observed compared with cells incubated with the same volume of phosphate-buffered saline (PBS). Specifically, approximately 45% of bone marrow cells cultured for 48 hours survived in the absence of PF4 and 67% survived in the presence of PF4 at 1 μg/mL, whereas greater than 80% survived in the presence of PF4 at 5 to 30 μg/mL (Fig 1A). When the cells were preincubated with PF4 5 μg/mL, this positive effect of PF4 on the viability of bone marrow cells (Fig 1B) and CB CD34+ cells (Fig 1C) persisted for 5 days. These results indicate a direct supporting effect of PF4 on hematopoietic cells and their progenitors.
Viability of normal human bone marrow cells (A and B) and CB CD34+ cells (C) in the presence or absence of PF4. (A) Effect of PF4 at different concentrations on the viability of normal bone marrow cells treated for 48 hours in culture. (B) Viability of cells incubated with or without PF4 (5 μg/mL) for 1 to 5 days. (C) Effect of PF4 on the viability of CB CD34+ cells with or without preincubation of PF4 (5 μg/mL) for 1 to 5 days. The arrow in (A) indicates a significant increase (P < .01) from this concentration point (1 μg/mL) or greater, and the arrow in (B) and (C) indicates that incubation of cells with PF4 from this day (day 1) up to day 5 resulted in a significant increase (P < .01) in viability.
Viability of normal human bone marrow cells (A and B) and CB CD34+ cells (C) in the presence or absence of PF4. (A) Effect of PF4 at different concentrations on the viability of normal bone marrow cells treated for 48 hours in culture. (B) Viability of cells incubated with or without PF4 (5 μg/mL) for 1 to 5 days. (C) Effect of PF4 on the viability of CB CD34+ cells with or without preincubation of PF4 (5 μg/mL) for 1 to 5 days. The arrow in (A) indicates a significant increase (P < .01) from this concentration point (1 μg/mL) or greater, and the arrow in (B) and (C) indicates that incubation of cells with PF4 from this day (day 1) up to day 5 resulted in a significant increase (P < .01) in viability.
IL-8 and NAP-2, which have been shown to inhibit megakaryocytopoiesis at concentrations of 10 to 100 ng/mL,5 were also tested using the same method. In our experiments, both IL-8 and NAP-2 at 0.04 to 1 μg/mL did not show a statistically significant supporting effect on viability (Fig 2A), although a slight increase, 6% to 8%, in cell viability was observed when cells were incubated with 0.1, 0.6, or 1 μg/mL IL-8. However, IL-8 and NAP-2 support the viability of bone marrow cells treated by 5-FU (Fig 2B). Protamine sulfate at 1, 10, and 100 μg/mL had no effect on the viability of normal bone marrow cells (data not shown).
Effects of IL-8 and NAP-2 on the viability of normal human bone marrow cells (A) and on the sensitivity of the cells to 5-FU (B). Cells (2 × 106/mL) were preincubated with IL-8 or NAP-2 for 48 hours. 5-FU (1 μg/mL) was then added respectively for an additional 24 hours. Cell viability was measured with an MTT assay. *P < .05 v control cells.
Effects of IL-8 and NAP-2 on the viability of normal human bone marrow cells (A) and on the sensitivity of the cells to 5-FU (B). Cells (2 × 106/mL) were preincubated with IL-8 or NAP-2 for 48 hours. 5-FU (1 μg/mL) was then added respectively for an additional 24 hours. Cell viability was measured with an MTT assay. *P < .05 v control cells.
Effect on viability of cancer cell lines.The effects of PF4 on the viability of cancer cells were studied using HEL, Dami, HL-60, 7721, and MKN-45 cells in comparison to normal bone marrow cells. It was found that PF4 at 0.05, 1, and 5 μg/mL had no significant effect on the viability of HEL and Dami cells. Interestingly, PF4 at 5 μg/mL induced a significant decrease in the viability of HL-60 cells. In the presence of PF4, the viability of HL-60 cells decreased by 33%. As for the two nonhematopoietic cell lines, incubation of the cells with PF4 (5 μg/mL) for 1 to 5 days did not cause any significant change in cell viability (data not shown).
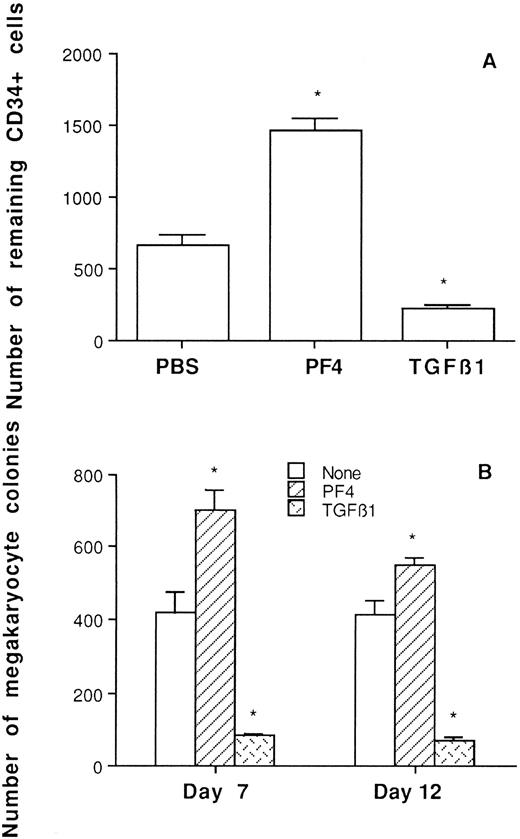
Effect on survival of hematopoietic progenitors.When CD34+ CB cells were incubated with PF4 or TGFβ1 in liquid cultures containing 10% AAS for 7 or 12 days followed by immunofluorescence and flow cytometry analysis, an increased number of CD34+ cells was observed in PF4-treated cells compared with cells incubated with PBS. Unlike PF4, TGFβ1 reduced the number of remaining CD34+ cells (Fig 3A).
Effects of PF4 and TGFβ1 on survival of hematopoietic progenitors. (A) Effects on the number of remaining CD34+ cells after 12 days of incubation from an initial 5 × 103 CD34+ cells. (B) Effects on the survival of CD34+ cells as measured by megakaryocyte colony-regenerating capacity. *P < .05 v cells without PF4 and TGFβ1 treatment.
Effects of PF4 and TGFβ1 on survival of hematopoietic progenitors. (A) Effects on the number of remaining CD34+ cells after 12 days of incubation from an initial 5 × 103 CD34+ cells. (B) Effects on the survival of CD34+ cells as measured by megakaryocyte colony-regenerating capacity. *P < .05 v cells without PF4 and TGFβ1 treatment.
In another line of experiments, cells were preincubated with PF4 or TGFβ1 for 7 and 12 days and then washed and replated in secondary cultures using a plasma clot optimal for megakaryocyte colony formation. The results showed that cells treated with PF4, but not with TGFβ1, were able to regenerate more megakaryocyte colonies than control cells in secondary cultures (Fig 3B).
Effect on chemosensitivity of normal cells.To study the effects of PF4 on cell chemosensitivity, bone marrow cells were cultured in suspension for 48 hours in the presence of PF4 and then incubated with various cytotoxic drugs for 24 hours. PF4 significantly decreased the chemosensitivity of cells in response to cytotoxic drugs. Pretreatment of cells with PF4 resulted in a significant increase in the 50% inhibition concentration (IC50) of several drugs, although the degree varied (Table 1). In a typical experiment, PF4 was found to reduce the sensitivity of cells to treatment with DNR and ETP. The cytotoxic effects of ETP and DNR on bone marrow cells pretreated with PF4 (5 μg/mL) were significantly decreased compared with the effects on cells without PF4 treatment (Fig 4).
Effects of PF4 on the IC50 of Cytotoxic Drugs In Vitro
| Drug . | IC50 ( μg/mL) . | |
|---|---|---|
| . | Without PF4 . | With PF4 . |
| DNR | 0.01 ± 0.002 | 2.20 ± 0.15* |
| ETP | 0.15 ± 0.001 | 2.78 ± 0.11* |
| Adriamycin | 0.11 ± 0.004 | 2.10 ± 0.09* |
| Methotrexate | 0.09 ± 0.01 | 1.90 ± 0.12* |
| 5-FU | 2.50 ± 0.45 | 5.60 ± 0.50* |
| Vincristine | 0.58 ± 0.03 | 1.76 ± 0.50* |
| Drug . | IC50 ( μg/mL) . | |
|---|---|---|
| . | Without PF4 . | With PF4 . |
| DNR | 0.01 ± 0.002 | 2.20 ± 0.15* |
| ETP | 0.15 ± 0.001 | 2.78 ± 0.11* |
| Adriamycin | 0.11 ± 0.004 | 2.10 ± 0.09* |
| Methotrexate | 0.09 ± 0.01 | 1.90 ± 0.12* |
| 5-FU | 2.50 ± 0.45 | 5.60 ± 0.50* |
| Vincristine | 0.58 ± 0.03 | 1.76 ± 0.50* |
Normal human bone marrow cells were incubated with PF4 (5 μg/mL) for 48 hours. Cytotoxic drugs at concentrations of 0.1, 1, 5, 10, 20, 50, 100, 250, and 500 ng/mL and 1, 2.5, 5, and 10 μg/mL were respectively added to the wells and the cells were further cultured for an additional 24 hours. Cell chemosensitivity was measured by an MTT assay. Data are expressed as the mean ± SEM from triplicate determinations of 3 separate experiments.
P < .01 v control cells without PF4, as determined by Student's t-test.
Effect of PF4 on the chemosensitivity of normal bone marrow cells in response to DNR (A) and ETP (B). Cells (2 × 106/mL) were preincubated with or without PF4 (5 μg/mL) in a volume of 100 μL per well in 24-well culture plates for 48 hours. DNR and ETP at various concentrations were then added respectively for an additional 24 hours. Cell viability was measured with an MTT assay. One hundred percent corresponds to the number of viable cells in drug- and PF4-free control samples after incubation for 3 days. There was a statistically significant difference between the drug alone and the drug plus PF4 for every concentration point (P < .05).
Effect of PF4 on the chemosensitivity of normal bone marrow cells in response to DNR (A) and ETP (B). Cells (2 × 106/mL) were preincubated with or without PF4 (5 μg/mL) in a volume of 100 μL per well in 24-well culture plates for 48 hours. DNR and ETP at various concentrations were then added respectively for an additional 24 hours. Cell viability was measured with an MTT assay. One hundred percent corresponds to the number of viable cells in drug- and PF4-free control samples after incubation for 3 days. There was a statistically significant difference between the drug alone and the drug plus PF4 for every concentration point (P < .05).
In another experiment, CD34+ CB cells were first preincubated with or without PF4 (5 μg/mL) for 3 or 5 days and then incubated with 5-FU (5.5 μmol/L) for an additional 2 days. After 5-FU, the cells were washed and replated at 3 × 103/well in a plasma clot culture system for 12 days. The results are expressed as the mean ± SEM of triplicate wells in three separate experiments. A positive effect of PF4 on the survival of megakaryocyte progenitors derived from CD34+ cells was observed, as shown by a significant increase in the number of megakaryocyte colonies from cells incubated with PF4 for 3 days (585 ± 36) or 5 days (765 ± 72) in comparison to cells incubated with PBS for 3 days (308 ± 12) or 5 days (439 ± 43) followed by 5-FU treatment.
Table 2 shows the results of the chemoprotective effects of PF4, IL-8, and NAP-2 on the survival of different stages of CB hematopoietic progenitors. All three chemokines caused an increase in the number of remaining progenitors including CFU-GEMM, CFU-GM, CFU-MK, and BFU-E after 5-FU treatment, although the degree of increase varied among the chemokines and different types of progenitors.
In Vitro Effects of PF4, NAP-2, and IL-8 on the Survival of Different Types of CB Hematopoietic Progenitors Treated With 5-FU
| Treatment of Cells (2.5 × 105) . | Total Hematopoietic Progenitors . | |||
|---|---|---|---|---|
| . | After Treatment . | |||
| . | CFU-GEMM . | BFU-E . | CFU-GM . | CFU-MK . |
| 1.PBS + PBS | 25 ± 3 | 104 ± 10 | 145 ± 7 | 67 ± 4 |
| 2.PBS + 5-FU | 6 ± 1† | 14 ± 3† | 31 ± 5† | 23 ± 3† |
| 3.PF4 1 μg/mL + 5-FU | 12 ± 2* | 42 ± 5* | 97 ± 4* | 55 ± 3* |
| 4.NAP-2 0.1 μg/mL + 5-FU | 5 ± 1 | 25 ± 2* | 59 ± 3* | 36 ± 4* |
| 5.NAP-2 0.6 μg/mL + 5-FU | 12 ± 2* | 54 ± 4* | 104 ± 11* | 42 ± 5* |
| 6.IL-8 0.1 μg/mL + 5-FU | 9 ± 2 | 48 ± 3* | 92 ± 5* | 41 ± 3* |
| 7.IL-8 0.6 μg/mL + 5-FU | 12 ± 1* | 36 ± 4* | 81 ± 3* | 39 ± 3* |
| 8.Protamine 10 μg/mL + 5-FU | 9 ± 2 | 21 ± 3 | 42 ± 5 | 28 ± 4 |
| Treatment of Cells (2.5 × 105) . | Total Hematopoietic Progenitors . | |||
|---|---|---|---|---|
| . | After Treatment . | |||
| . | CFU-GEMM . | BFU-E . | CFU-GM . | CFU-MK . |
| 1.PBS + PBS | 25 ± 3 | 104 ± 10 | 145 ± 7 | 67 ± 4 |
| 2.PBS + 5-FU | 6 ± 1† | 14 ± 3† | 31 ± 5† | 23 ± 3† |
| 3.PF4 1 μg/mL + 5-FU | 12 ± 2* | 42 ± 5* | 97 ± 4* | 55 ± 3* |
| 4.NAP-2 0.1 μg/mL + 5-FU | 5 ± 1 | 25 ± 2* | 59 ± 3* | 36 ± 4* |
| 5.NAP-2 0.6 μg/mL + 5-FU | 12 ± 2* | 54 ± 4* | 104 ± 11* | 42 ± 5* |
| 6.IL-8 0.1 μg/mL + 5-FU | 9 ± 2 | 48 ± 3* | 92 ± 5* | 41 ± 3* |
| 7.IL-8 0.6 μg/mL + 5-FU | 12 ± 1* | 36 ± 4* | 81 ± 3* | 39 ± 3* |
| 8.Protamine 10 μg/mL + 5-FU | 9 ± 2 | 21 ± 3 | 42 ± 5 | 28 ± 4 |
CB NAMCS (106/mL and 2.5 × 105/well) were preincubated with or without different concentrations of PF4, NAP-2, or IL-8 for 48 hours. 5-FU at 2.5 μg/mL was then added for an additional 24-hour incubation. After incubation, cells were counted, washed 3 times, and then cultured in triplicate at 5 × 104 cells/0.25 mL/well in a 24-well plate in a methylcellulose system containing 10% FCS, recombinant human IL-3 (20 ng/mL), GM-CSF (20 ng/mL), and EPO (4 U/mL) for 14 days for CFU-GEMM, BFU-E, and CFU-GM, and in plasma clot for CFU-MK. Data are expressed as the mean ± SEM from 3 separate experiments.
P < .05 v PBS + 5-FU.
P < .05 v PBS + PBS.
Effect on chemosensitivity of cancer cell lines.The effects of PF4 on the viability and chemosensitivity of tumor cell lines were also studied in comparison to normal bone marrow cells. PF4 had no effect on either the viability or chemosensitivity of two nonhematopoietic tumor cell lines, SMMC 7721 and MKN-45 (Table 3). The effects of PF4 on the chemosensitivity of three leukemia cell lines were also studied. PF4 at 0.05, 1, and 5 μg/mL did not show any effect on the viability of HEL, Dami, and HL-60 cells (data not shown).
Chemoprotective Effect on Normal Bone Marrow Cells and Two Tumor Cell Lines, SMMC 7721 and MKN-45
| Drug . | Cell Viability (%) . | ||
|---|---|---|---|
| . | Bone Marrow . | SMMC 7721 . | MKN-45 . |
| None | 100 ± 9.2 | 100 ± 15 | 100 ± 8 |
| PF4 | 143 ± 133-151 | 101 ± 15 | 96 ± 1 |
| DNR | 34 ± 5 | 48 ± 4 | 54 ± 12 |
| DNR + PF4 | 64 ± 83-150 | 47 ± 4 | 49 ± 10 |
| ETP | 27 ± 3 | 40 ± 7 | 51 ± 9 |
| ETP + PF4 | 63 ± 53-150 | 43 ± 4 | 56 ± 5 |
| Drug . | Cell Viability (%) . | ||
|---|---|---|---|
| . | Bone Marrow . | SMMC 7721 . | MKN-45 . |
| None | 100 ± 9.2 | 100 ± 15 | 100 ± 8 |
| PF4 | 143 ± 133-151 | 101 ± 15 | 96 ± 1 |
| DNR | 34 ± 5 | 48 ± 4 | 54 ± 12 |
| DNR + PF4 | 64 ± 83-150 | 47 ± 4 | 49 ± 10 |
| ETP | 27 ± 3 | 40 ± 7 | 51 ± 9 |
| ETP + PF4 | 63 ± 53-150 | 43 ± 4 | 56 ± 5 |
Cells (2 × 106/mL) were preincubated with or without PF4 (5 μg/mL) in a volume of 100 μL per well in 24-well culture plates for 48 hours. DNR 1 μg/mL and ETP 1 μg/mL were then added respectively for an additional 24 hours. Cell viability was measured with an MTT assay. Data are expressed as the mean ± SEM from triplicate determinations of 3 separate experiments. 100% corresponds to the number of viable cells in drug- and PF4-free control samples after incubation for 3 days.
P < .05 v drug.
P < .01 v control group.
Effect on apoptosis of CD34+ CB cells.TGFβ1 is a typical inducer of apoptosis, and 5-FU and ETP also are capable of inducing apoptosis.17 18 Therefore, the response of CD34+ CB cells to TGFβ1, 5-FU, or ETP after incubation of the cells with or without PF4 was evaluated to determine whether PF4 protects cells from apoptosis induced by these compounds. TGFβ1, 5-FU, and ETP did not induce apoptosis of CD34+ cells (Table 4). Pretreatment of cells with PF4 resulted in a significant decrease in the percentage of apoptotic cells as compared with cells treated using TGFβ1, 5-FU, or ETP.
Effects of PF4 on Apoptosis Induced by TGFβ1, 5-FU, or ETP in CD34+ CB Cells
| PF4 ( μg/mL) . | TGFβ1 (1 ng/mL) . | 5-FU (2.5 μg/mL) . | Etoposide (1 μg/mL) . | |||
|---|---|---|---|---|---|---|
| . | Viability (%) . | Apoptotic Cells (%) . | Viability (%) . | Apoptotic Cells (%) . | Viability (%) . | Apoptotic Cells (%) . |
| 0 | 72 ± 6 | 10 ± 3 | 45 ± 3 | 17 ± 2 | 41 ± 3 | 21 ± 3 |
| 5 | 91 ± 34-150 | 2 ± 14-150 | 87 ± 64-150 | 3 ± 24-150 | 79 ± 64-150 | 4 ± 14-150 |
| PF4 ( μg/mL) . | TGFβ1 (1 ng/mL) . | 5-FU (2.5 μg/mL) . | Etoposide (1 μg/mL) . | |||
|---|---|---|---|---|---|---|
| . | Viability (%) . | Apoptotic Cells (%) . | Viability (%) . | Apoptotic Cells (%) . | Viability (%) . | Apoptotic Cells (%) . |
| 0 | 72 ± 6 | 10 ± 3 | 45 ± 3 | 17 ± 2 | 41 ± 3 | 21 ± 3 |
| 5 | 91 ± 34-150 | 2 ± 14-150 | 87 ± 64-150 | 3 ± 24-150 | 79 ± 64-150 | 4 ± 14-150 |
CD34+ CB cells at 104/mL were first incubated in liquid culture in the presence or absence of PF4 5 μg/mL for 2 days. TGFβ1, 5-FU, or ETP were separately added to the culture for an additional incubation for 24 hours. Results are expressed as the mean ± SEM of triplicate determinations.
P < .05 v control cells without PF4 treatment.
DISCUSSION
It has been shown that HGFs and hematopoietic growth inhibitors regulate not only the proliferation but also the survival of hematopoietic progenitors.17-23 Several HGFs, in addition to an ability to stimulate cell proliferation, are also capable of supporting the survival of murine and human hematopoietic progenitor cells in the absence of detectable colony formation.20,21 In contrast, some inhibitors such as TGFβ1, as well as chemotherapeutic agents, inhibit the viability-promoting activity of HGFs.17,22,23 Although PF4 has been demonstrated to be a potent inhibitor of megakaryocytopoiesis with an inhibitory action on cell proliferation different from that of TGFβ1,4-9 12 it remained unclear whether PF4 modulates the viability and survival of hematopoietic cells.
In the present study, we have provided the first evidence that PF4 supports the viability and survival of normal hematopoietic cells. Using an MTT assay, we observed that normal human bone marrow cells incubated with PF4 showed an increased viability compared with control cells. Similar results were also observed using CB CD34+ cells, suggesting a direct action of PF4 on the viability of hematopoietic progenitors. PF4 also promoted the survival of hematopoietic progenitor cells, as determined by an increased number of colonies regenerated in secondary cultures and of remaining CD34+ cells after treatment of CD34+ cells by PF4. To date, no hematopoietic inhibitor has been reported to support cell viability and survival while inhibiting cell proliferation. Our data demonstrate a unique action of PF4 on the viability and survival of hematopoietic progenitors, which has not been described for other hematopoietic inhibitors such as TGFβ1.
Chemotherapy is an important treatment of most cancer; however, chemotherapeutic agent–induced myelosuppression may lead to infections, anemia, and/or hemorrhage, which are limiting factors for most chemotherapy agents.24 In addition, a major impediment to successful chemotherapy is the failure of some tumor types to respond to either form of treatment and the appearance of a resistant cell population upon relapse of an originally responsive malignancy. Therefore, the prevention of myelosuppression and the underlying basis of cellular resistance to anticancer agents have been the focus of many experimental studies. In fact, HGFs such as G-CSF, GM-CSF, and EPO are generally used to accelerate hematopoietic recovery during cancer therapy. However, the restricted effect of these factors on a specific cell lineage and the decrease in the therapeutic effect of these factors after multiple courses of chemotherapy suggest a need for other approaches.19,20 Indeed, it has been demonstrated that HGFs inhibit apoptosis induced by a variety of chemotherapeutic compounds.17 Consequently, HGFs not only may stimulate the recovery of normal hematopoietic cells, but also may enhance the viability of tumor cells in cancer therapy.
Several physiologic hematopoietic inhibitors including tetrapeptide AcSDKP, the pentapeptide pEEDCK, the macrophage inflammatory protein 1α, and TGFβ have been suggested to prevent myelosuppression from chemotherapeutic drugs.25-28 An application of PF4 to such an aim has also been made in our recent studies showing a protective effect of PF4 on hematopoietic progenitors in vitro in CD34+ CB cells and in vivo in mice.11,12 However, from a clinical perspective, it is becoming increasingly evident that an understanding of the mechanism of action of PF4 contributes to our insight in the use of PF4 as a chemoprotector of normal hematopoietic progenitors during cancer chemotherapy. The present study has further provided evidence that PF4 reduces the chemosensitivity of cells to several cytotoxic drugs. Pretreatment of human bone marrow cells with PF4 resulted in a significant increase in the IC50 , although the ratio of increase varied among drugs. These results are consistent with the data obtained from our previous studies,11 12 and indicate that PF4 does protect normal hematopoietic cells from the cytotoxicity of a variety of chemotherapeutic drugs in vitro.
The mechanism whereby PF4 reduces cell chemosensitivity is unclear at present. It has long been thought that anticancer agents selectively target rapidly dividing cells. However, this explanation is not satisfactory, since curable cancers may be relatively slow-growing and many rapidly dividing cancers are resistant to treatment. Emerging evidence suggests that chemotherapeutic drugs may induce apoptosis in tumor cells, but merely induce a cell cycle pause in their normal cell counterparts. Correspondingly, a major mode of resistance to anticancer treatment may be insensitivity to apoptosis induction.29 Because PF4 reversibly inhibits cell proliferation by blocking cells in S phase,30 31 cells treated by PF4 are still viable but at a relatively quiescent state, so they are less sensitive to damage by cytotoxic drugs. Alternatively, PF4 reduces the chemosensitivity of normal hematopoietic cells through an ability to protect these cells from apoptosis induced by cytotoxic drugs.
PF4 is a cationic protein with a strong charge, permitting its high-affinity binding to cell-surface heparin-like molecules. To determine whether this action of PF4 is simply due to a nonspecific charge effect, the effects of protamine sulfate on the viability and chemosensitivity of bone marrow and CB cells were studied as a control. Because protamine at the concentrations tested showed no PF4-like activities, the possibility that a nonspecific charge interaction of PF4 with cells causes a modification of cell viability and survival seems unlikely.
PF4 has considerable amino acid homology with other members of CXC chemokines such as IL-8 and NAP-2. Several recent reports described receptors for NAP-2 and IL-8 on neutrophils and the pathways of these chemokines.32-34 It has been shown that the effect and mechanism of action of PF4 on neutrophils is different from that of IL-8 and other chemokines. Unlike IL-8 and NAP-2, PF4 by itself does not induce functional changes in neutrophils, but is a selective mediator of marker release from specific granules in the presence of the proinflammatory cytokine TNF-α. PF4 and IL-8 use different receptors and activate different mechanisms leading to neutrophil activation.35 However, Gewirtz et al5 have provided evidence that IL-8 and NAP-2, as well as some CC chemokines, are able to exert similar inhibitory effects on megakaryocytopoiesis at lower concentrations than are required for PF4. Inhibition is observed when chemokines are added to progenitor cell cultures at concentrations equivalent to those at which they activate neutrophils and monocytes, suggesting that the inhibitory signals they generate may be of physiologic significance. Furthermore, α and β IL-8 receptors have been found in normal megakaryocytes and platelets.5 Therefore, the effects of the two chemokines on the viability, survival, and chemosensitivity of hematopoietic cells were also evaluated. We have noted that at 0.1 to 1 μg/mL, both IL-8 and NAP-2 did not increase the viability but significantly supported the survival of normal hematopoietic progenitors and reduced the chemosensitivity to the cytotoxicity of 5-FU. These data suggest that the effects of these α chemokines on the survival and chemosensitivity of hematopoietic stem cells may involve a similar mechanism that certainly needs to be further clarified.
It was interesting that PF4, as well as IL-8 and NAP-2, did not significantly increase cell viability and chemosensitivity of three leukemia cell lines, HEL, Dami, and HL-60, and two cancer cell lines, MNK-45 and SMMC 7721. Although further studies using different types of normal and tumor cells should be performed, these results suggest that the effects of PF4 on cell viability and chemosensitivity may be selective or predominant on normal hematopoietic cells.
In conclusion, the present study has provided several lines of evidence that PF4 supports the viability and survival of normal hematopoietic progenitor cells. In addition, PF4, as well as IL-8 and NAP-2, protects normal hematopoietic cells but not cancer cell lines evaluated against the cytotoxicity of chemotherapeutic drugs. These results can have important implications for the use of PF4 and related chemokines to correct therapy-associated myelosuppression and apoptosis of normal hematopoietic progenitors during cancer chemotherapy and radiotherapy. Better understanding of the mechanism of action of PF4 in modulating cell proliferation, viability, and survival and reducing chemosensitivity will require further experiments involving cell cycle progression, apoptosis, and multidrug-resistant genes.
ACKNOWLEDGMENT
We thank Dr J. Amiral for providing recombinant human PF4 and François Schaller for organizing the cord blood collection.
Supported by a grant from the Association pour la Recherche sur le Cancer (France) to Z.C.H.
Address reprint requests to Zhong Chao Han, MD, PhD, Institut des Vaisseaux et du Sang, 8 rue Guy-Patin, 75475 Paris, France.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal