Abstract
To examine the relationship between dietary iron exposure through the consumption of traditional beer and the presence of iron overload in black Africans not related by birth, we studied 28 husband and wife pairs from a rural Zimbabwean community. Lifetime traditional beer consumption was estimated by questioning subjects and iron status was assessed by repeated measurements of serum ferritin and transferrin saturation in subjects who were fasting and had received vitamin C supplementation. Each of the 56 study subjects had an estimated lifetime traditional beer consumption <1,000 L. The mean ± standard deviation (SD) concentration of iron in the supernatants of nine samples of traditional beer from the community was 46 ± 10 mg/L. Four of 28 men (14.3%) and no women had the combination of an elevated serum ferritin and a transferrin saturation <70%, suggestive of substantial iron overload. Significant correlations were not found between the iron status of the husbands and their wives or between dietary iron exposure and iron stores. Our findings suggest that dietary iron exposure may not fully explain the development of iron overload in Africans and are consistent with the hypothesis that an iron-loading gene may also be implicated in pathogenesis.
DIETARY IRON OVERLOAD is a common yet under-recognized problem in rural Zimbabwe1 and many other parts of sub-Saharan Africa.2 Traditional beer, a home-brewed beverage that has a high content of bioavailable iron, is the source of the excess iron.3 To the clinician, iron overload in Africa usually presents as hepatic portal fibrosis and cirrhosis.4,5 The condition is also related to the appearance of scurvy and osteoporosis,6 and it has been implicated in the development of diabetes mellitus,7 hepatocellular carcinoma and tuberculosis.8 The prevalence in sub-Saharan Africa of iron overload is the highest in the world, with more than 10% of certain populations being affected.2
The observation that not all individuals who consume traditional beer develop iron overload1 raised the possibility that a factor other than the ingestion of excess dietary iron may contribute to the etiology of this condition. Recently, a segregation analysis of transferrin saturation in 36 African pedigrees suggested that individuals develop iron overload when they have both exposure to high dietary iron content and a common iron-loading gene that is not linked to the HLA locus.9 If iron overload in Africa is caused by an interaction between an inherited tendency and dietary iron content, then, in a given community, some individuals with a certain level of traditional beer consumption would have iron overload and others with a comparable consumption would not have excess iron stores.
Because the evidence for a genetic pattern to African iron overload so far derives from only a single study, we deemed it worthwhile to examine members of a rural Africa community to determine if unrelated subjects from similar environments and with similar dietary iron exposures are concordant or discordant for iron status. We, therefore, performed an investigation of the iron status of 28 husband and wife pairs from a rural Zimbabwean community, and we examined their customs related to the preparation and consumption of traditional beer. The study was characterized by the use of repeated fasting measures of serum ferritin and transferrin saturation to determine iron status and by the engagement of health professionals with an intimate knowledge of the local customs and languages to characterize cultural practices.
MATERIALS AND METHODS
Study hypotheses.The study was designed to determine if the occurrence of iron overload in a rural Zimbabwe community could be explained by dietary iron exposure alone or whether the pattern was consistent with some additional factor, such as an inherited tendency. The primary hypothesis of the study was that, if iron overload is fully explained by dietary iron exposure, then both members of spouse pairs with similar living environments and traditional beer consumption patterns would have similar or parallel iron status. The subsidiary hypothesis was that, in the community as a whole, individuals with similar habits of traditional beer consumption would have similar iron status and that increasing levels of traditional beer consumption would be associated with increasing body iron stores.
Selection of the community.The study was conducted in the Zaka District of Masvingo Province, about 300 km southeast of Harare in rural Zimbabwe from February to May of 1994. The community was chosen because of an index subject who had a diagnostic liver biopsy demonstrating increased hepatocellular and macrophage iron. This patient was a male, 61 years of age, who had been drinking traditional beer for 32 years. His presenting symptoms were vague right upper quadrant abdominal discomfort and low back pain. On examination he had a nontender, firm hepatomegaly and hyperpigmentation. The needle liver biopsy showed grade 4 hepatocellular iron,10 heavy macrophage iron, and features of early cirrhosis. The serum ferritin concentration was 3,403 μg/L and the transferrin saturation was 93%. The patient was negative for hepatitis B surface antigen, but positive for antibody to hepatitis C.
Brewing of traditional beer and analysis of samples.The production of traditional beer in the Zaka community entails both the preparation of a malt and the brewing of the beer. Before the brewing begins, a malt is prepared from a grain known as mumera or rapoko (Eleucine coracana ). A bucketful of mumera is placed in a burlap sack and soaked in water overnight. The sack is then removed from the water, but the grain is left in the sack for at least an additional 24 hours until the grain begins to germinate. The grain is then spread out in a shed for 2 or 3 additional days until it has completely germinated. Finally, it is placed in the sun to dry for 2 or 3 days and then ground into a fine meal called chimera.
The brewing of traditional beer lasts 7 days during which the beverage under preparation is contained in an iron drum. On day 1, one-fourth of a bucket of malt or chimera is mixed with maize meal in a 1:5 ratio and then water is added to make a thin porridge. The porridge is heated over a slow fire with continuous stirring for about 1 hour, but is not boiled. The mixture is then removed from the fire and allowed to ferment for 2 days. On day 3, the mixture is returned to the fire and left to boil for 6 to 8 hours and then the mixture is cooled overnight. On day 4, another one-fourth of the chimera is added to the mixture and fermentation is allowed to proceed for 2 more days. On day 6, the last one-half of the chimera is mixed with water to make a thick porridge and is heated over the fire with continuous stirring, but is not boiled. It is left to cool and is then mixed with the substance that has been in preparation for 6 days. After about 1 hour, the mixture is poured through a sieve into clay pots and is ready for consumption on the same or the next day.
In the present study, traditional beer samples were collected from nine different villages of the Zaka community and the iron concentrations in the beer supernatants were measured using bathophenanthroline disulphonate as the chromagen. The absorbance was read at 535 nm and standards for the assay consisted of ferric chloride solutions.
Estimation of traditional beer consumption.An estimate was made of the amount of traditional beer consumed over each subject's lifetime based on the information obtained by members of our research team who were fluent in the local languages and knowledgeable of the local culture. The number of liters of traditional beer consumed in a typical beer-drinking day was multiplied by the number of days the individual usually drank per month, and this monthly total was then multiplied by 12 times the number of years in which the individual had drunk traditional beer. This estimate probably only provides a broad approximation of the lifetime traditional beer consumption because consumption may not have been uniform over time and information was obtained by recollection.
Selection of the study subjects.Husband and wife pairs from the Zaka community were invited to participate in the study if they met the criterion of having an estimated total lifetime consumption of greater than 1,000 L of traditional home-brewed beer. Written informed consent was obtained from all study participants. Ethnically, the subjects were of Shona and of Shangaan origin, and none had European ancestry. The individual husband and wife pairs were not closely related to each other, for in traditional African culture it is generally regarded as taboo to marry in one's own extended family or totem (the totem is an animal, plant, or natural object that serves as the emblem of the clan or the extended family). The study subjects were questioned regarding symptoms of the complications of iron overload and regarding conditions leading to secondary iron overload such as multiple blood transfusions or iron-loading anemia. They were also questioned about the intake of other forms of alcohol and about inflammatory conditions that might affect serum ferritin and transferrin saturation values. Women were questioned regarding their menopausal status, the number of pregnancies they had experienced, and the number of children who were carried to term.
Collection and analysis of blood samples.Venous blood samples were obtained in the morning from fasting subjects on 3 separate days. Because serum iron and serum ferritin may be inappropriately lowered in iron-loaded subjects with vitamin C deficiency,11 2.0 grams of vitamin C were given orally to each subject 24 hours before the second and third venesections. Subjects were also asked to refrain from taking any form of alcohol for at least 24 hours before the first venesection and to continue abstaining until the third blood sample was collected. Serum iron and total iron binding capacity were determined by methods modified from those recommended by the International Committee for Standardization in Haematology.12 13 The transferrin saturation was calculated by dividing the serum iron by the total iron binding capacity and multiplying by 100. Serum ferritin was measured using an enzyme immunoassay (Department of Haematology, University of the Witwatersrand, Johannesburg, South Africa). The means of the transferrin saturation and serum ferritin values obtained on the second and third days were used to assign iron status. Full blood counts (Coulter, Hialeah, FL), reticulocyte counts, and erythrocyte sedimentation rates were determined. Liver function tests were measured on a Cobas Bio autoanalyzer using reagents from Roche Diagnostic Systems, South Africa. Hepatitis B and hepatitis C markers were screened using enzyme immunoassay techniques (Abbott Laboratories, North Chicago, IL).
Determination of bone marrow iron concentration.In seven subjects with either an elevated serum ferritin or an elevated transferritin saturation, a bone marrow biopsy was performed and the tissue iron concentration was measured by the method of Torrance and Bothwell.14
Ratio of serum ferritin concentration to aspartate amminotransferase activity.Because in rural Africa the excess dietary iron is derived from an alcoholic beverage, we wished to assess the degree to which elevated serum ferritin may reflect increased body iron stores versus alcohol-induced hepatocellular damage. The ratio of serum ferritin to aspartate amminotransferase (ferritin:AST) has been shown to correlate well with the hepatic iron concentration15 and to be constant in a given patient both in the setting of acute alcohol ingestion and after prolonged abstention from alcohol.16 We calculated the ferritin:AST ratio for each study subject by dividing the serum ferritin determined on day 1 by the aspartate aminotransferase determined on day 1, and we compared these values according to categories of iron status.
Definition of categories of iron status.We followed the example of an assessment of the iron nutritional status of the United States population17 and regarded a raised serum ferritin to be greater than 150 μg/L in women 20 to 44 years of age; greater than 200 μg/L in men 20 to 44 and women 45 to 65 years; greater than 300 μg/L in men 45 to 64 and women over 64 years of age; and greater than 400 μg/L in men over 64 years of age. In keeping with the recommendation of Dr C.A. Finch18 and the example of the United States nutritional survey,17 we considered individuals with the combination of an elevated serum ferritin and a transferrin saturation more than 70% as being especially likely to have established iron overload. Each subject was classified as being a part of one of three groups: Group I in whom the serum ferritin was not elevated, Group II in whom the serum ferritin was elevated, but the transferrin saturation was less than 70%, and Group III in whom the serum ferritin was raised and the transferrin saturation was over 70%.
Statistical analysis.The Student's t test or analysis of variance were used to compare continuous variables that were not skewed and the Mann-Whitney U test or Kruskal-Wallis test were used to compare skewed data. The Fisher's exact test or Pearson's chi square test were used to compare proportions. Relationships between continuous variables were assessed with linear correlation. Repeated measures analysis of variance was used to examine the effect of vitamin C supplementation on serum iron and leukocyte vitamin C measures.
RESULTS
Iron concentration in traditional beer.The mean ± standard deviation (SD) iron concentration in the supernatants of nine samples of traditional beer from the Zaka Community was 46 ± 10 mg/L. For comparison, the iron concentration of commercially prepared beer in the United States is less than 0.2 mg/L.1
Relationship between bone marrow iron concentration and serum ferritin.In seven subjects, the bone marrow iron concentrations ranged from 3.7 to 41.4 μmoles/g dry weight. In the same subjects, the serum ferritins ranged from 265 to 3,709 μg/L, and there was a significant relationship between bone marrow iron concentration and serum ferritin (r = 0.871, P = .011).
Clinical characteristics of study subjects.Twenty-eight spouse pairs were studied, and each of the 56 subjects had consumed more than an estimated lifetime total of 1,000 L of traditional beer. The general clinical characteristics of the study participants are summarized in Table 1 according to sex. In general, men were older and had drunk more traditional beer. Most women were postmenopausal and the median number of pregnancies was nine. Mean values for hemoglobin, reticulocytes, sedimentation rate, and liver functions were within the normal range. None of the subjects had a history of multiple blood transfusions or of a chronic anemia. Iron-related measurements and leukocyte vitamin C levels, determined repeatedly over 3 days, are summarized in Table 2. On day 1, mean serum levels for iron, total iron binding capacity, transferrin saturation and ferritin and mean leukocyte vitamin C concentrations were in the normal range. The administration of vitamin C, in a dose of 2.0 grams orally 24 hours before each of the second and third day blood samples, led to significant rises in leukocyte vitamin C concentrations, but had no significant effects on serum iron, total iron binding capacity, transferrin saturation or serum ferritin.
Clinical Characteristics According to Sex
| . | Men (n = 28) . | Women (n = 28) . | P . | (Normal Range) . |
|---|---|---|---|---|
| Age (yr; mean ± SD) | 66 ± 11 | 56 ± 13 | .007 | |
| Pre-:postmenopausal | 9:19 | |||
| Number of pregnancies (median and range)* | 9 (0-13) | |||
| Pregnancies carried to term (median and range)† | 8 (0-12) | |||
| Estimated lifetime traditional beer consumption (L; median and range) | 18,720 | 13,869 | .049 | |
| (4,140-113,400) | (1,920-72,000) | |||
| Years of beer consumption (mean ± SD) | 40 ± 11 | 31 ± 15 | .011 | |
| Abstention from beer at the time of study (days; median and range) | 2 (1->30) | 4 (1->30) | .6 | |
| Lactate dehydrogenase (U/L; mean ± SD)† | 157 ± 50 | 162 ± 45 | .7 | (105-190) |
| Hemoglobin (g/dL; mean ± SD)‡ | 14.2 ± 1.3 | 13.6 ± 0.9 | .036 | (12.0-18.0) |
| Reticulocytes (no/μL; mean ± SD)‡ | 23,800 ± 14,600 | 24,200 ± 16,100 | .9 | (15,000-75,000) |
| Erythrocyte sedimentation rate (mm/h; median and range)‡ | 10 (1-70) | 16 (0-115) | .6 | (0-20) |
| Protein (g/L; mean ± SD) | 76 ± 8 | 78 ± 8 | .5 | (55-85) |
| Albumin (g/L; mean ± SD) | 42 ± 6 | 41 ± 5 | .7 | (35-55) |
| Bilirubin (mmol/L; mean ± SD) | 7.9 ± 4.6 | 7.9 ± 5.3 | 1.0 | (1.7-17.1) |
| Alanine aminotransferase (U/L; median and range) | 25 (13-124) | 19 (11-81) | .1 | (6-37) |
| Aspartate aminotransferase (U/L; median and range) | 28 (13-125) | 22 (15-92) | .007 | (10-30) |
| Gamma glutamyl transferase (U/L; median and range) | 31 (5-171) | 15 (3-89) | .002 | (5-35) |
| . | Men (n = 28) . | Women (n = 28) . | P . | (Normal Range) . |
|---|---|---|---|---|
| Age (yr; mean ± SD) | 66 ± 11 | 56 ± 13 | .007 | |
| Pre-:postmenopausal | 9:19 | |||
| Number of pregnancies (median and range)* | 9 (0-13) | |||
| Pregnancies carried to term (median and range)† | 8 (0-12) | |||
| Estimated lifetime traditional beer consumption (L; median and range) | 18,720 | 13,869 | .049 | |
| (4,140-113,400) | (1,920-72,000) | |||
| Years of beer consumption (mean ± SD) | 40 ± 11 | 31 ± 15 | .011 | |
| Abstention from beer at the time of study (days; median and range) | 2 (1->30) | 4 (1->30) | .6 | |
| Lactate dehydrogenase (U/L; mean ± SD)† | 157 ± 50 | 162 ± 45 | .7 | (105-190) |
| Hemoglobin (g/dL; mean ± SD)‡ | 14.2 ± 1.3 | 13.6 ± 0.9 | .036 | (12.0-18.0) |
| Reticulocytes (no/μL; mean ± SD)‡ | 23,800 ± 14,600 | 24,200 ± 16,100 | .9 | (15,000-75,000) |
| Erythrocyte sedimentation rate (mm/h; median and range)‡ | 10 (1-70) | 16 (0-115) | .6 | (0-20) |
| Protein (g/L; mean ± SD) | 76 ± 8 | 78 ± 8 | .5 | (55-85) |
| Albumin (g/L; mean ± SD) | 42 ± 6 | 41 ± 5 | .7 | (35-55) |
| Bilirubin (mmol/L; mean ± SD) | 7.9 ± 4.6 | 7.9 ± 5.3 | 1.0 | (1.7-17.1) |
| Alanine aminotransferase (U/L; median and range) | 25 (13-124) | 19 (11-81) | .1 | (6-37) |
| Aspartate aminotransferase (U/L; median and range) | 28 (13-125) | 22 (15-92) | .007 | (10-30) |
| Gamma glutamyl transferase (U/L; median and range) | 31 (5-171) | 15 (3-89) | .002 | (5-35) |
P gives significance level for comparison between men and women.
N = 25 women.
N = 26 men, N = 26 women.
N = 25 men, N = 27 women.
Serum Iron Measures and Leukocyte Vitamin C Levels Before (Day 1) and After (Days 2 and 3) Vitamin C Supplementation
| . | Day 1 . | Day 2 . | Day 3 . | P . |
|---|---|---|---|---|
| Serum iron (μg/dL) | ||||
| Men* | 124 ± 46 | 125 ± 51 | 123 ± 65 | .9 |
| Women* | 101 ± 36 | 104 ± 37 | 104 ± 36 | .96 |
| Total iron binding capacity (μg/dL) | ||||
| Men* | 261 ± 39 | 269 ± 56 | 262 ± 58 | .5 |
| Women* | 288 ± 57 | 289 ± 51 | 281 ± 49 | .2 |
| Transferrin saturation (%) | ||||
| Men* | 48 ± 18 | 47 ± 18 | 47 ± 21 | .99 |
| Women* | 35 ± 10 | 36 ± 11 | 37 ± 12 | .4 |
| Serum ferritin (μg/L)† | ||||
| Men* | 387 (117-1279) | 358 (113-1135) | 346 (111-1076) | .2 |
| Women* | 93 (32-266) | 90 (30-271) | 87 (24-323) | .3 |
| Vitamin C (ng/108 leukocytes) | ||||
| Men‡ | 43 ± 13 | 52 ± 13 | 53 ± 17 | .036 |
| Womenρ | 51 ± 17 | 62 ± 17 | 58 ± 20 | .001 |
| . | Day 1 . | Day 2 . | Day 3 . | P . |
|---|---|---|---|---|
| Serum iron (μg/dL) | ||||
| Men* | 124 ± 46 | 125 ± 51 | 123 ± 65 | .9 |
| Women* | 101 ± 36 | 104 ± 37 | 104 ± 36 | .96 |
| Total iron binding capacity (μg/dL) | ||||
| Men* | 261 ± 39 | 269 ± 56 | 262 ± 58 | .5 |
| Women* | 288 ± 57 | 289 ± 51 | 281 ± 49 | .2 |
| Transferrin saturation (%) | ||||
| Men* | 48 ± 18 | 47 ± 18 | 47 ± 21 | .99 |
| Women* | 35 ± 10 | 36 ± 11 | 37 ± 12 | .4 |
| Serum ferritin (μg/L)† | ||||
| Men* | 387 (117-1279) | 358 (113-1135) | 346 (111-1076) | .2 |
| Women* | 93 (32-266) | 90 (30-271) | 87 (24-323) | .3 |
| Vitamin C (ng/108 leukocytes) | ||||
| Men‡ | 43 ± 13 | 52 ± 13 | 53 ± 17 | .036 |
| Womenρ | 51 ± 17 | 62 ± 17 | 58 ± 20 | .001 |
P represents significance level for repeated measures analysis of variance of mean values over 3 days. Statistical comparison between men and women: serum ferritins differ at significance level of <.0005 and transferrin saturations at level of <.05. Normal ranges: serum iron 60 to 150; total iron binding capacity 250 to 400; transferrin saturation 20 to 50; serum ferritin (see Materials and Methods section); vitamin C ≥20 ng/108 leukocytes.
N = 28 day 1 and 27 days 2 and 3.
Geometric mean and SD range.
N = 22 on day 1 and 20 on days 2 and 3.
ρ N = 21 on day 1 and 20 on days 2 and 3.
Prevalence of iron categories.As shown in Table 3, 14 of 28 males (50%) and 21 of 28 females (75%) did not have raised serum ferritins (group I). Ten men (35.7%) and seven women (25%) had elevated serum ferritins and transferrin saturations less than 70% (group II). Four of 28 males (14.3%) and none of 28 females had the combination of an elevated serum ferritin and a transferrin saturation >70% (group III). The proportions in males were different from the proportions in females at a significance level of 0.052. Postmenopausal women predominated and the median number of pregnancies was more than six in both groups I and II.
Proportions Belonging to Three Categories of Iron Status According to Sex, Menopause Status, and Pregnancy History in Women
| . | Men . | Women (n = 28) . | |||
|---|---|---|---|---|---|
| . | (n = 28) . | No. (%) . | Premenopausal: . | Total Pregnancies (median and range) . | Pregnancies Carried to Term (median and range) . |
| . | No. (%) . | . | Postmenopausal . | . | . |
| Group I: serum ferritin not raised | 14 (50.0) | 21 (75.0) | 8:13 | 7 (0-13)3-150 | 6 (0-12)3-150 |
| Group II: serum ferritin elevated and transferrin saturation <70% | 10 (35.7) | 7 (25.0) | 1:6 | 11 (10-12)3-151 | 9 (7-12)3-151 |
| Group III: serum ferritin elevated and transferrin saturation >70% | 4 (14.3) | 0 (0) | |||
| . | Men . | Women (n = 28) . | |||
|---|---|---|---|---|---|
| . | (n = 28) . | No. (%) . | Premenopausal: . | Total Pregnancies (median and range) . | Pregnancies Carried to Term (median and range) . |
| . | No. (%) . | . | Postmenopausal . | . | . |
| Group I: serum ferritin not raised | 14 (50.0) | 21 (75.0) | 8:13 | 7 (0-13)3-150 | 6 (0-12)3-150 |
| Group II: serum ferritin elevated and transferrin saturation <70% | 10 (35.7) | 7 (25.0) | 1:6 | 11 (10-12)3-151 | 9 (7-12)3-151 |
| Group III: serum ferritin elevated and transferrin saturation >70% | 4 (14.3) | 0 (0) | |||
N = 19.
N = 6.
Concordance of spouse pairs for iron categories.One-half of the spouse pairs were concordant for iron category and one-half were not (Table 4). Of the four men who formed group III (combination of elevated serum ferritin and transferrin saturation >70%), three had spouses in group I (serum ferritin not raised) and one had a spouse in group II (serum ferritin raised and transferrin saturation <70%). The clinical characteristics of the members of Group III and their spouses are shown in Table 5. All four subjects with the combination of an elevated serum ferritin and a transferrin saturation >70% had total estimated lifetime traditional beer consumptions of over 15,000 L. One of these four subjects was positive for antibody to hepatitis C and none was positive for hepatitis B surface antigen.
Concordance of Iron Categories Within Spouse Pairs
| . | No. of Spouse Pairs . | % of Spouse Pairs . |
|---|---|---|
| Concordant for iron status | 14 | 50.0 |
| Both members group I (ferritins not raised) | 11 | 39.3 |
| Both members group II (raised ferritins and transferrin saturations <70%) | 3 | 10.7 |
| Both members group III (combination of raised ferritins and transferrin saturation >70%) | 0 | 0 |
| Discordant for iron status | 14 | 50.0 |
| One member group I and one member group II | 10 | 35.7 |
| One member group I and one member group III | 3 | 10.7 |
| One member group II and one member group III | 1 | 3.6 |
| . | No. of Spouse Pairs . | % of Spouse Pairs . |
|---|---|---|
| Concordant for iron status | 14 | 50.0 |
| Both members group I (ferritins not raised) | 11 | 39.3 |
| Both members group II (raised ferritins and transferrin saturations <70%) | 3 | 10.7 |
| Both members group III (combination of raised ferritins and transferrin saturation >70%) | 0 | 0 |
| Discordant for iron status | 14 | 50.0 |
| One member group I and one member group II | 10 | 35.7 |
| One member group I and one member group III | 3 | 10.7 |
| One member group II and one member group III | 1 | 3.6 |
Clinical Characteristics of the Husbands With the Combination of a Raised Serum Ferritin and a Transferrin Saturation <70% and of Their Wives
| . | Iron Status Group . | Age (yr) . | Total No. of Pregnancies . | Total Beer Consumption . | Ferritin (μg/L) . | Transferrin Saturation (%) . | Erythrocyte Sedimentation Rate (mm/h) . | Lactate Dehydrogenase (IU/L) . | Hemoglobin (g/dL) . | Reticulocytes (no/10−3/μL) . | Bilirubin (μmol/L) . | Alanine Aminotransferase (IU/L) . | Aspartate Aminotransferase (IU/L) . | Gamma Glutamyltransferase (IU/L) . | Hepatitis B Surface Antigen . | Hepatitis C Antibody . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Husband | III | 46 | 113,400 | 1,058 | 79 | 20 | 176 | 15.0 | 12.0 | 13.0 | 21 | 24 | 58 | − | − | |
| Wife | I | 42 | 7 | 24,000 | 57 | 40 | 50 | 127 | 13.4 | 28.8 | 15.8 | 15 | 17 | 34 | − | − |
| Husband | III | 62 | 15,600 | 1,279 | 77 | 5-150 | 5-150 | 5-150 | 5-150 | 13.0 | 34 | 36 | 40 | − | − | |
| Wife | I | 43 | 5-150 | 1,920 | 22 | 30 | 5-150 | 5-150 | 5-150 | 5-150 | 11.0 | 22 | 28 | 12 | − | − |
| Husband | III | 65 | 17,380 | 1,379 | 81 | 55 | 172 | 12.9 | 18.0 | 13.0 | 18 | 27 | 27 | − | − | |
| Wife | II | 60 | 10 | 72,000 | 814 | 61 | 3 | 266 | 14.6 | 51.0 | 17.0 | 33 | 33 | 30 | − | − |
| Husband | III | 69 | 52.800 | 3,709 | 78 | 5 | 111 | 14.9 | 11.6 | 7.1 | 124 | 125 | 171 | − | + | |
| Wife | I | 61 | 6 | 14,760 | 187 | 30 | 0 | 171 | 14.1 | 33.6 | 6.5 | 29 | 35 | 10 | − | − |
| . | Iron Status Group . | Age (yr) . | Total No. of Pregnancies . | Total Beer Consumption . | Ferritin (μg/L) . | Transferrin Saturation (%) . | Erythrocyte Sedimentation Rate (mm/h) . | Lactate Dehydrogenase (IU/L) . | Hemoglobin (g/dL) . | Reticulocytes (no/10−3/μL) . | Bilirubin (μmol/L) . | Alanine Aminotransferase (IU/L) . | Aspartate Aminotransferase (IU/L) . | Gamma Glutamyltransferase (IU/L) . | Hepatitis B Surface Antigen . | Hepatitis C Antibody . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Husband | III | 46 | 113,400 | 1,058 | 79 | 20 | 176 | 15.0 | 12.0 | 13.0 | 21 | 24 | 58 | − | − | |
| Wife | I | 42 | 7 | 24,000 | 57 | 40 | 50 | 127 | 13.4 | 28.8 | 15.8 | 15 | 17 | 34 | − | − |
| Husband | III | 62 | 15,600 | 1,279 | 77 | 5-150 | 5-150 | 5-150 | 5-150 | 13.0 | 34 | 36 | 40 | − | − | |
| Wife | I | 43 | 5-150 | 1,920 | 22 | 30 | 5-150 | 5-150 | 5-150 | 5-150 | 11.0 | 22 | 28 | 12 | − | − |
| Husband | III | 65 | 17,380 | 1,379 | 81 | 55 | 172 | 12.9 | 18.0 | 13.0 | 18 | 27 | 27 | − | − | |
| Wife | II | 60 | 10 | 72,000 | 814 | 61 | 3 | 266 | 14.6 | 51.0 | 17.0 | 33 | 33 | 30 | − | − |
| Husband | III | 69 | 52.800 | 3,709 | 78 | 5 | 111 | 14.9 | 11.6 | 7.1 | 124 | 125 | 171 | − | + | |
| Wife | I | 61 | 6 | 14,760 | 187 | 30 | 0 | 171 | 14.1 | 33.6 | 6.5 | 29 | 35 | 10 | − | − |
Missing values.
Analysis of iron status of wives according to iron categories of husbands.Although none of the wives fell into group III, suggestive of substantial iron overload, we wished to determine if the iron status of the wives would parallel that of their husbands. We, therefore, compared mean values for ferritin, ferritin:AST ratio and transferrin saturation in the wives according to the iron status of their husbands. The results, shown in Table 6, did not show significant differences in these iron parameters according to iron categories of the husbands.
Iron Status of Wives According to Iron Categories of Husbands
| Iron Category of Husbands . | N . | Indirect Measures of Iron Status in Wives and Husbands . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Ferritin (μg/L)6-150 . | Ferritin: AST Ratio6-150 . | Transferrin Saturation(%)6-151 . | . | . | . | |||
| . | . | Husbands . | Wives . | Husbands . | Wives . | Husbands . | Wives . | . | . | . |
| Group I (normal ferritin) | 14 | 159 (62-406) | 69 (19-255) | 6.7 (3.2-13.6) | 3.6 (1.1-11.6) | 42 ± 19 | 35 ± 10 | |||
| Group II (ferritin raised and transferrin saturation <70%) | 10 | 667 (479-929) | 104 (45-238) | 22.1 (14.8-32.9) | 3.7 (1.8-7.5) | 44 ± 11 | 37 ± 12 | |||
| Group III (raised ferritin and transferrin saturation >70%) | 4 | 1,622 (925-2,844) | 117 (25-558) | 41.8 (33.7-51.9) | 5.1 (1.3-20.5) | 79 ± 12 | 40 ± 14 | |||
| P | <.0005 | .6 | <.0005 | .8 | .001 | .8 | ||||
| Iron Category of Husbands . | N . | Indirect Measures of Iron Status in Wives and Husbands . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Ferritin (μg/L)6-150 . | Ferritin: AST Ratio6-150 . | Transferrin Saturation(%)6-151 . | . | . | . | |||
| . | . | Husbands . | Wives . | Husbands . | Wives . | Husbands . | Wives . | . | . | . |
| Group I (normal ferritin) | 14 | 159 (62-406) | 69 (19-255) | 6.7 (3.2-13.6) | 3.6 (1.1-11.6) | 42 ± 19 | 35 ± 10 | |||
| Group II (ferritin raised and transferrin saturation <70%) | 10 | 667 (479-929) | 104 (45-238) | 22.1 (14.8-32.9) | 3.7 (1.8-7.5) | 44 ± 11 | 37 ± 12 | |||
| Group III (raised ferritin and transferrin saturation >70%) | 4 | 1,622 (925-2,844) | 117 (25-558) | 41.8 (33.7-51.9) | 5.1 (1.3-20.5) | 79 ± 12 | 40 ± 14 | |||
| P | <.0005 | .6 | <.0005 | .8 | .001 | .8 | ||||
P represents significance level for comparison of mean values according to the iron category of the husbands.
Geometric mean and SD range.
Mean ± SD.
Comparison of the subjects according to iron category based on serum ferritins and transferrin saturations.As shown in Table 7, only the sex ratio, hepatic enzyme activities, and ferritin/AST ratio differed significantly according to the three categories of iron status. Because the serum ferritins and transferrin saturations were employed to define the categories, these variables were not analyzed statistically. Neither the estimated total lifetime consumption of traditional beer nor the total number of years the beverage had been consumed differed significantly according to the three categories of iron status.
Clinical Characteristics According to Iron Category
| . | Group I: Ferritin Not Elevated . | Group II: Ferritin Elevated and Transferrin Saturation <70% (n = 17) . | Group III: Ferritin Elevated and Transferrin Saturation >70% . | P . |
|---|---|---|---|---|
| . | (n = 35) . | . | (n = 4) . | . |
| Age (yr; mean ± SD) | 61 ± 14 | 60 ± 11 | 61 ± 10 | .96 |
| Sex (M:F) | 14:21 | 10:7 | 4:0 | .052 |
| Beer consumption (liters; median and range) | 19,200 | 10,944 | 35,040 | .3 |
| (1,920-94,896) | (2,880-90,000) | (15,600-113,400) | ||
| Years of beer consumption (mean ± SD) | 37 ± 15 | 33 ± 12 | 33 ± 8 | .7 |
| Abstention from beer (days; median and range) | 4 (1->30) | 2 (1->30) | 2 (1-3) | .4 |
| Serum ferritin (μg/L; geometric mean and SD range) | 86 (28-260) | 478 (272-838) | 1,621 (925-2,844) | — |
| Transferrin saturation (%; mean ± SD) | 37 ± 14 | 45 ± 10 | 79 ± 1 | — |
| Ferritin:AST ratio (median and range) | 4.5 (0.2-18.3) | 17.0 (2.9-39.5) | 42.5 (33.8-51.2) | <.0005 |
| Hemoglobin (g/dL; mean ± SD) | 13.7 ± 1.27-150 | 14.3 ± 0.9 | 14.3 ± 1.27-151 | .13 |
| Reticulocytes (mean ± SD) | 22,000 ± 13,2007-150 | 29,600 ± 18,500 | 23,900 ± 3,6007-151 | .13 |
| Erythrocyte sedimentation rate (mm/h; median and range) | 12 (0-90)7-150 | 10 (2-115) | 20 (5-55)7-151 | .7 |
| Lactate dehydrogenase (U/L; mean ± SD) | 155 ± 467-152 | 170 ± 53ρ | 153 ± 367-151 | .6 |
| Protein (g/L; mean ± SD) | 76 ± 9 | 77 ± 7 | 84 ± 5 | .3 |
| Albumin (g/L; mean ± SD) | 41 ± 6 | 42 ± 4 | 44 ± 5 | .4 |
| Bilirubin (mmol/L; mean ± SD) | 7.0 ± 4.6 | 8.9 ± 5.5 | 11.5 ± 3.0 | .14 |
| Alkaline phosphatase (U/L; median and range) | 93 (34-153) | 84 (58-185) | 77 (70-99) | .4 |
| Alanine aminotransferase (U/L; median and range) | 18 (13-51) | 26 (11-98) | 28 (18-124) | .02 |
| Aspartate aminotransferase (U/L; median and range) | 23 (13-58) | 28 (18-94) | 32 (24-125) | .027 |
| Gamma glutamyltransferase (U/L; median and range) | 15 (3-84) | 25 (10-123) | 49 (27-171) | .005 |
| Vitamin C (ng/108 white blood cells; median and range) | 44 (35-93)7-155 | 41 (19-70)7-154 | 36 (25-48)7-167 | .3 |
| . | Group I: Ferritin Not Elevated . | Group II: Ferritin Elevated and Transferrin Saturation <70% (n = 17) . | Group III: Ferritin Elevated and Transferrin Saturation >70% . | P . |
|---|---|---|---|---|
| . | (n = 35) . | . | (n = 4) . | . |
| Age (yr; mean ± SD) | 61 ± 14 | 60 ± 11 | 61 ± 10 | .96 |
| Sex (M:F) | 14:21 | 10:7 | 4:0 | .052 |
| Beer consumption (liters; median and range) | 19,200 | 10,944 | 35,040 | .3 |
| (1,920-94,896) | (2,880-90,000) | (15,600-113,400) | ||
| Years of beer consumption (mean ± SD) | 37 ± 15 | 33 ± 12 | 33 ± 8 | .7 |
| Abstention from beer (days; median and range) | 4 (1->30) | 2 (1->30) | 2 (1-3) | .4 |
| Serum ferritin (μg/L; geometric mean and SD range) | 86 (28-260) | 478 (272-838) | 1,621 (925-2,844) | — |
| Transferrin saturation (%; mean ± SD) | 37 ± 14 | 45 ± 10 | 79 ± 1 | — |
| Ferritin:AST ratio (median and range) | 4.5 (0.2-18.3) | 17.0 (2.9-39.5) | 42.5 (33.8-51.2) | <.0005 |
| Hemoglobin (g/dL; mean ± SD) | 13.7 ± 1.27-150 | 14.3 ± 0.9 | 14.3 ± 1.27-151 | .13 |
| Reticulocytes (mean ± SD) | 22,000 ± 13,2007-150 | 29,600 ± 18,500 | 23,900 ± 3,6007-151 | .13 |
| Erythrocyte sedimentation rate (mm/h; median and range) | 12 (0-90)7-150 | 10 (2-115) | 20 (5-55)7-151 | .7 |
| Lactate dehydrogenase (U/L; mean ± SD) | 155 ± 467-152 | 170 ± 53ρ | 153 ± 367-151 | .6 |
| Protein (g/L; mean ± SD) | 76 ± 9 | 77 ± 7 | 84 ± 5 | .3 |
| Albumin (g/L; mean ± SD) | 41 ± 6 | 42 ± 4 | 44 ± 5 | .4 |
| Bilirubin (mmol/L; mean ± SD) | 7.0 ± 4.6 | 8.9 ± 5.5 | 11.5 ± 3.0 | .14 |
| Alkaline phosphatase (U/L; median and range) | 93 (34-153) | 84 (58-185) | 77 (70-99) | .4 |
| Alanine aminotransferase (U/L; median and range) | 18 (13-51) | 26 (11-98) | 28 (18-124) | .02 |
| Aspartate aminotransferase (U/L; median and range) | 23 (13-58) | 28 (18-94) | 32 (24-125) | .027 |
| Gamma glutamyltransferase (U/L; median and range) | 15 (3-84) | 25 (10-123) | 49 (27-171) | .005 |
| Vitamin C (ng/108 white blood cells; median and range) | 44 (35-93)7-155 | 41 (19-70)7-154 | 36 (25-48)7-167 | .3 |
P represents significance level for comparison of values among the three groups.
n = 32.
n = 3.
n = 34.
ρ n = 15.
n = 27.
n = 14.
n = 2.
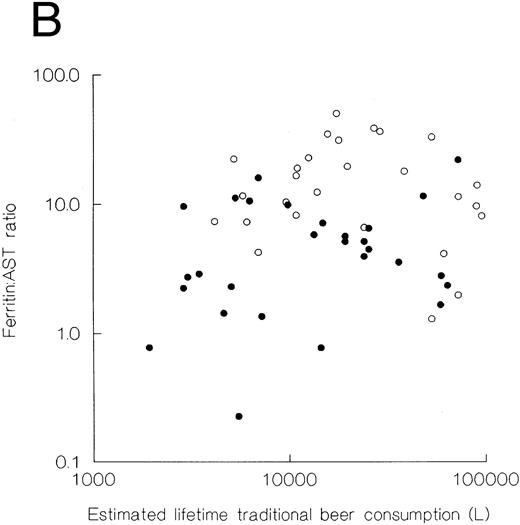
Relationships of serum ferritin and transferrin saturation with estimates of traditional beer consumption.Serum ferritin concentrations, ferritin:AST ratios and transferrin saturations according to estimates of traditional beer consumption are shown in Fig 1. The correlation coefficients of .206, .238, and .169 respectively, were not statistically significant (P > .05).
Plots of serum ferritin (A), ferritin:AST ratio (B), and transferrin saturation (C) as a function of estimated lifetime traditional beer consumption. When analyzed by regression, the correlation coefficients were all less than 0.26 and the significance levels <.05 whether the subjects were considered overall or divided according to sex. (•) Women; (○) men.
Plots of serum ferritin (A), ferritin:AST ratio (B), and transferrin saturation (C) as a function of estimated lifetime traditional beer consumption. When analyzed by regression, the correlation coefficients were all less than 0.26 and the significance levels <.05 whether the subjects were considered overall or divided according to sex. (•) Women; (○) men.
DISCUSSION
The principal limitations to this study are that dietary iron exposure in the form of traditional beer consumption was estimated by questioning study subjects and that the iron status was determined primarily by indirect measures.
Dietary iron exposure.With the retrospective recall method of quantifying traditional beer consumption, there is an inherent degree of uncertainty to the estimates obtained. To ensure the highest quality results possible, individuals who knew the local language and culture took the histories of traditional beer consumption and did so in a relaxed, friendly, and unhurried manner. Furthermore, the reproducibility of these histories was verified in a subset of subjects. We are confident that these histories provide valid broad approximations of traditional beer consumption.
Indirect measures of iron status.While the transferrin saturation is the best single indirect test for an iron-loading defect in HLA-linked hereditary hemochromatosis in whites,19 the serum ferritin is also useful.20,21 In African iron overload, it is not known whether the serum ferritin or the transferrin saturation is a better indirect measure of iron status. In our previous work, we employed both the serum ferritin and transferrin saturation to estimate the prevalence of iron overload in a rural Zimbabwean community1 and we employed the transferrin saturation as the measure of iron status in our study, which suggested that dietary iron content and an iron-loading locus may both contribute to the pathogenesis of African iron overload.9 In the present study, we again used both the serum ferritin and the transferrin saturation for classifying iron status and to ensure the validity of the results we performed repeated fasting measurements (Table 2). Our use of the serum ferritin is supported by the finding of a strong correlation between bone marrow nonheme iron concentration and serum ferritin in the present data set and by the fact that other investigators have reported a good correlation between bone marrow iron concentration and hepatic iron concentration in African iron overload.22 Because ascorbic acid deficiency seems to block the release of iron from reticuloendothelial cells and in iron overload may be associated with a low serum iron and transferrin saturation,11 we administered vitamin C before drawing two of the three blood samples. It is of note, in this regard, that most of our subjects and even those with elevated serum ferritins had normal leukocyte ascorbic acid levels. Furthermore, administration of vitamin C significantly raised leukocyte ascorbic acid levels, but did not significantly influence serum iron, total iron binding capacity, transferrin saturation, or serum ferritin (Table 2). It may be worthwhile to underline the difference between African iron overload and European HLA-linked hemochromatosis in regard to bone marrow iron. Bone marrow iron concentration correlates well with hepatic iron concentration and total body iron burden in African iron overload, but not HLA-linked hemochromatosis.22 In contrast to African iron overload, HLA-linked hemochromatosis is marked by a relatively reduced content of iron in the macrophages of the bone marrow, liver, and spleen.22 23
All 56 subjects in the present study had consumed more than an estimated 1,000 L of traditional beer in their lifetime. Twenty-one subjects, 14 men and seven women had elevated serum ferritins (Table 3) and four subjects, all men, had the combination of an elevated serum ferritin and a transferrin saturation of >70% (Tables 3-5), which is suggestive of substantial iron overload.18 In interpreting these elevated serum ferritins and transferrin saturations, it is important to consider secondary causes of iron overload such as iron-loading anemias and multiple blood transfusions2 and also to consider other conditions that could lead to a rise in serum ferritin inappropriate to the level of storage iron, namely, liver disease,15,24,25 inflammation from infections, malignancies, or rheumatological conditions,26-29 and excess alcohol ingestion.30 31 None of the subjects in the present study with the combination of raised serum ferritin and transferrin saturation >70% had received blood transfusions and only one had a borderline low hemoglobin of 12.9 g/dL, indicating that secondary iron overload did not contribute to the elevated serum ferritins. Because none of these subjects were positive for hepatitis B surface antigen, it is not likely that the elevated iron levels are due to chronic hepatitis B infection. One of the subjects was positive for antibody to hepatitis C and had associated abnormal liver function tests, raising the possibility of chronic hepatitis C infection contributing to the elevation in serum ferritin and transferrin saturation in one subject. The subjects with raised serum ferritins and transferrin saturations >70% did not have malignancies or rheumatologic conditions or any obvious infections. The positive correlation between bone marrow iron and serum ferritin, the marked elevations in the ferritin/AST ratio and the absence, in most subjects, of marked elevations in hepatic enzymes all suggest that the elevated serum ferritins reflect iron stores, rather than the effects of alcohol. Thus, the majority of subjects with elevated serum ferritins and high transferrin saturations would appear to have iron overload.
The primary hypothesis of the present study was that, if iron overload is fully explained by dietary iron exposure, then both members of spouse pairs with similar living environments and traditional beer consumption patterns would have a similar or parallel iron status. Table 4 shows that half of the spouse pairs, all the members of whom had consumed more than an estimated 1,000 L of traditional beer and lived in the same circumstances, were discordant for iron categories based on serum ferritin and transferrin saturation. Table 6 shows that, as a group, the iron status of the wives did not parallel that of the husbands. These results seem to be compatible with the possibility that some factor other than dietary iron exposure may influence iron status. Of particular interest are the four members of group III (elevated serum ferritin and transferrin saturation >70%), three of whom had spouses in group I (normal serum ferritin) and one a spouse in group II (Table 5). While these results are consistent with the hypothesis that a factor other than dietary iron exposure influences iron status, it should be noted that the three spouses with normal serum ferritins had substantially lower estimates for traditional beer consumption than their husbands and that a firm conclusion cannot be drawn.
The subsidiary hypothesis of the present study was that, if iron overload is fully explained by dietary iron content, then in the community as a whole, individuals with similar habits of traditional beer consumption would have similar iron status and increasing levels of traditional beer consumption would be associated with increasing body iron stores. The mean ionic iron concentration of the traditional beer samples from the Zaka community was 46 ± 10 mg/L. Since it is common to drink 2 or more liters on certain days, the daily intake of iron in this African community may often exceed 100 mg as compared with a daily intake of about 14 mg of iron in a typical Western diet.32 The high prevalence of the combination of an elevated serum ferritin and a transferrin saturation >70% (14.3%) among men who drink traditional beer in the Zaka community is consistent with our earlier studies1,33 and with an association between dietary iron and iron overload in Africa.3 Interestingly, however, half or more of both men and women with substantial traditional beer consumption had normal serum ferritins. Furthermore, estimates of traditional beer consumption did not differ significantly among the three groups with different iron status based on serum ferritin and transferrin saturation (Table 7). Finally, in the subjects as a whole, the correlations of serum ferritin, transferrin saturation and ferritin:AST ratio with estimated lifetime beer consumption were low (Fig 1). These findings seem to suggest that high intake of dietary iron in the form of traditional beer does not completely account for the differences in serum ferritins and hence iron stores observed in our study, and they are consistent with our previous study of 36 pedigrees that suggested a possible genetic factor in the pathogenesis of African iron overload.9 The facts that the members of the spouse pairs were not related, that they exhibited similar drinking patterns and that they often were divergent as to iron status support the opinion that African iron overload is more complex than the originally perceived exclusively dietary condition.
Supported in part by a grant from the Office of Minority Health to the Cell Biology and Metabolism Branch, National Institute of Child Health and Human Development (Bethesda, MD); by National Institute of Child Health and Human Development Contract No. 1-HD 3-3196; by a grant from the J.F. Kapnek Charitable Trust (Philadelphia, PA); and by a grant allocation from the Research Board of the University of Zimbabwe (Harare, Zimbabwe).
Address reprint requests to Victor R. Gordeuk, MD, Division of Hematology and Oncology, The George Washington University Medical Center, Suite 3-428, 2150 Pennsylvania Ave, NW, Washington DC 20037.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal