Abstract
Human hemoglobins (Hbs) are known to be immunogenic, and both normal and variant forms of Hb have been shown to stimulate antibody formation in a variety of animal species. In patients who are homozygous for the sickle Hb (HbS) mutation, transfusion of normal, HbA-containing erythrocytes provides a potential stimulus for HbA alloimmunization. We tested serum samples for the presence of anti-Hb antibody by a solid-phase enzyme-linked immunosorbent assay (ELISA) using Hb-coated polystyrene microtiter plates. Hb-bound antibody was identified using an antihuman IgG antibody. Serum samples from 89 patients with sickle cell disease were initially tested for evidence of Hb antibody. The serum from three individuals exhibited antibody activity against HbA with little or no activity against HbS. Only one of them, a multiply transfused adult with HbSS, was available for further study. When this patient's antibody was tested against a variety of normal and mutant Hbs using antibody either to human IgG or to κ chains, the anti-Hb antibody demonstrated specificity for the region of the Hb β chain corresponding to the site of the amino acid substitution of HbS. The level of activity of the patient's anti-HbA showed no significant change over 1.5 years of observation. The transfusion of erythrocytes containing Hb structurally different from that of the recipient appeared to be capable of stimulating the production of Hb-specific alloimmune antibody.
HEMOGLOBINS (Hbs) are immunogenic proteins, and a variety of anti-Hb antibodies, both polyclonal1-6 and monoclonal,7-10 have been produced in various animal species. Many of these antibodies have been characterized as having a high order of specificity for well-defined epitopes.5,7,8 10
Inasmuch as HbA can be regarded as a “foreign” protein in individuals who are homozygous for structural Hb mutations, transfusion of such individuals with erythrocytes from normal donors could potentially elicit an alloimmune response. To test this hypothesis, we screened a group of previously transfused HbSS patients for evidence of Hb-specific antibody.
MATERIALS AND METHODS
Hb preparation and purification.HbF (fetal hemoglobin) was prepared from cord blood samples from normal infants. The HbF0 fraction was isolated from lysates of washed erythrocytes by CM-cellulose column chromatography.11 This method was also used for the isolation of HbC and HbE. HbA, HbA2 , HbGPhiladelphia, and Hb-Leiden were prepared from erythrocytes of normal or heterozygous individuals, and were purified by DEAE-cellulose chromatography.12 Each of the isolated Hb fractions migrated as a single band by isoelectric focusing.13
IgG preparation from serum samples. The procedure was modified from a published method.14 Ammonium sulfate precipitates from the patient's serum were subjected to DEAE-cellulose chromatography. The recovered IgG preparations were shown to be free of contaminating proteins by immunoelectrophoresis.15
Enzyme-linked immunoassay for Hb antibody. This procedure was adopted from the method described by Garver et al.16 The wells of a polystyrene microtiter plate were activated with 0.25% glutaraldehyde, and 5 μg purified Hb in a volume of 100 μL was added to each well. To block nonspecific Ig binding sites, 200 μL 0.5% bovine serum albumin (BSA) in Tris-buffered saline [TBS] 20 mmol/L Tris in 500 mmol/L NaCl containing 0.2% Tween 80, pH 7.5) was added to each of the wells, followed by incubation at room temperature for 1 hour. The patient samples, either whole serum or purified IgG, diluted in 2% BSA in TBS were added to the wells in a volume of 100 μL, followed by incubation at room temperature for an additional hour, and subsequently by the addition of 100 μL developing antibody. For total IgG assays, horseradish peroxidase–conjugated affinity-purified goat antihuman IgG (heavy and light chain–specific) antibody (Vector Laboratories Inc, Burlingame, CA) was used as the developing antibody. For Ig light chain assays, peroxidase-conjugated affinity-purified sheep antihuman κ or λ chain antibodies (The Binding Site, San Diego, CA) were used. Peroxidase substrate (tetramethylbenzidine 0.1 mg/mL, in 0.1 mol/L sodium acetate/citrate buffer containing 0.3% hydrogen peroxide) was added to each of the wells, and after 30 minutes the absorbance of the samples was estimated at 630 nm.
RESULTS
Screening results from individuals with sickle cell disease and normal subjects.Serum samples were obtained from 89 patients with sickle cell disease, most of whom were multiply transfused. As normal controls, 20 serum samples from individuals with normal Hb phenotypes were also tested. Each serum, at a dilution of 1:10, was assayed in duplicate by the enzyme-linked immunosorbent assay (ELISA) procedure using goat antihuman IgG as a developing antibody against both HbA and HbS. Serum samples from three of these patients produced positive results against HbA, with little or no demonstrable activity against HbS. Only one of these individuals was available for more detailed analysis, and the subsequent studies used IgG prepared from samples of this patient's serum.
Clinical findings from the patient.The patient, an African-American man, was born in 1951. A diagnosis of sickle cell anemia was made when he was 10 years old. Since that time, he has had frequent episodes of pain crises, as well as multiple other complications related to the disease. These included at least five episodes of acute chest syndrome, aseptic necrosis involving both shoulders and the right hip, septic arthritis of one knee, cholelithiasis with cholecystitis, and chronic leg ulcers. He has been significantly obese for many years (a recent weight was 137 kg), and for more than 10 years he has had hypertension (blood pressure before treatment, 190/94 mm Hg). He has had at least five major surgical procedures with general anesthesia. His present medications include hydrochlorothiazide, nifedipine, and a variety of analgesics; he has received many different antibiotics on numerous occasions.
A representative blood cell count from this patient, more than 3 months after any transfused blood, showed the following: Hb, 80.0 g/L; red blood cell count, 3.40 × 1012/L; mean corpuscular volume, 91 fL; reticulocyte count, 7.8%; platelet count, 210 × 109/L; and white blood cell count, 10.8 × 109/L, with 50% neutrophils, 28% lymphocytes, 16% monocytes, 4% eosinophils, and 2% basophils. His recent transfusion history is summarized in Table 1. His blood group phenotype is [B, ccDee, Ms/Ns, P1 , Jka, Fyb, Leb]. Despite the extensive number of previous transfusions and the considerable likelihood that he has received at least some transfused erythrocytes with blood group antigen incompatibility, he has never shown evidence of blood group alloimmunization, and his direct antiglobulin test has always remained negative.
Blood Transfusions Received by the Patient
| Year . | Clinical Indication . | No. of Blood Units . |
|---|---|---|
| 1976 | Pneumonia | 1 |
| 1978 | Hemolytic crisis/pneumonia | 6 |
| 1980 | Cholecystitis/cholelithiasis | 6 |
| 1981 | Acute chest syndrome | 4 |
| 1984 | Joint surgery | 6 |
| 1986 | Septic arthritis | 2 |
| 1992 | Acute chest syndrome | 2 |
| 1994 | Acute chest syndrome | 2 |
| 1994 | Ileostomy | 6 |
| 1995 | Ileostomy bleeding | 2 |
| 1995 | Ileostomy revision | 2 |
| Year . | Clinical Indication . | No. of Blood Units . |
|---|---|---|
| 1976 | Pneumonia | 1 |
| 1978 | Hemolytic crisis/pneumonia | 6 |
| 1980 | Cholecystitis/cholelithiasis | 6 |
| 1981 | Acute chest syndrome | 4 |
| 1984 | Joint surgery | 6 |
| 1986 | Septic arthritis | 2 |
| 1992 | Acute chest syndrome | 2 |
| 1994 | Acute chest syndrome | 2 |
| 1994 | Ileostomy | 6 |
| 1995 | Ileostomy bleeding | 2 |
| 1995 | Ileostomy revision | 2 |
This patient also has not shown any indication of autoimmune disease. His anti–nuclear antibody test has consistently been negative, and screening tests for circulating antibody against rheumatoid factor and smooth muscle also were negative.
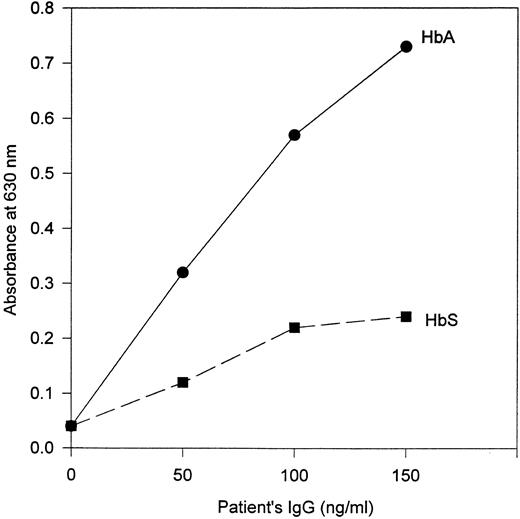
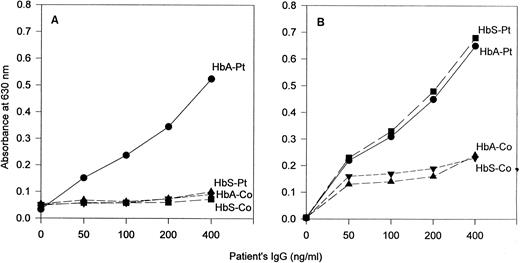
Properties of the patient's anti-Hb antibodies.IgG prepared from the patient's serum demonstrated antibody activity against HbA but little or no activity against HbS in ELISAs that used goat antihuman IgG as the developing antibody (Fig 1). This specificity was also observed in assays that used antihuman κ chain antibody (Fig 2A). However, in ELISAs in which antihuman λ chain antibody was used as the developing antibody, the patient's IgG showed binding activity to both HbA and HbS (Fig 2B).
Response of antibody from the patient to HbA and HbS by ELISA. Goat antihuman IgG (heavy and light chain–specific) antibody was used as the developing antibody.
Response of antibody from the patient to HbA and HbS by ELISA. Goat antihuman IgG (heavy and light chain–specific) antibody was used as the developing antibody.
Comparison by ELISA of the antibody response to HbA and HbS with IgG from the patient (Pt) and from a representative normal control (Co). The developing antibodies were (A) sheep antihuman κ chain antibody and (B) sheep antihuman λ chain antibody.
Comparison by ELISA of the antibody response to HbA and HbS with IgG from the patient (Pt) and from a representative normal control (Co). The developing antibodies were (A) sheep antihuman κ chain antibody and (B) sheep antihuman λ chain antibody.
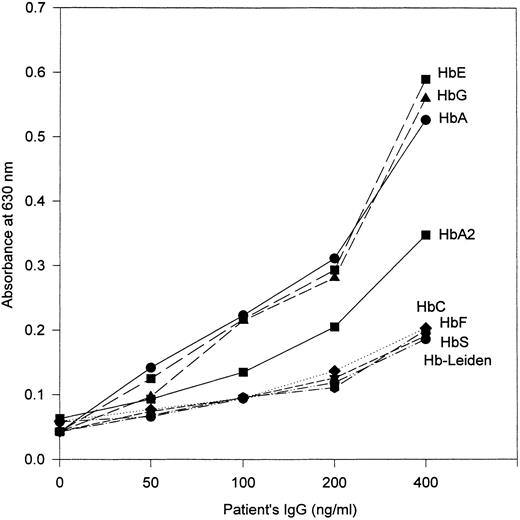
Antibody-binding specificity against other human Hbs.To gain some definition of the antigen-binding epitope of the HbA-specific antibody, we substituted a number of other purified human Hbs in the ELISA. These included fetal Hb (HbF ), HbA2 , HbGPhiladelphia (an α-chain variant with structurally normal β chains), HbC (β6 Lys), Hb-Leiden (β5 or β6 Glu deleted), and HbE (β26 Lys). The results of representative experiments are shown in Fig 3.
Activity of the anti-Hb antibody from the patient against various purified human Hbs. Sheep antihuman κ chain antibody was used as the developing antibody in the ELISA.
Activity of the anti-Hb antibody from the patient against various purified human Hbs. Sheep antihuman κ chain antibody was used as the developing antibody in the ELISA.
In the studies that used other Hb variants with structural changes at the β6 position (the site of the βs mutation), namely HbC and Hb-Leiden, negligible antibody activity was detected with either antihuman IgG antibody or anti–κ chain antibody, with the data points being virtually indistinguishable from those obtained with HbS. With HbG and HbE, whose β6 regions are structurally normal, the antibody exhibited activity of a similar degree as against HbA (Fig 3).
Results obtained with HbA2 and HbF are also shown in Fig 3. With HbA2 , the activity of the patient's anti-Hb antibody was approximately intermediate between that against HbA and against HbS. HbA2 contains α subunits that are identical to those of HbA; the sequence of amino acid residues 1 to 8 of its δ chains is also identical to those of the β chains of HbA, but at amino acid positions 9 and 12 their sequences differ. HbF also contains α subunits that are identical to those of HbA, but the amino acid sequence of its γ chains is substantially different from that of βA; a notable difference is that the γ6 position is occupied by aspartate and β6 by glutamate. Correspondingly, the patient's HbA-specific antibody exhibited no apparent activity against HbF (Fig 3).
The specificity of the antibody-binding epitope of the patient's anti-Hb IgG was also examined in an experiment that used a blocking antibody. For this purpose, mouse monoclonal antibody with antigenic specificity for β6 to β9 of human HbA17 was used. When preparations of this antibody (Immuno-rx, Augusta, GA) were added to wells containing HbA, subsequent binding of antibody from the patient's IgG was completely inhibited. Nonimmune mouse IgG exhibited no blocking effect.
When antihuman λ chain antibody was used as the developing antibody in the ELISA, the data points from the patient's IgG showed no significant difference from those against HbA and HbS (Fig 2B) when HbF, HbA2 , HbG, HbC, Hb-Leiden, and HbE were substituted (data not shown). Each produced a virtually identical curve.
DISCUSSION
The antibody activity identified in this study represents, to our knowledge, the first known demonstration of human antibody formation against Hb. Earlier efforts by Reichlin18 to identify HbA antibody in multiply transfused HbSS patients yielded only negative results.
The patient in the present study appeared to have at least two distinct anti-Hb antibodies. One of these, an IgG with λ light chains, exhibited no apparent specificity against any of the various normal and mutant Hbs we examined. This antibody could have specificity for an epitope on the Hb α subunits, or on a region of the β subunits relatively distant from the sites of the βS or βE mutations, but we have no experimental evidence to address these possibilities directly. Inasmuch as there was no apparent indication of the presence of this seemingly nonspecific anti-Hb antibody in the ELISAs that employed antihuman IgG antibody (Fig 1), it would appear either that the antihuman IgG antibody we used had preferential binding to IgG with κ chains rather than λ chains, or that the patient's anti-Hb IgG that contained λ chains represented only a minor component of his anti-Hb antibody.
The other anti-Hb antibody identified in this patient, in which the light chains are of the κ type, exhibited many of the features of an alloantibody: the patient's major adult Hb is of the sickle cell type, and therefore does not contain the normal β6 glutamate; he has been exposed repeatedly to HbA from his multiple transfusions; and this antibody displays a high order of specificity for structurally normal Hb in the region corresponding to the site of the sickle mutation.
We also consistently observed that this antibody exhibited activity against normal HbA2 , but of a lesser degree than that against HbA (Fig 3). The β chains of HbA and the δ chains of HbA2 share identical amino acid sequences for amino acid positions 1 through 8, including the presence of glutamate at position 6, which is the site of the βS mutation. At sequence positions 9, 12, and 22, the β and δ chains contain different amino acids, with the structures of their amino termini otherwise being the same as far as amino acid position 49. From these considerations, it would appear that the antigen-binding epitope of this antibody encompasses, at a minimum, amino acid positions β6 through β9, and could also include position β12.
Studies of antibody formation against human HbA in animals4,17 have identified the β6 site as being potentially immunogenic. A murine monoclonal antibody with specificity for this region has been shown to be nonreactive against HbS, HbF, HbC, and HbA2 ,17 and another mouse monoclonal antibody with anti-HbC activity showed no cross-reactivity with HbA, HbS, HbA2 , or HbF.19 The Hb-binding properties of the κ chain–specific antibody from our patient exhibited a degree of specificity similar but apparently nonidentical to that observed with these murine monoclonal anti-Hb antibodies.
Our patient was one of only three among 89 patients with sickle cell disease we screened in whom we could detect the presence of Hb antibody. This apparently rare event could possibly be accounted for by the unusually large number of blood transfusions this patient received; however, many of the other adults with sickle cell disease that we studied also had a history of multiple blood transfusions. Considering the findings that suggest that this patient's anti-Hb antibody may have arisen by an alloimmune process, it is of interest that he did not show evidence of blood group alloimmunity. This is particularly notable in light of his relatively rare Rh phenotype. Studies of patients with sickle cell disease who have been transfused have shown that the rate of blood group alloimmunization may exceed 30%.20-22 This patient's clinical and laboratory findings also provide no indication of his having any other form of autoimmune or immune complex disease.
In mice, the development of anti-Hb antibody has been shown to be related to the presence of specific immune response genes.23 Whether comparable forms of genetic control might play a role in human anti-Hb antibody formation can at present only be a point of speculation.
Over the period of about 1.5 years that we studied our patient, the activity of his anti-Hb antibody remained virtually unchanged despite his having been transfused during this interval. There has been no indication that the presence of the antibody has contributed to the severity of his sickle cell disease, or to a shortening of survival of transfused erythrocytes.
ACKNOWLEDGMENT
We thank Dr Mabel Koshy for allowing us to include her patients in this study, and for her advice and help.
Address reprint requests to George R. Honig, MD, PhD, Department of Pediatrics (M/C 856), The University of Illinois at Chicago, 840 S Wood St, Chicago, IL 60612.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal