Abstract
HLA class II molecules, expressed on the surface of antigen-presenting cells, are responsible for the presentation of antigen-derived peptides to CD4+ helper T lymphocytes. Signaling via these molecules initiates the generation of second messengers leading to programed cell death (PCD) of activated B lymphocytes. The present study examined the mechanism of HLA class II–mediated apoptosis and describes the essential role of the molecule Fas and its ligand (FasL). FasL was expressed in B lymphocytes after stimulation via HLA class II or with phorbol esters. Expression of FasL protein was significantly increased in 50% of B lymphocytes after stimulation via HLA class II, and the level of FasL mRNA was also increased either by activation with phorbol esters and ionomycin or by signaling via HLA class II. Although HLA class II signaling did not change the expression of the Fas molecule, it did lead to increased sensitivity to Fas-mediated apoptosis. The crucial role of Fas/FasL interactions was confirmed by the absence of cell death via HLA class II in B cells lacking Fas expression, and by the significant inhibition of HLA class II–mediated apoptosis in the presence of either an antagonistic anti-Fas or anti-FasL antibody. These data demonstrate FasL expression on activated human B lymphocytes and support the idea that antigen presentation could contribute to the regulation of lymphocyte populations via Fas and FasL interactions.
RECENT STUDIES HAVE demonstrated that homeostasis of the immune system is actively mediated by cellular differentiation, proliferation, and death. Either recognition of specific antigen or polyclonal stimulation leads to the expansion of T- and B-lymphocyte populations that can be limited by a process referred to as activation-induced cell death (AICD), essentially the apoptosis of activated cells.1,2 The first clues to the mechanism of this cell death in T cells came with the finding that T lymphocytes from the spontaneous mutant lpr mouse did not die after activation.3 The defects in these mice, which develop lymphadenopathy, were traced to mutations in the CD95 (Fas) gene.4 The spontaneous gld mutant has a phenotype similar to the lpr mouse, but contains a point mutation in the CD95 ligand (Fas ligand, FasL) gene.5 A human lymphoproliferative syndrome has been recently described that has immunologic features resembling those seen in lpr mice.6,7 This disorder was characterized by mutations or deletions in the Fas gene.6 7
Cognate activation of T cells requires the encounter of the T-cell receptor (TCR) with a specific peptide presented by the major histocompatibility complex (MHC). The principle role of HLA class II molecules is the presentation of peptides to antigen-specific CD4+ T cells, and their ligands include both the TCR/CD4 complex on T cells and the bacterial superantigens.8,9 HLA class II molecules can exist as dimers and have been recently detected as dimers of dimers (superdimers) on both murine and human antigen-presenting cells.10-13 It has been proposed that superdimers could enhance the affinity for the TCR complex and activate signaling pathways in both the antigen-presenting cell and the T cell.10 HLA class II molecules are capable of signal transduction leading to the generation of second messengers, homotypic aggregation, and either cellular activation or programmed cell death (PCD),14-19 and a role for HLA class II signaling in increasing sensitivity to apoptosis via the Fas molecule has also been recently described in B-cell lines.20
Fas (Apo-1, CD95) is a cell surface molecule of the tumor necrosis factor (TNF )/nerve growth factor receptor families,21,22 whereas FasL is a type II membrane protein also belonging to the TNF family.21,23 Stimulation of the Fas molecule with monoclonal antibodies (MoAbs) induces apoptosis of certain Fas-expressing cells.24,25 The interaction of Fas with FasL has been implicated in the induction of apoptosis by cytotoxic T cells and in natural killer cell–mediated killing.26-28 A role for Fas-induced apoptosis during thymic selection has also been proposed,29 although the deletion of autoreactive T cells is apparently normal in the lpr mouse, suggesting that the major role of Fas is in peripheral deletion.30 31
Fas does not always trigger apoptosis, but instead may signal for cellular activation, since a synergy with anti-CD3–mediated activation has been observed.32 Consequently, the level of Fas-mediated killing is minimal on normal, nonactivated T and B lymphocytes32,33 (and personal observation). The regulation of Fas sensitivity to apoptotic signaling appears to be activation-mediated, so that after several days of activation Fas-mediated apoptosis increases dramatically in both B and T lymphocytes.33,34 The Fas-associating molecules in the cytoplasm that could determine whether an activating or a death-inducing signal is transduced are only now being identified (reviewed in Peter et al35 ).
Recent studies have shown that TCR-mediated AICD can occur via cell-autonomous Fas and FasL interactions.36-38 Whether the interaction of Fas and FasL occurs through an association at the cell surface or via a soluble form of the FasL has yet to be fully characterized.36,39 This has led to the suggestion that Fas-mediated killing of these cells is a “suicidal” event, since single cells can undergo AICD via Fas/FasL association.37 However, the possibility of Fas/FasL interactions between different cells cannot be ruled out in vivo. Probing the activated T-cell hybridoma (A1.1) with a Fas fusion protein showed that greater than 50% of the cells expressed FasL after activation and that the level of Fas expression was also activation-dependent.37 Coupled with the observation that Fas-mediated apoptosis increases after activation, this suggests that activated T cells have only a limited time in which to react to a given stimulus.34 Another study concerning superantigen-mediated depletion of T cells showed that only proliferating cells were susceptible to death.40 These data underline the importance of Fas/FasL interactions in normal immune homeostasis.
We have continued our study of HLA class II molecule signaling on antigen-presenting cells by examining the mechanism of HLA class II–mediated PCD. We report that FasL protein was consistently induced after HLA class II ligation in a large proportion of primary B cells. Induction of FasL via HLA class II was confirmed by reverse transcriptase–polymerase chain reaction (RT-PCR) detection of FasL mRNA. Furthermore, we show that the sensitivity of primary B cells to Fas-induced apoptosis is increased after HLA class II stimulation. Confirmation of the importance of Fas/FasL interactions in HLA class II–mediated PCD was provided by the absence of apoptosis in a B-cell line that does not express Fas (Fas−) isolated from a patient with autoimmune lymphoproliferative disease.6 Furthermore, HLA class II–mediated apoptosis was inhibited in the presence of either an anti-Fas or anti-FasL antibody. These results indicate that (1) AICD via a Fas/FasL pathway in B lymphocytes occurs in a manner analogous to that observed in activated T lymphocytes, and (2) HLA class II molecules mediate PCD via Fas and FasL in human B lymphocytes. Finally, these data underline the importance of HLA class II molecules in superantigen-mediated diseases.
MATERIALS AND METHODS
Media and cells.Cells were suspended in RPMI 1640 (Seromed, Berlin, Germany) supplemented with 10% fetal calf serum (FCS; Boehringer, Mannheim, Germany) and 2 mmol/L L-glutamine, 500 IU/mL penicillin, 500 μg/mL streptomycin, 1 mmol/L sodium pyruvate (Seromed), and 1% Mycoplasma removal agent (ICN Flow Laboratories, Orsay, France). This complete medium will be referred to as RPMI-10 herein. Human spleens were generously provided by the transplantation center of Cochin Hospital (Paris, France). Human B lymphocytes were prepared by aseptically passing splenic fragments through a metal gauze, and washed with RPMI-10. T cells were removed by addition of a 10% (vol/vol) solution of aminoethylisothiouronium bromide (Sigma, St Guentin-Fallavier, France)–treated sheep red blood cells and centrifugation on Ficoll (Eurobio, Les Ulis, France). B lymphocytes were recovered at the interface. Macrophages and monocytes were depleted by adherence to plastic. Nonadherent cells were used throughout these experiments, and double-labeling has shown that greater than 95% of the cells were both CD19+ and HLA-DR–positive. A second method of B-cell separation by positive selection using anti-CD19 magnetic beads (Dynabeads; Dynal, Oslo, Norway) was also used according to the manufacturer's instructions, to obtain highly purified B lymphocytes and to further reduce the risk of T-cell contamination. This method of purification yielded a population that was 99% CD19+ as determined by cytofluorometry. Generation of second messengers via HLA class II was comparable in cells prepared by either method of separation (data not shown). Epstein-Barr virus (EBV)-transformed human B-cell line, from patient no. 1, which contains a deletion in the cytoplasmic domain of the Fas molecule (Fas−), has been described in detail elsewhere, its most important feature being the total lack of Fas expression.6
B-lymphocyte treatments — Cytofluorometry.Cells (0.5 × 106) were incubated with either 5 μg/mL L227 and 20 μg/mL of a cross-linking goat antimouse F(ab′)2 antibody (Organon Teknika N.V., Veedijk, Belgium; L227 XL in the text) or 10 μg/mL biotinylated toxic shock syndrome toxin-1 (TSST-1; Toxin Technology Inc, Sarasota, FL) cross-linked with 10 μg/mL streptavidin (Sigma). PMA (Sigma) was used at 20 ng/mL and ionomycin (Sigma) at 5 ng/mL. As a control, the anti-HLA class I MoAb W6.32 was used at 5 μg/mL with 20 μg/mL of a cross-linking goat antimouse F(ab′)2 antibody, abbreviated as W6.32 XL throughout the text. Anti-Fas IgM MoAb (Upstate Biotechnology Inc, Lake Placid, NY) was used at 1 μg/mL for 24 hours before preparation of the cells for FACS analysis. For studies of the inhibition of HLA class II–mediated apoptosis, the cells were first incubated with L227 XL for 48 hours before the addition of either 10 μg/mL of the Fas-antagonistic MoAb ZB4 (Kamiya Biomedical Co, Thousand Oaks, CA) or 5 μg/mL of the anti-FasL antibody PE62.41 To confirm that inhibition in the presence of either the anti-Fas or anti-FasL antibodies was specific, the effect of 10 μg/mL anti-CD19 (Becton Dickinson, San Jose, CA) MoAb was also examined after 48 hours' incubation with L227 XL. Apoptosis was quantified after a further 48 hours of incubation in the presence of the relevant antibody.
B-lymphocyte stimulation for RT-PCR detection of FasL.For RT-PCR, 5 × 106 cells in 1.5 mL RPMI-10 were stimulated with anti–HLA class II-DR MoAb L227 at a concentration of 6.7 μg/mL, and a cross-linking secondary antibody, a goat antimouse F(ab′)2 , was used at 20 μg/mL. The biotinylated staphylococcal superantigen TSST-1 was used at a final concentration of 13.3 μg/mL. Streptavidin was used at 13.3 μg/mL. The positive control for B-cell activation was obtained by stimulating the cells with 20 ng/mL PMA (Sigma) and 13 ng/mL of the calcium ionophore ionomycin.
Expression of FasL — FACS analysis.Cells (0.5 × 106) were incubated as described earlier. After 0 or 96 hours of stimulation, the cells were fixed for 5 minutes in 1 mL ice-cold 95% ethanol and 5% (vol/vol) acetic acid, followed by centrifugation and resuspension in PBS containing 0.02% sodium azide (Sigma) and 1% FCS. This method of fixation permeabilizes the cell membrane, and therefore allows detection of FasL expressed either at the cell surface or in the cytoplasm. To control for nonspecific fixation of the primary antibody, polyclonal rabbit anti–cyclin B (Santa Cruz Biotechnology) was used in place of the anti-FasL antibody. The cells were washed and incubated with 2 μg anti-FasL antibody N-20 (Santa Cruz Biotechnology) or with 1 μg anti-FasL antibody PE62 for 30 minutes on ice. The cells were again washed and then incubated on ice in the dark for 30 minutes with a polyclonal goat antirabbit FITC-conjugated antibody (Immunotech). After further washing, the cells were analyzed using the green fluorescence detector of a Becton Dickinson FACScan. FasL staining in Fas− cells was performed as described for splenic B cells, except that the monoclonal rat IgM anti-FasL, AL9842 was used as the primary antibody. The secondary fluorescent MoAb for AL98 detection was a monoclonal goat anti–rat IgM F(ab′)2 FITC (Immunotech). Apoptotic and living cells were distinguished by their forward (size) and side (granularity) scatters.
Western blotting.PE62 has been used to detect FasL by immunoblotting,41,42 and a modification of this protocol was used. Cell lysates were prepared under both reducing and nonreducing conditions.42 Briefly, 2.5 × 106 cells were lysed in 30 μL cell lysis buffer (0.5% Triton X-100 with 300 mmol/L NaCl in 50 mmol/L Tris-HCl, pH 7.6) containing 1 mmol/L phenylmethylsulfonyl fluoride, 1/100 aprotinin, 10 nmol/L leupeptin, 0.5 mmol/L trypsin inhibitor, and 0.1 mmol/L iodoacetamide, all purchased from Sigma. The postnuclear fraction was separated on 10% SDS-PAGE and transferred to nitrocellulose. PE62 was used to detect FasL protein, which was shown using goat antirabbit IgG coupled to horseradish peroxidase. The blots were developed using the Enhanced Chemiluminescence kit from Amersham (UK).
Quantification of apoptotic cells.Apoptotic cells were quantified according to a modification of the protocol devised by Nicoletti et al.43 Briefly, 0.5 × 106 cells were used per treatment. After stimulation, the cells were resuspended in 1 mL of a hypotonic solution containing 0.1% sodium citrate, 0.1% Triton X-100, and 50 μg/mL propidium iodide (all from Sigma). These were left at 4°C overnight in the dark, before measuring the red fluorescence using a Becton Dickinson FACScan. Debris was removed by increasing the forward-scatter threshold. Ten thousand events were counted per sample and analyzed using CellQuest V1.1.1 software (Becton Dickinson) on a MacIntosh Quadra computer (Apple). The hypodiploid nuclei, corresponding to apoptotic nuclei, were counted. This is a method of choice for determining HLA class II–mediated apoptosis, since it circumvents problems resulting from HLA class II–induced cellular aggregation. Furthermore, this method permits quantitative studies, which is not the case for methods based on DNA fragmentation detected in agarose gels.
Staining of apoptotic cells.Cells at a final concentration of 5 × 106/mL were mixed with 0.5 μg/mL of the DNA-binding dye Hoescht 33342 (Sigma). The cells were left in the dark for 5 minutes at room temperature before identification of apoptotic nuclei by fluorescence microscopy; these nuclei are distinguished by their irregular (or crescent) shapes and bright fluorescence.
RNA isolation.Total RNA was prepared from human splenic B cells using a rapid RNA isolation kit (TRIzol; Life Technologies, Cergy Pontoise, France). RNA isolation from the cells was performed according to the manufacturer's instructions, and was quantified by measuring optical density at 260 nm.
RT-PCR.A quantity of 3.5 μg total RNA was used per test. The reaction was performed using the GeneAmp EZ rTth RNA PCR kit (Perkin Elmer, Branchburg, NJ) in the presence of 5 μCi γ32P-dATP (Amersham) per reaction tube. The following primers were used to amplify FasL transcripts at a final concentration of 20 pmol/L: 5′-CAGCTCTTCCACCTACAGAAGG-3′ and 5′-AGATTCCTCAAAATTGACCA-GAGAGAG-3′. The oligonucleotides used as PCR primers for human FasL were designed from the human sequence (Genbank). The predicted size of the FasL PCR product was 510 bp. GAPDH loading controls are detailed elsewhere.38 The reaction was performed by reverse transcription at 60°C for 30 minutes, followed by 40 cycles of 1 minute of denaturation at 92°C, 1 minute of annealing at 55°C, and 1 minute of extension at 72°C. The PCR products were analyzed after migration on a 2% agarose gel. The gel was dried and autoradiographed for either 2 hours or 18 hours.
RESULTS
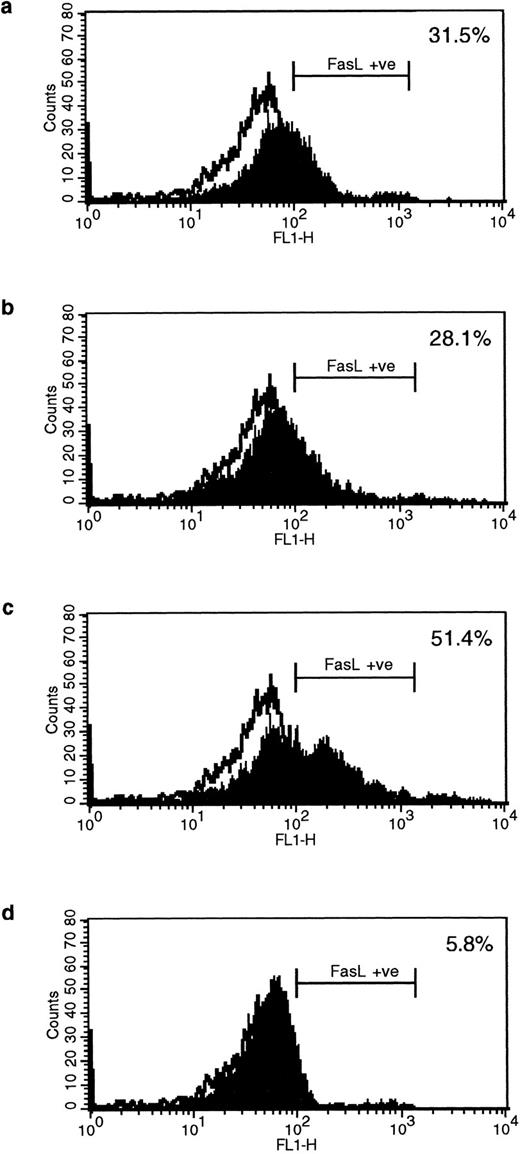
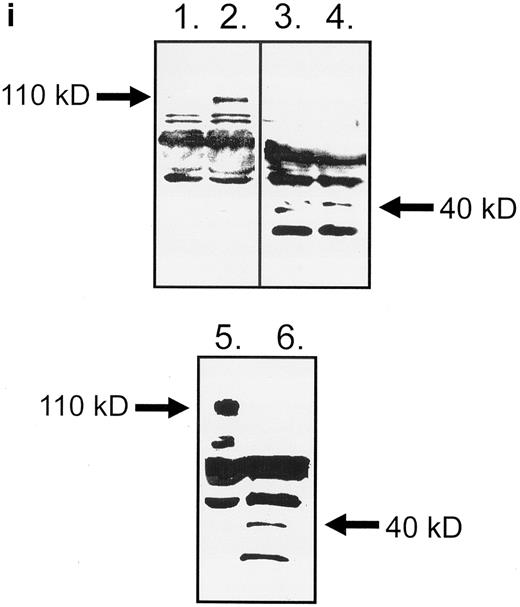
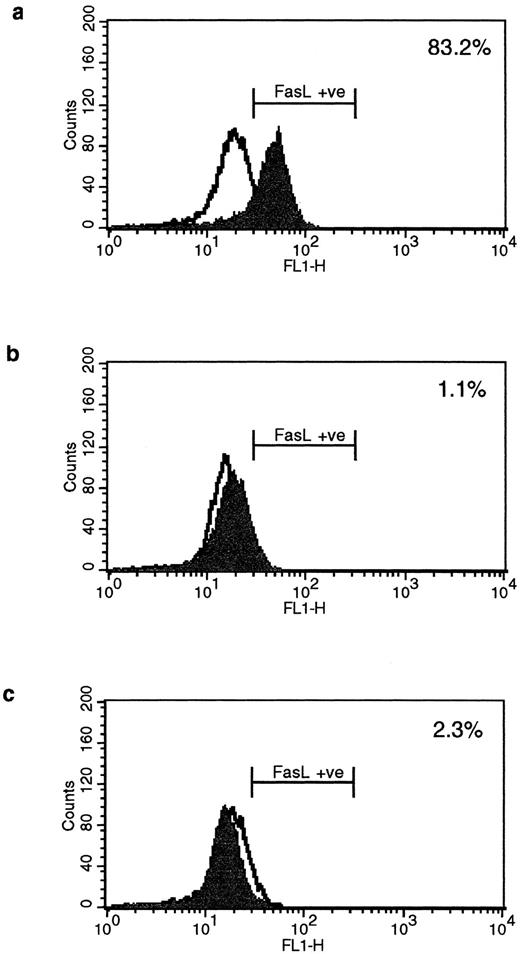
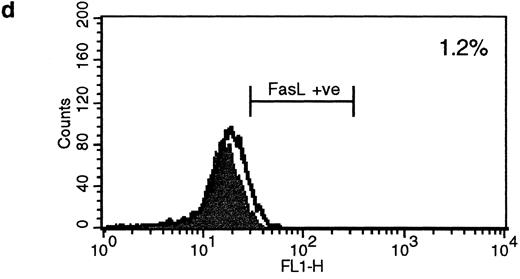
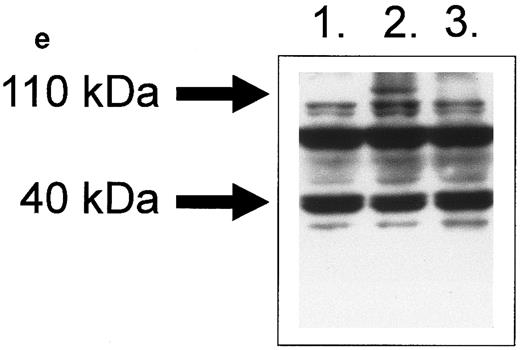
HLA class II–mediated signaling induces expression of FasL on B lymphocytes.Fas and FasL interactions play a major role in the AICD of T lymphocytes. Since we have previously detected HLA class II–mediated apoptosis, we examined the expression of FasL on primary B lymphocytes stimulated via HLA class II after 96 hours of stimulation. The N-20 anti-FasL antibody was used for this study. Figure 1a shows that 31.5% of splenic B cells express FasL protein after treatment with PMA and ionomycin. Figure 1b shows FasL expression on 28% of cells treated with streptavidin cross-linked biotinylated TSST-1. Figure 1c shows FasL expression in 51% of B cells treated with L227 XL, and Fig 1d shows that stimulation with an isotype-matched anti–HLA class I MoAb did not significantly alter expression of FasL. FasL expression in nonstimulated cells is shown by the unfilled trace, and FasL expression is shown by the filled trace. To control for nonspecific binding of the anti-FasL antibody, species fixation of an isotype-matched control antibody, a rabbit polyclonal IgG anti–cyclin B, was also examined before and after stimulation. Figures 1e and h demonstrate that the binding of anti-FasL was specific, since there was no difference in the level of binding of the control antibody before or after stimulation. To confirm these data, immunoblotting was performed using the anti-FasL PE62 polyclonal antibody. The data illustrated in Fig 1i show FasL expression after stimulation for 24 hours (lanes 1 and 3, nonstimulated B cells; lanes 2 and 4, L227 XL). A newly detected protein that migrates at an apparent molecular weight of 110 kD was observed after stimulation via HLA class II molecules in lysates prepared in nonreducing buffer. On the contrary, this protein was absent from lysates of stimulated cells prepared in reducing buffer, but a significant band at 40 kD was observed. The soluble FasL is biologically active as a trimer,39 and we therefore suggest that the newly detected 110-kD protein observed after HLA class II stimulation is trimeric FasL, whereas only the monomeric 40-kD form is detected under reducing conditions. Oligomerization of the FasL protein has previously been detected by this antibody when lysates were prepared under nonreducing conditions.41
Expression of FasL in B lymphocytes before and after stimulation via HLA class II. (a) PMA and ionomycin; (b) bacterial superantigen TSST-1; (c) anti–HLA-DR MoAb L227 XL; (d) anti–HLA class I MoAb W6.32 XL. The level of fluorescence in nonstimulated cells is shown as an unfilled profile and is compared with the filled profile of stimulated cells. Profiles e to h show binding of an isotype-matched control antibody (polyclonal rabbit IgG, anti–cyclin B) before and after stimulation as above. For a to h, the cells were fixed in a solution containing 95% ethanol with 5% acetic acid and then stained with either 2 μg/mL anti-FasL antibody N-20 or 1 μg/mL anti-FasL PE62, followed by 10 μg/mL secondary MoAb conjugated with FITC. (i) Immunoblot with the anti-FasL antibody PE62 in which cell lysates were prepared either under nonreducing conditions (lanes 1, 2, and 5) or under reducing conditions (lanes 3, 4, and 6). Lanes 1 and 3, nonstimulated cells; lanes 2 and 4, L227 XL; lanes 5 and 6, bacterial superantigen TSST-1.
Expression of FasL in B lymphocytes before and after stimulation via HLA class II. (a) PMA and ionomycin; (b) bacterial superantigen TSST-1; (c) anti–HLA-DR MoAb L227 XL; (d) anti–HLA class I MoAb W6.32 XL. The level of fluorescence in nonstimulated cells is shown as an unfilled profile and is compared with the filled profile of stimulated cells. Profiles e to h show binding of an isotype-matched control antibody (polyclonal rabbit IgG, anti–cyclin B) before and after stimulation as above. For a to h, the cells were fixed in a solution containing 95% ethanol with 5% acetic acid and then stained with either 2 μg/mL anti-FasL antibody N-20 or 1 μg/mL anti-FasL PE62, followed by 10 μg/mL secondary MoAb conjugated with FITC. (i) Immunoblot with the anti-FasL antibody PE62 in which cell lysates were prepared either under nonreducing conditions (lanes 1, 2, and 5) or under reducing conditions (lanes 3, 4, and 6). Lanes 1 and 3, nonstimulated cells; lanes 2 and 4, L227 XL; lanes 5 and 6, bacterial superantigen TSST-1.
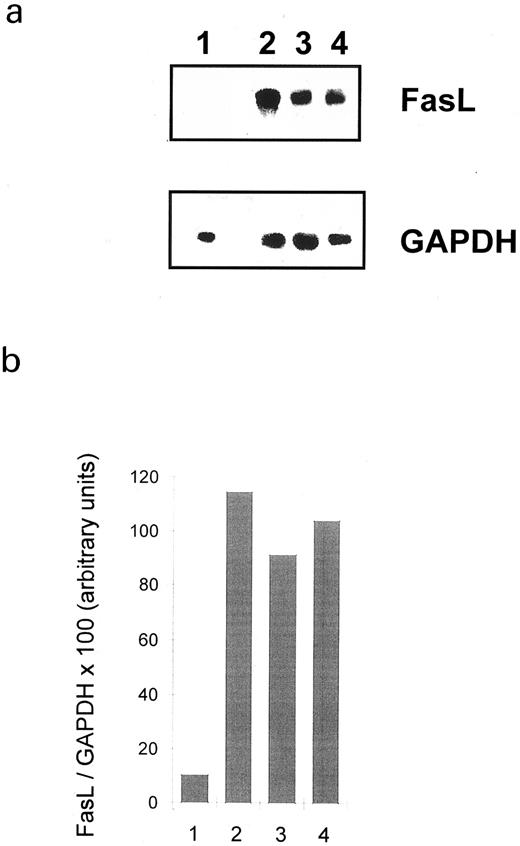
FasL expression is induced after HLA class II stimulation: Detection of FasL mRNA.We tested whether HLA class II stimulation could induce FasL and therefore apoptosis via the Fas/FasL pathway by searching for FasL mRNA transcripts in splenic B cells. The oligonucleotide pair gave rise to a single amplification product of the predicted molecular size (510 bp). Figure 2a shows that FasL mRNA was not detected in nonstimulated B cells. However, FasL mRNA was present after 4 hours' treatment with either cross-linked L227, streptavidin cross-linked biotinylated TSST-1, or PMA and ionomycin. The high level of cellular aggregation induced following HLA class II ligation made it difficult to include later time points, since extremely little RNA could be recovered, probably due to either mechanical shearing of the cells or background apoptosis, damaging the overall RNA quality.44 The relative number of FasL transcripts in stimulated and nonstimulated B lymphocytes was compared by densitometry of the autoradiograph, which was normalized by comparing them with the densities of the GAPDH bands. The ratio of FasL/GAPDH for nonstimulated versus stimulated cells is shown in Fig 2b. These data confirm that HLA class II ligation results in a significant increase in the level of FasL mRNA.
Effect of HLA class II molecule signaling on the expression of FasL mRNA. Human splenic B lymphocytes were stimulated as indicated for 4 hours. RT-PCR amplification was performed, and PCR products were visualized after electrophoresis in a 2% agarose gel (a). The autoradiograph was subsequently analyzed with a densitometer, which was normalized by dividing the FasL PCR product with the density of the GAPDH profile (b). The PCR product of FasL amplification migrated at the predicted size of 510 bp. Lane 1, nonstimulated; lane 2, 6.7 μg/mL anti–HLA class II MoAb (L227 XL); lane 3, bacterial superantigen TSST-1; lane 4, PMA and ionomycin.
Effect of HLA class II molecule signaling on the expression of FasL mRNA. Human splenic B lymphocytes were stimulated as indicated for 4 hours. RT-PCR amplification was performed, and PCR products were visualized after electrophoresis in a 2% agarose gel (a). The autoradiograph was subsequently analyzed with a densitometer, which was normalized by dividing the FasL PCR product with the density of the GAPDH profile (b). The PCR product of FasL amplification migrated at the predicted size of 510 bp. Lane 1, nonstimulated; lane 2, 6.7 μg/mL anti–HLA class II MoAb (L227 XL); lane 3, bacterial superantigen TSST-1; lane 4, PMA and ionomycin.
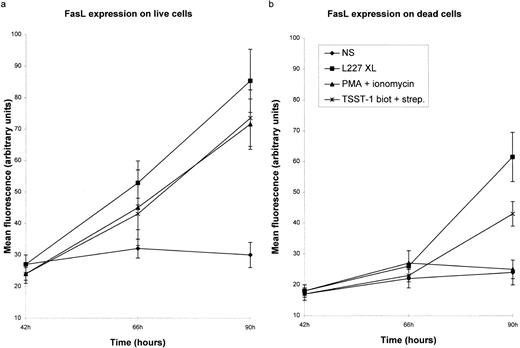
Significant levels of FasL protein are detected in B lymphocytes after 42 hours of stimulation.We examined the expression of FasL on primary B lymphocytes stimulated via HLA class II over a period of 96 hours. Expression of FasL was detected 48 hours after HLA class II stimulation. Following this time point, FasL expression increases dramatically after treatment with either L227, PMA and ionomycin, or TSST-1 (Fig 3a). Because FasL was detected on both live and dead B cells, we examined each population separately. FasL expression was detected on B lymphocytes stimulated either via HLA class II or in the presence of PMA and ionomycin. Expression of FasL in dead cells (Fig 3b) was observed following stimulation with either L227 or TSST-1. FasL expression was not detected on cells rendered necrotic by incubation in 10% dimethylsulfoxide (data not shown).
FasL is detected on B lymphocytes after HLA class II signaling. (a and b) The mean fluorescence reflecting the expression of FasL after stimulation with either 5 μg/mL L227 and goat antimouse F(ab′)2 MoAb (L227 XL), 10 μg/mL biotinylated TSST-1 and 10 μg/mL streptavidin, or 20 ng/mL PMA and 5 ng/mL ionomycin for up to 90 hours. For all of the tests, cells were fixed in a solution containing 95% ethanol with 5% acetic acid and then stained with either 2 μg/mL anti-FasL antibody N-20 or 1 μg/mL anti-FasL PE62, followed by 10 μg/mL secondary MoAb conjugated with FITC. Live and dead cells were distinguished by forward and side scatters. The background level of fluorescence in nonstimulated cells (NS) is also shown. The mean fluorescence was calculated after subtraction of the background fluorescence in the presence of the FITC-labeled secondary MoAb alone. The bars in (a) and (b) show the standard error.
FasL is detected on B lymphocytes after HLA class II signaling. (a and b) The mean fluorescence reflecting the expression of FasL after stimulation with either 5 μg/mL L227 and goat antimouse F(ab′)2 MoAb (L227 XL), 10 μg/mL biotinylated TSST-1 and 10 μg/mL streptavidin, or 20 ng/mL PMA and 5 ng/mL ionomycin for up to 90 hours. For all of the tests, cells were fixed in a solution containing 95% ethanol with 5% acetic acid and then stained with either 2 μg/mL anti-FasL antibody N-20 or 1 μg/mL anti-FasL PE62, followed by 10 μg/mL secondary MoAb conjugated with FITC. Live and dead cells were distinguished by forward and side scatters. The background level of fluorescence in nonstimulated cells (NS) is also shown. The mean fluorescence was calculated after subtraction of the background fluorescence in the presence of the FITC-labeled secondary MoAb alone. The bars in (a) and (b) show the standard error.
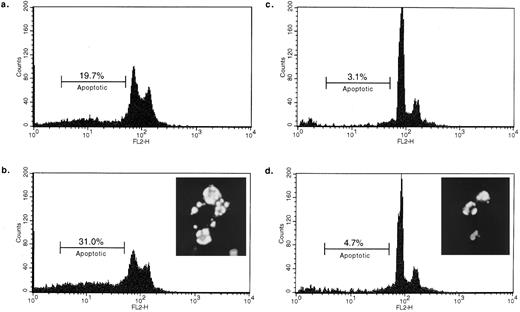
Fas− B cells are not susceptible to HLA class II–mediated apoptosis.The Fas− B-cell line was established from a patient with a lymphoproliferative autoimmune disorder characterized by the absence of Fas at the cell surface.6 We have verified that these cells are equally positive for HLA class II expression as normal activated human B cells (data not shown). Incubation of Fas− cells with L227 XL for either 24 or 48 hours did not significantly increase the number of apoptotic nuclei (Fig 4c and d, respectively). The background level of dead cells was 3.7% for the Fas− cell line and 2.5% for the Burkitt's lymphoma–derived cell line RAJI, which was examined in parallel. After 48 hours, over 30% of apoptotic RAJI cells are detected (Fig 4b). We then confirmed that the Fas− cells can undergo apoptosis by incubating them with 50 nmol/L of the general kinase inhibitor staurosporine for 24 hours before staining with Hoescht 33342. The inset to Fig 4d demonstrates that Fas− cells are capable of apoptosis and that the morphology of the cells is indistinguishable from the morphology of apoptotic RAJI cells (Fig 4b, inset) after treatment with L227 MoAb. These data support the idea that HLA class II–mediated apoptosis requires Fas.
B-lymphocyte line lacking Fas is not susceptible to HLA class II–mediated death. 0.5 × 106 cells were incubated for 24 hours (a, RAJI; c, Fas−) or 48 hours (b, RAJI; d, Fas−) with L227 XL before quantifying the apoptotic nuclei. EBV-transformed Burkitt's lymphoma cell line RAJI was compared with the EBV-transformed Fas− cells. The background level of apoptotic nuclei in nonstimulated cells was 2.5% for RAJI and 3.7% for Fas−. Insets showing the morphology typical of apoptosis depict either Hoescht 33342–stained RAJI incubated for 48 hours with L227 (b) or Hoescht 33342–stained Fas− incubated for 24 hours with 50 ng/mL staurosporine (d).
B-lymphocyte line lacking Fas is not susceptible to HLA class II–mediated death. 0.5 × 106 cells were incubated for 24 hours (a, RAJI; c, Fas−) or 48 hours (b, RAJI; d, Fas−) with L227 XL before quantifying the apoptotic nuclei. EBV-transformed Burkitt's lymphoma cell line RAJI was compared with the EBV-transformed Fas− cells. The background level of apoptotic nuclei in nonstimulated cells was 2.5% for RAJI and 3.7% for Fas−. Insets showing the morphology typical of apoptosis depict either Hoescht 33342–stained RAJI incubated for 48 hours with L227 (b) or Hoescht 33342–stained Fas− incubated for 24 hours with 50 ng/mL staurosporine (d).
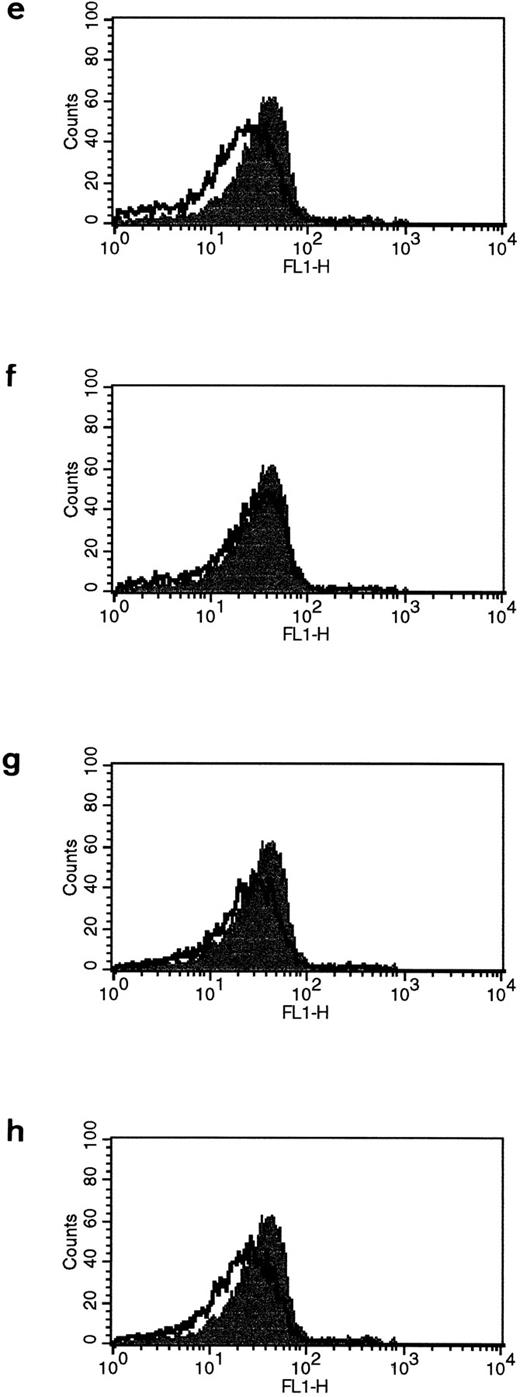
Fas− B cells express FasI.It was verified that Fas− cells express FasL after HLA class II stimulation by FACS staining using the monoclonal anti-FasL AL98. Interestingly, only L227 XL induced FasL expression in Fas− cells (Fig 5a). L227 alone (Fig 5b) did not induce significant FasL expression. The control in Fig 4c shows the goat antimouse F(ab′)2 alone, and Fig 5d is the W6.32 XL control antibody. Lysates were prepared from Fas− cells under nonreducing conditions, and immunoblotting was performed using the PE62 antibody (Fig 5e: lane 1, nonstimulated; lane 2, L227 XL; and lane 3, the antibody control W6.32 XL). Similar to the human primary B lymphocytes, stimulation via HLA class II results in the expression of a novel 110-kD protein.
Fas− cells express FasL after strong cross-linking of HLA class II molecules. Fas− cells were incubated for 96 hours with (a) L227 XL, (b) L227, (c) goat antimouse F(ab′)2 , or (d) W6.32 XL. The level of expression of nonstimulated cells is also shown as an unfilled profile. FACS profiles were obtained according to the method used in Fig 1a to d. (e) FasL detection after SDS-PAGE in nondenaturing conditions as a 110-kD trimer in lane ii. Lane 1, nonstimulated; lane 2, L227 XL; lane 3, W6.32 XL.
Fas− cells express FasL after strong cross-linking of HLA class II molecules. Fas− cells were incubated for 96 hours with (a) L227 XL, (b) L227, (c) goat antimouse F(ab′)2 , or (d) W6.32 XL. The level of expression of nonstimulated cells is also shown as an unfilled profile. FACS profiles were obtained according to the method used in Fig 1a to d. (e) FasL detection after SDS-PAGE in nondenaturing conditions as a 110-kD trimer in lane ii. Lane 1, nonstimulated; lane 2, L227 XL; lane 3, W6.32 XL.
Fas-induced apoptosis is increased after HLA class II stimulation.Having demonstrated that HLA class II signals stimulate the expression of FasL, we continued our study by examining the effect of HLA class II signaling on apoptosis via the Fas molecule. HLA class II molecules were cross-linked for 24 or 96 hours with 5 μg/mL L227 without cross-linking secondary antibody, to avoid any possible cross-reactivity with the anti-Fas MoAb, and anti-Fas MoAb was added for the final 24 hours of incubation. Fas does not significantly induce apoptosis in normal, resting B cells (Fig 6). Following pretreatment with anti–HLA class II MoAb or TSST-1, the level of Fas-mediated apoptosis was significantly increased by over 12% (after 96 hours' treatment with L227, P < .001, n = 3). On the other hand, PMA and ionomycin treatment did not increase sensitivity to anti-Fas–mediated death (after 96 hours' incubation, P = .515, n = 3), even though it did induce splenic B-cell proliferation (data not shown). The Fas molecule was expressed on 98% of the B lymphocytes, and the level of expression was not significantly increased by HLA class II stimulation (data not shown). These data suggest that activation via HLA class II primes resting B lymphocytes for apoptosis via Fas.
Stimulation of B lymphocytes via HLA class II increases sensitivity to Fas-induced apoptosis. Splenic B lymphocytes (0.5 × 106) were incubated for 24 or 96 hours with either L227 XL, biotinylated TSST-1 cross-linked with streptavidin, or PMA ionomycin. 1 μg/mL anti-Fas MoAb was added 24 hours before treatment of the cells for FACS analysis. Bars correspond to the standard error for each time point.
Stimulation of B lymphocytes via HLA class II increases sensitivity to Fas-induced apoptosis. Splenic B lymphocytes (0.5 × 106) were incubated for 24 or 96 hours with either L227 XL, biotinylated TSST-1 cross-linked with streptavidin, or PMA ionomycin. 1 μg/mL anti-Fas MoAb was added 24 hours before treatment of the cells for FACS analysis. Bars correspond to the standard error for each time point.
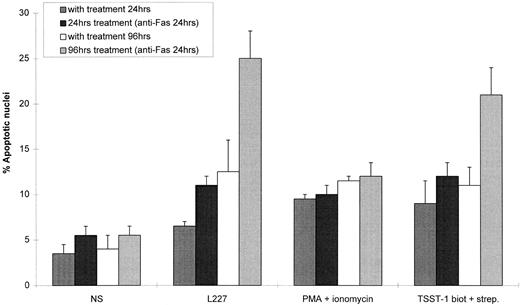
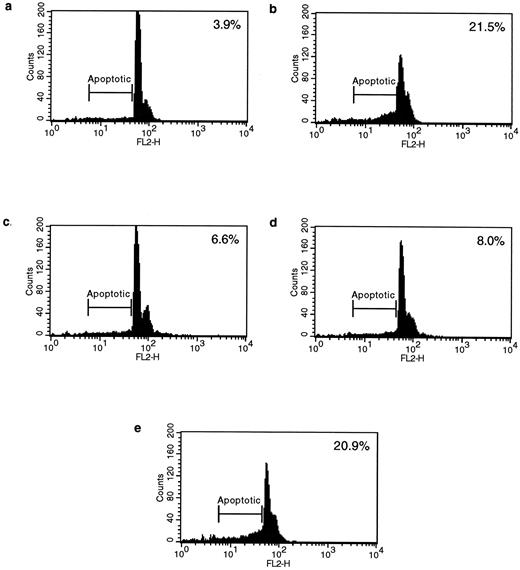
Blocking Fas/FasL interactions inhibits HLA class II–mediated apoptosis.To verify the hypothesis that Fas and FasL are involved in HLA class II–mediated apoptosis, we examined the ability of either an antagonistic anti-Fas or an anti-FasL antibody to inhibit HLA class II–mediated apoptosis. Splenic B cells were stimulated via HLA class II for 48 hours, followed by a further 48 hours in the presence of either the antagonistic anti-Fas MoAb ZB4 or the anti-FasL antibody PE62. Although ZB4 is an isotype of IgG and does not induce apoptosis, it does inhibit efficient cross-linking and subsequent signaling of the Fas molecule, whereas the FasL-specific PE62 would also inhibit any FasL/Fas interactions. Of the nuclei, 21.5% were apoptotic after stimulation via HLA class II for 96 hours, compared with only 3.9% in the nonstimulated cells (Fig 7a and b). However, stimulation via HLA class II in the presence of either PE62 or ZB4 significantly reduced the number of apoptotic nuclei to 6.6% and 8.0%, respectively (P < .001, n = 3; Fig 7c and d). Finally, stimulation via HLA class II in the presence of an MoAb directed against CD19 failed to inhibit the degree of apoptosis via HLA class II (Fig 7e). These data strongly support the idea that HLA class II–mediated cell death involves Fas and FasL.
HLA class II–induced apoptosis is mediated via Fas and FasL interactions. Anti-Fas and anti-FasL antibodies significantly inhibited HLA class II–mediated apoptosis. 0.5 × 106 splenic B lymphocytes were either left untreated for 96 hours or incubated for 96 hours with L227 XL (b) and for 48 hours with L227 XL with the addition of either 5 μg/mL anti-FasL antibody PE62 (c), 10 μg/mL antagonistic anti-Fas MoAb ZB4 (d), or 10 μg/mL control MoAb anti-CD19 (e) for a further 48 hours. 10,000 events were counted by FACS for each experiment.
HLA class II–induced apoptosis is mediated via Fas and FasL interactions. Anti-Fas and anti-FasL antibodies significantly inhibited HLA class II–mediated apoptosis. 0.5 × 106 splenic B lymphocytes were either left untreated for 96 hours or incubated for 96 hours with L227 XL (b) and for 48 hours with L227 XL with the addition of either 5 μg/mL anti-FasL antibody PE62 (c), 10 μg/mL antagonistic anti-Fas MoAb ZB4 (d), or 10 μg/mL control MoAb anti-CD19 (e) for a further 48 hours. 10,000 events were counted by FACS for each experiment.
DISCUSSION
Signal transduction via HLA class II molecules on B lymphocytes can lead to either activation or apoptosis. AICD in T lymphocytes is mediated by Fas/FasL interactions,36-38 but a mechanism for AICD in B lymphocytes has not yet been determined, although receptor-triggered pathways do induce apoptosis of activated B lymphocytes.2 The data presented herein support the idea that Fas/FasL interaction is a major mechanism of apoptosis in B lymphocytes and that this mechanism is mediated after signal transduction via HLA class II molecules.
Although FasL expression in the immune system was initially believed to be restricted to T lymphocytes,45 recent studies have detected FasL in a wide variety of cell types, including hepatocytes (in patients with alcoholic liver damage), retina, and testis.46-48 FasL has also been detected in certain natural killer cell lymphomas, in T-cell large granular lymphocyte leukemias, in dendritic cells, and in a colon cancer cell line.26,49,50 FasL mRNA was initially described as absent from the murine B-cell lineage following studies in a B lymphoma and in a plasmacytoma,51 although it has since been detected on primary activated B cells.52 This report is the first description of induction of FasL on human primary B cells stimulated with either a phorbol ester (PMA) and a calcium ionophore (ionomycin) or via HLA class II molecules. It is noteworthy that cross-linking of HLA class II molecules leads to induction of an intracellular calcium flux and activation of PKC18,53 in primary B lymphocytes. Induction of FasL via sIgM has not been detected in B-cell lymphomas, even though apoptosis was induced via sIgM.51 Interestingly, our preliminary studies of sIgM stimulation of primary human B cells failed to detect any induction of expression of FasL (results not shown), and stimulation of murine B lymphocytes via sIgM has actually been described as a means of providing protection from Fas-mediated apoptosis.54 Since previous studies on FasL expression in B cells have concentrated on stimulation via sIgM, we conclude that this model is sufficiently different from HLA class II stimulation of primary B lymphocytes as to preclude useful comparison.
The different separation techniques (either positive or negative selection) used to isolate B lymphocytes yielded cells that responded identically after stimulation. Although apoptosis via HLA class II was consistently detected in approximately 20% of the primary B-cell population, this is probably an underestimate, since the degradation of apoptotic bodies cannot be estimated and since the degradation products of apoptotic bodies are dismissed as debris. The level of apoptosis induced in primary B cells via HLA class II corresponds to levels previously reported after cross-linking of CD19, CD22, or IgM.55 Our data suggest that HLA class II signaling has a direct role in the homeostasis of lymphocyte populations, and that Fas/FasL interactions are crucial to this role. In a physiologic situation, this influence could be exercised following interaction of the TCR with HLA class II. We propose that signaling via HLA class II first leads to an increased sensitivity of the B cell to Fas-mediated apoptosis and a concomitant upregulation of FasL. The increased sensitivity to Fas may be a result of the second messengers induced via HLA class II molecules throughout B-cell ontogeny. After a minimum of 72 hours, the B cells can terminate their response by apoptosis. It is increasingly clear that successful termination of an immune response is as important as its induction in order to prevent aberrant proliferation or autoimmune responses.
Bacterial superantigens have been shown to activate B cells in either a costimulatory or direct fashion.14 15 The observed induction of FasL after stimulation with TSST-1 reveals a role for HLA class II–mediated apoptosis in pathologic situations, and underlines its importance in superantigen-mediated disease (eg, Kawasaki disease). Furthermore, these data support the idea that bacterial superantigens could initiate the depletion of activated B cells.
A combination of FasL expression and cellular activation suggests that the time course of susceptibility to HLA class II–mediated apoptosis is tightly regulated. We cannot distinguish whether apoptosis via Fas/FasL occurs on the same B cell or whether homotypic cell-cell interactions are required. HLA class II signaling leads to rapid aggregation of B cells in a homotypic or heterotypic fashion,15 which could theoretically encourage Fas/FasL interactions between cells. Similar to TNF, matrix metalloprotease action cleaves the membrane-bound FasL to produce the soluble trimeric form.26,39 Furthermore, the description of a secreted FasL that induces apoptosis increases the possibility that FasL interacts with Fas on the secreting cell.39 We have not distinguished between the potential roles of membrane-bound or secreted FasL in this study. However, the immunoblotting experiments in this study suggest that the trimeric form of soluble FasL is implicated in HLA class II–mediated apoptosis.
The dynamics of the interactions between Fas and FasL could be as follows. In the first instance, T-cell/B-cell interactions would principally have a role in activation, and the increased expression of FasL following activation would allow trimerization of Fas and subsequent cell death. Fas-mediated signals on already activated cells would therefore lead to apoptosis rather than to further activation. It has been demonstrated that activated T cells become sensitive to Fas-mediated death over a period of about 6 days.34 This limits the time in which the T lymphocyte can react to stimulation. Similarly, the results presented herein suggest that from day 3 after HLA class II signaling, the Fas molecule at the B-cell surface begins to signal for death (Fig 6). Dead cells expressing FasL become more abundant after this time point, reflecting the corresponding increase in Fas sensitivity (Fig 3). The initial levels of FasL are lower than in the live B cells, which is probably due to the delay in FasL induction and Fas sensitivity. These data suggest that the role of FasL in B lymphocytes may be important in the control of B-lymphocyte populations, but do not discount the possibility of B-lymphocyte–mediated death of other Fas-expressing cells. These data also indicate that the phenomenon of AICD, which has previously been attributed to T cells, is equally a B-cell phenomenon.
A number of secondary messengers have been identified after HLA class II–mediated signaling, but the signaling pathway from Fas to death remains unclear.35 Since PMA and ionomycin mediate the expression of mRNA transcripts of FasL, we suggest that the intracellular calcium flux and the activation of PKC, which have previously been described in the context of HLA class II–mediated activation,18,53 are also important for PCD in our model.56 However, PMA and ionomycin treatment did not increase the sensitivity to apoptosis via Fas, whereas this has been shown to induce FasL expression in cytotoxic T cells.41
A recent study describes the lysis of Fas-bearing target cells by activated FasL-bearing murine B cells, and therefore demonstrates that B cells have a role in cell-mediated cytotoxicity.52 The induction of FasL and mediation of death via Fas strongly suggest that HLA class II signaling could contribute to a form of B-lymphocyte–mediated cytotoxicity. It is clear that T cells become susceptible to Fas-mediated apoptosis after activation, but their contact with activated B cells could also cause death. This would provide yet another means of control of the immune response to a foreign antigen, in this case, a “double killing.” This may also be useful in the limitation of autoimmune responses, since T-cell recognition of a self antigen presented by an activated B cell could ultimately lead to the death of both lymphocytes. The recent observation of FasL on a subpopulation of dendritic cells is pertinent to this notion.49
The question of B-cell differentiation into the memory subtype has not been addressed in the present studies. The signals needed for their differentiation and how they escape the time limit imposed on the other B cells merits further study, since this may also provide clues to the mechanisms of B-cell transformation.
In summary, interaction with HLA class II molecules is one method by which human B cells can acquire FasL, and thereby participate in their homeostasis. Further studies will be required to understand the control of activation and apoptosis by antigen presentation.
Supported in part by the Association pour la recherche contre le cancer and the Ligue Contre le cancer (comité de Paris).
Address reprint requests to Nuala Mooney, PhD, INSERM U396, 15 rue de l'Ecole de Médecine, 75006 Paris, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal