Abstract
Recent evidence has supported the hypothesis that chemotherapeutic drugs and radiation induce an apoptotic pathway that requires the active participation of the cell. One pathway of apoptosis in malignant lymphoid cells is mediated by the Fas antigen. We studied the human myeloma (8226) and T-cell leukemia (CEM) cell lines selected for resistance to the anthracenes, doxorubicin or mitoxantrone, by continuous culture in the presence of either agent. We found that these drug-resistant cell lines were also resistant to Fas-mediated apoptosis in a dose-dependent manner. The degree of resistance to Fas-mediated apoptosis correlated directly with the level of resistance to chemotherapeutic drugs. These observations indicate that, as cancer cell lines develop mechanisms of drug resistance, they may also develop mechanisms of resistance to physiologic signals of apoptosis. Two mechanisms of resistance to Fas-mediated apoptosis were observed in these cell lines. One mechanism was associated with a dose-dependent reduction in the surface expression of Fas antigen. Analysis of RNA by reverse transcriptase-polymerase chain reaction assays showed that the reduction of Fas antigen expression occurred at the level of transcription. A second mechanism of drug resistance showed no decrease of Fas antigen expression; however, the apoptotic response was diminished. In this situation, removal of the chemotherapeutic agent resulted in a partial reversion to chemosensitivity and re-expression of the Fas antigen, but these cell lines did not regain the ability to undergo apoptosis in response to cross-linking by anti-Fas antibody. These findings support the hypothesis that apoptosis mediated by both chemotherapeutic agents and physiologic stimuli may share a common downstream effector. The demonstration that selection for drug resistance in hematopoietic cell lines results in a simultaneous resistance to Fas-mediated apoptosis may have clinical implications in the development of strategies for the treatment of resistant disease. Further analysis of the molecular mechanisms of Fas expression and function will facilitate the design of biological response modifying agents for the treatment of malignancy.
CHEMOTHERAPEUTIC agents commonly used in the treatment of both solid tumors and hematopoietic disease have been shown to induce apoptosis in target cells.1-3 However, cancers that initially respond to standard chemotherapeutic agents often relapse, with the selective outgrowth of a subpopulation of tumor cells that are resistant to further treatment. These tumors frequently display resistance not only to the specific agent initially used in treatment, but also to a variety of structurally and functionally unrelated agents. This multidrug-resistant phenotype is particularly prevalent in hematopoietic diseases and has been shown, in many cases, to be mediated by the expression of the ATP-dependent efflux pump P-glycoprotein (P-gp).4-6 P-gp has been shown to be induced by treatment with anthracenes both in vitro7 and in patient populations.8 Drug resistance mediated by P-gp is thought to be primarily due to its ability to transport cytotoxic agents out of the cell, resulting in reduced intracellular concentrations of drug and failure of the drug to reach an intracellular target.9 However, the recent identification of MRP, LRP, and glutathione S-transferase, among others, as mechanisms of drug resistance underscores the diversity of a tumor's adaptive response to cytotoxic stimuli.10-12 Thus, selection for resistance to one agent may co-select for characteristics influencing the tumor cell's response to apparently unrelated cytotoxic signals.
The Fas antigen, also known as Apo-1/CD95, is a cell surface protein that has been shown to initiate a signal for apoptosis when engaged by specific ligand or antibody. Programmed cell death induced by Fas/Fas ligand interactions has been implicated in the elimination of chronically activated lymphocytes in peripheral circulation and in the killing of tumor targets by immune cytotoxic effector cells.13,14 This 45-kD transmembrane receptor is structurally related to the tumor necrosis factor receptor (TNF-R) family, characterized by a cysteine rich extracellular region and a short, highly conserved cytoplasmic domain.15,16 This cytoplasmic region, known as the death domain, has been shown to be critical for transducing an apoptotic signal when the Fas antigen is cross-linked by ligand or specific antibody.17,18 Several recent reports have identified cytoplasmic proteins that interact with the death domain, including MORT1/FADD,19,20 and MACH/FLICE,21,22 forming a death-inducing signaling complex.23 These proteins participate in a proteolytic cascade leading to the activation of cysteine proteases that are central to programmed cell death induced by the Fas antigen and other members of the TNF-R family.24 25
Previous reports have shown that myeloma cell lines and myeloma patient cells express the Fas antigen. However, only some of them are sensitive to Fas-mediated apoptosis.26,27 We have previously developed and characterized human myeloma cell lines that are resistant to various cytotoxic agents.7 28 We report here that these cell lines selected for resistance to anthracene-type agents are also resistant to apoptosis induced by cross-linking of the Fas antigen with specific antibody.
The Fas ligand has recently been implicated as a mechanisms of apoptosis in the cytotoxicity of doxorubicin.29 Friesen et al29 showed that treatment of drug-sensitive cell lines with physiologic doses of doxorubicin induced the expression of the Fas ligand. Inhibition of protein synthesis with cyclohexamide or cyclosporin A abrogated the cytotoxic activity of the drug, as did blocking with anti-Fas antibody fragments. These data would suggest that signal transduction through the Fas/Fas ligand pathway may be involved in doxorubicin-mediated apoptosis.
We believe our data to be the first to demonstrate that cross-resistance between cytotoxic drugs and Fas-induced apoptosis is mediated by at least two distinct mechanisms involving the Fas antigen. One is a dose-dependent suppression of Fas antigen expression, which occurs during selection for resistance to high levels of doxorubicin or mitoxantrone. The other mechanism, seen with the removal of the selection agent and partial reversal to drug sensitivity, likely involves a downstream event that is common between Fas-mediated and drug-induced apoptosis.
MATERIALS AND METHODS
Cell lines.The human myeloma cell line RPMI 8226 and CEM, a human T-cell leukemia cell line, were obtained from the American Type Culture Collection (Rockville, MD). All cells were grown in suspension in RPMI 1640 media supplemented with 5% heat-inactivated fetal bovine serum, 1% pen/strep, and 100 mmol/L L-glutamine (Gemini, Calabasas, CA). Drug-resistant cell lines were established as previously described.7 28
Apoptosis assay.All assays were performed on cells that had been maintained in drug-free medium for 3 days and fed an equal volume of fresh medium on the day before analysis to ensure log phase growth. Cells were plated at 5 × 105/mL in 96-well plates with 200 μL of media. Anti-Fas antibody (murine IgM clone CH-11; MBL [Nagoya, Japan] or UBI [Lake Placid, NY]) or whole mouse control serum was added at the indicated concentrations. After incubation at 37°C, apoptotic cells were stained with 7-amino-actinomycin D (7-AAD; Calbiochem, La Jolla, CA) at a concentration of 20 μg/mL for 20 minutes on ice in the dark. Samples were directly measured on a FACScan flow cytometer and 7-AAD uptake by apoptotic cells analyzed with LYSYS II software (Becton Dickinson, Mountain View, CA). The TUNEL assay was used in selected experiments to verify that cell death was occurring via an apoptotic pathway. Briefly, anti-Fas and control serum-treated cells were fixed in 1% formaldehyde and labeled with biotin-d-UTP by terminal deoxynucleotidyl transferase. They were then incubated with avidin-fluorescein isothiocyanate (FITC) and stained with propidium iodide (PI) before analysis by flow cytometry. Two-channel analysis of FITC and PI staining indicated the presence of end-labeled DNA fragments and the characteristic sub-G1 population of cells (data not shown).
Surface expression.Cell surface expression of the Fas antigen was measured by flow cytometry using UB2 (MBL), a murine IgG anti-Fas antibody that does not induce apoptosis in target cells. One million cells were preincubated with 50 μL of 0.2 mg/mL mouse Ig serum (Sigma, St Louis, MO) for 10 minutes at room temperature. IgG1 isotype control serum (Becton Dickinson) or anti-Fas (UB2) was then added and incubated for an additional 30 minutes at room temperature. Cells were washed once in phosphate-buffered saline and reacted with FITC-goat antimouse F(ab′ )2 (Dako, Carpinteria, CA) for 20 minutes at 4°C, at which time they were washed twice in phosphate-buffered saline and held at 4°C in the dark until analysis by flow cytometry as described above.
RNA extraction and polymerase chain reaction (PCR). Total RNA was extracted from 10 × 106 cells in log growth phase by lysis in guanidine isothiocyanate followed by cesium chloride density centrifugation and ethanol precipitation. PCR primers (Biosynthesis, Lewisville, TX) used were 5′-(CAAGGTTCTCATGAATCTCCAAC) and 5′-(GAAATCCAAAGCTTGGTCTAGAG). One hundred nanograms of RNA was reverse transcribed and a 392-bp fragment of the Fas antigen was amplified with 29 cycles of PCR in a 9600 Perkin Elmer Thermocycler (Foster City, CA). This was within the exponential range of amplification as determined by serial cycle analysis (data not shown). Conditions used were as follows: 60 seconds at 94°C; denaturing for 15 seconds at 94°C; annealing for 15 seconds at 52°C; extension for 15 seconds at 72°C; and final extension for 60 seconds at 72°C. After amplification, 10% of the PCR products were separated on a 5% nondenaturing acrylamide gel, and the incorporated radioactivity was quantitated on a Molecular Dynamics phosphorimager using ImageQuant software (Sunnyvale CA). The identity of the product was established by restriction digest and direct sequencing. A 201-bp fragment of Histone 3.3 was amplified as a control for RNA integrity and quantitation.
RESULTS
Cross-linking by the anti-Fas antibody induces cell death in 8226 myeloma cells.To determine the sensitivity of drug-resistant myeloma cells to Fas-induced apoptosis, the 8226 human myeloma cell line was treated with the mouse IgM anti-Fas antibody CH-11 for 16 to 18 hours and the percentage of apoptotic cells was analyzed by 7-AAD staining and flow cytometry. Treatment of the parental, drug-sensitive cell line, 8226/S, anti-Fas antibody induced apoptosis in 60% of cells (Fig 1). In contrast, treatment of the doxorubicin-resistant subclone, 8226/Dox40 showed only 12% apoptotic response of the cells induced by anti-Fas antibody (Fig 1). To verify that cell death was occurring by an apoptotic pathway, cell lines treated with anti-Fas antibody were fixed and labeled with biotin-d-UTP followed by avidin-FITC and propidium iodide counterstain. Two-channel flow cytometry analysis showed a corresponding quantity of end-labeled DNA fragments and the characteristic sub-G1 population of cells undergoing programmed cell death (data not shown).
Apoptosis induced by anti-Fas (CH-11). Cell lines were treated with 200 ng/mL CH-11 for 16 to 18 hours at 37°C, stained with 20 μg/mL 7-AAD, and analyzed by flow cytometry. Apoptotic cells are 7-AADbright, whereas viable cells are 7-AADdim.
Apoptosis induced by anti-Fas (CH-11). Cell lines were treated with 200 ng/mL CH-11 for 16 to 18 hours at 37°C, stained with 20 μg/mL 7-AAD, and analyzed by flow cytometry. Apoptotic cells are 7-AADbright, whereas viable cells are 7-AADdim.
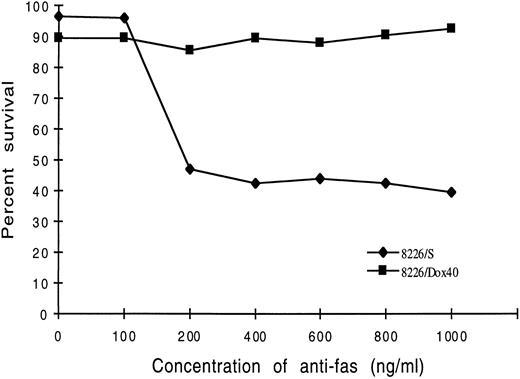
Dose-response analysis of 8226/S cells indicated that the minimum concentration of Fas antibody required for apoptosis was 200 ng/mL. This appears to be a saturable dose for this cell line, with no significant increase in cell death at higher concentrations of antibody. Similarly, the doxorubicin-resistant cell line, 8226/Dox40, did not respond to antibody concentrations up to 1,000 ng/mL (Fig 2). Extended exposure to the anti-Fas antibody for time periods up to 48 hours also did not appreciably effect the number of cells induced to undergo apoptosis (data not shown).
Dose-response of 8226/S and 8226/Dox40 myeloma cells with the anti-Fas antibody CH-11. 8226/S or 8226/Dox40 cells were incubated with the indicated dose of CH-11 antibody for 16 hours at 37°C. Cell death was assayed by flow cytometry of 7-AAD–stained cells, and the percentage of apoptotic cells (7-AADbright) was quantitated using LYSYS II software. Values are Fas-induced apoptosis minus nonspecific cell death. Each point represents the average of at least three independent trials.
Dose-response of 8226/S and 8226/Dox40 myeloma cells with the anti-Fas antibody CH-11. 8226/S or 8226/Dox40 cells were incubated with the indicated dose of CH-11 antibody for 16 hours at 37°C. Cell death was assayed by flow cytometry of 7-AAD–stained cells, and the percentage of apoptotic cells (7-AADbright) was quantitated using LYSYS II software. Values are Fas-induced apoptosis minus nonspecific cell death. Each point represents the average of at least three independent trials.
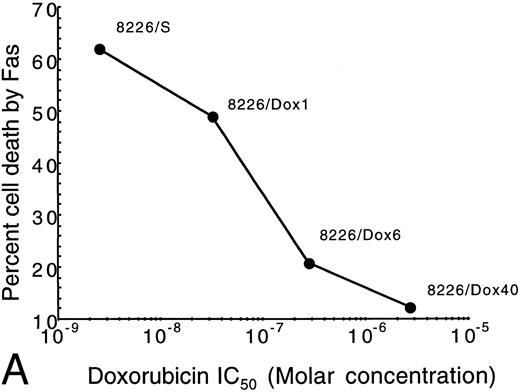
Resistance to Fas-induced apoptosis correlates with the degree of resistance to doxorubicin and mitoxantrone.To determine whether resistance to Fas-mediated apoptosis correlated with the degree of drug resistance, a panel of cells with varying degrees of resistance to doxorubicin was tested (Table 1). Sensitivity to drugs was determined by MTT assay, as previously described.30 The 8226 myeloma cell lines were incubated with anti-Fas antibody CH-11 for 18 hours at 37°C and analyzed for apoptosis by 7-AAD staining (Fig 3A). Fas-mediated apoptosis in the 8226/Dox1 cell line, which shows a 13-fold resistance as compared with the parental 8226/S, showed a 23% decrease in response to Fas-mediated apoptosis (Table 1). 8226/Dox6, which is 110-fold resistant to doxorubicin, showed a 75% reduction in cell death induced by anti-Fas antibody. The highly resistant 8226/Dox40, which is 1,350-fold resistance to doxorubicin, showed 80% reduction in Fas-induced cell death as compared with the parental cell line. In all doxorubicin cell lines tested, the degree of resistance to anti-Fas antibody-mediated apoptosis directly correlated with the IC50 of the drug-resistant cell line (Fig 3A).
Degree of Resistance to Cytotoxic Drugs and Fas-Mediated Apoptosis
| Cell Line . | IC50 of Selecting Agent (mol/L) . | DOR to . | % Fas-Induced . | % Decrease . |
|---|---|---|---|---|
| . | . | Drug . | Cell Death . | in Apoptosis . |
| 8226/S | Dox 2.5 × 10−9 | 1 | 62 | |
| MR 9.7 × 10−8 | ||||
| 8226/Dox1 | 3.2 × 10−8 | 13 | 49 | 21 |
| 8226/Dox6 | 2.8 × 10−7 | 110 | 21 | 66 |
| 8226/Dox40 | 2.7 × 10−6 | 1,350 | 12 | 81 |
| 8226/Dox40ood | 4.5 × 10−7 | 180 | 18 | 71 |
| 8226/MR4 | 1.0 × 10−6 | 11 | 42 | 32 |
| 8226/MR20 | 3.6 × 10−6 | 37 | 19 | 69 |
| 8226/MR20ood | 2.8 × 10−6 | 30 | 21 | 66 |
| CEM/S | 3.2 × 10−8 | 1 | 96 | |
| CEM/Dox1-F5 | 2.9 × 10−9 | 3 | 72 | 25 |
| Cell Line . | IC50 of Selecting Agent (mol/L) . | DOR to . | % Fas-Induced . | % Decrease . |
|---|---|---|---|---|
| . | . | Drug . | Cell Death . | in Apoptosis . |
| 8226/S | Dox 2.5 × 10−9 | 1 | 62 | |
| MR 9.7 × 10−8 | ||||
| 8226/Dox1 | 3.2 × 10−8 | 13 | 49 | 21 |
| 8226/Dox6 | 2.8 × 10−7 | 110 | 21 | 66 |
| 8226/Dox40 | 2.7 × 10−6 | 1,350 | 12 | 81 |
| 8226/Dox40ood | 4.5 × 10−7 | 180 | 18 | 71 |
| 8226/MR4 | 1.0 × 10−6 | 11 | 42 | 32 |
| 8226/MR20 | 3.6 × 10−6 | 37 | 19 | 69 |
| 8226/MR20ood | 2.8 × 10−6 | 30 | 21 | 66 |
| CEM/S | 3.2 × 10−8 | 1 | 96 | |
| CEM/Dox1-F5 | 2.9 × 10−9 | 3 | 72 | 25 |
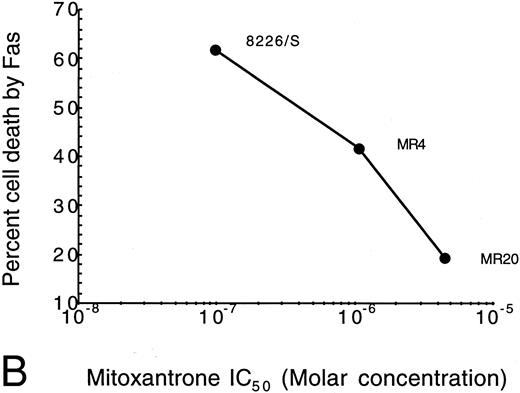
Correlation of drug resistance with Fas-mediated resistance as determined by IC50 for drug- versus Fas-induced cell death. The percentage of cell death induced by anti-Fas was assayed by flow cytometry analysis of 7-AAD cells. IC50 of the selection agent is determined by the concentration that inhibits growth by 50% in a modified tetrazolium-based assay as previously described.30 (A) Doxorubicin-resistant cells (Dox); (B) Mitoxantrone-resistant cells (MR).
Correlation of drug resistance with Fas-mediated resistance as determined by IC50 for drug- versus Fas-induced cell death. The percentage of cell death induced by anti-Fas was assayed by flow cytometry analysis of 7-AAD cells. IC50 of the selection agent is determined by the concentration that inhibits growth by 50% in a modified tetrazolium-based assay as previously described.30 (A) Doxorubicin-resistant cells (Dox); (B) Mitoxantrone-resistant cells (MR).
Selection of 8226 myeloma cells with mitoxantrone results in a drug-resistant phenotype that is cross-resistant to doxorubicin by a non–P-gp, non-MRP mechanism.28 Similar to the doxorubicin-selected cell lines, mitoxantrone resistance correlates with resistance to Fas-mediated apoptosis in a dose-dependent manner (Fig 3B and Table 1), indicating that the mechanism of resistance to Fas-mediated apoptosis is not related to P-gp expression. We also tested a T-cell leukemia cell line, CEM, with low-level resistance to doxorubicin. The parent CEM cell line is highly sensitive to Fas-mediated apoptosis, demonstrating 96% cell death. Similar to the myeloma cells, the CEM/Dox1-F5 doxorubicin-selected cell line, which is threefold resistant to doxorubicin, displayed reduced sensitivity to Fas-mediated apoptosis, with only 72% cell death (Table 1).
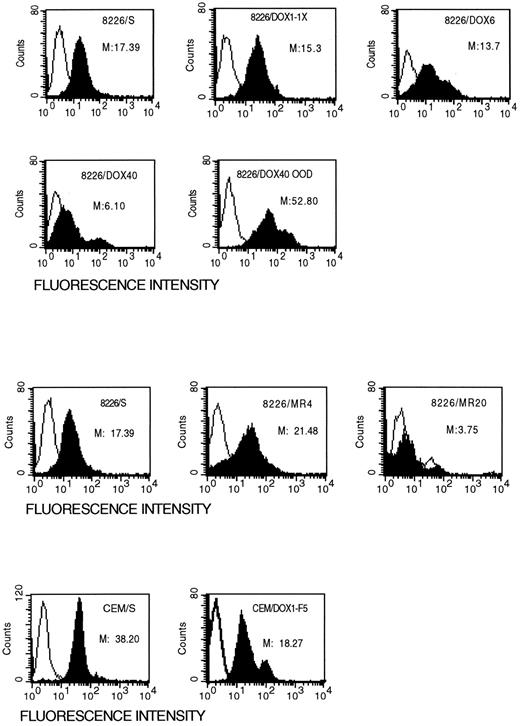
Expression of the Fas antigen is reduced in drug-resistant cell lines.To determine whether resistance to Fas-mediated apoptosis in the drug resistance cell lines is due to alterations in Fas expression or to downstream events in the signal transduction pathway, we examined the surface expression of the Fas antigen by flow cytometry. Drug-sensitive and drug-resistant cell lines were stained with the non–apoptosis-inducing antibody, UB2, and analyzed by flow cytometry. Cell lines selected for resistance to anthracenes appear to be more heterogeneic in Fas antigen surface expression as compared with the parental, drug-sensitive cell line (Fig 4). The median fluorescence intensity (MFI) of the low level resistant 8226/Dox1 cells is reduced to 15.3, compared with the parental 8226/S at 17.4. Surface expression of Fas antigen in 8226/Dox6 and 8226/Dox40 cell lines was reduced to 13.7 and 6.10, respectively. Although the majority of drug-resistant cells expressed less Fas antigen, there appears to be a small subpopulation of cells that express significantly more Fas on their surface than do the parental cells. However, in the drug-resistant cell lines, 15% of the 8226/Dox6 and 39% of the 8226/Dox40 cells appear to be entirely negative for Fas expression, compared with 4% in the parental 8226/S. This is true in the mitoxantrone-selected cell lines as well, in which the low-level resistant 8226/MR4 cell line actually displays a higher MFI than the parental, but 19% of the cells are Fas-negative, compared with 4% of the 8226/S. Selection at high doses of mitoxantrone in the 8226/MR20 cell line results in a dramatic reduction in the surface expression of the Fas antigen, with 59% of the cells displaying no Fas antigen. The doxorubicin-resistant T-cell leukemia cell line CEM/Dox1-F5 exhibited a similar pattern of Fas surface expression, with the majority of the cells expressing a lower level of Fas antigen on their surface, whereas a small percentage of the cells express levels of Fas antigen higher than the parental CEM/S. This subpopulation of cells that are Fas bright is likely to represent the population of cells that are induced to undergo apoptosis when treated with the anti-Fas antibody.
Surface expression of the Fas antigen in drug-sensitive and drug-resistant cell lines. Cells were incubated with the non–apoptosis-inducing monoclonal antibody, UB-2, and analyzed for surface expression by flow cytometry. The open peaks represent the isotype matched control; solid peaks are specific staining for Fas antigen. M, median fluorescence intensity.
Surface expression of the Fas antigen in drug-sensitive and drug-resistant cell lines. Cells were incubated with the non–apoptosis-inducing monoclonal antibody, UB-2, and analyzed for surface expression by flow cytometry. The open peaks represent the isotype matched control; solid peaks are specific staining for Fas antigen. M, median fluorescence intensity.
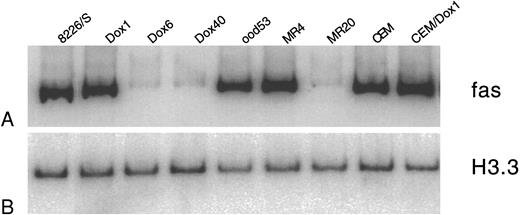
Suppression of Fas antigen expression in drug-resistant cell lines occurs at the level of mRNA transcription.To determine whether the suppression of Fas antigen expression in drug-resistant cell lines occurs at the level of transcription or translation, we examined the mRNA expression of the Fas antigen in drug-sensitive and -resistant cell lines (Fig 5).
Quantitative RT-PCR analysis of Fas antigen mRNA in myeloma cell lines. Total RNA was extracted from drug-sensitive and drug-resistant cell lines and analyzed for expression of Fas antigen RNA. One hundred nanograms was reverse transcribed, and a 392-bp fragment of the cytoplasmic region of the Fas antigen was amplified using 29 cycles of PCR. PCR products were labeled by the incorporation of α 32P-dCTP in the amplification reaction. (A) Ten percent of the Fas antigen PCR products were separated on a 5% acrylamide gel and quantitated by phosphorimage analysis using ImageQuant software. (B) A 201-bp fragment of histone 3.3 was amplified from the drug-sensitive and -resistant cDNA using 26 cycles of PCR. Lanes are as labeled above.
Quantitative RT-PCR analysis of Fas antigen mRNA in myeloma cell lines. Total RNA was extracted from drug-sensitive and drug-resistant cell lines and analyzed for expression of Fas antigen RNA. One hundred nanograms was reverse transcribed, and a 392-bp fragment of the cytoplasmic region of the Fas antigen was amplified using 29 cycles of PCR. PCR products were labeled by the incorporation of α 32P-dCTP in the amplification reaction. (A) Ten percent of the Fas antigen PCR products were separated on a 5% acrylamide gel and quantitated by phosphorimage analysis using ImageQuant software. (B) A 201-bp fragment of histone 3.3 was amplified from the drug-sensitive and -resistant cDNA using 26 cycles of PCR. Lanes are as labeled above.
The low-level resistant cell line 8226/Dox1 expressed equivalent amounts of Fas antigen mRNA as the parental 8226/S. In the 8226/Dox6 and 8226/Dox40 cell lines, Fas antigen mRNA expression is reduced by 92% and 94%, respectively. Selection with low levels of mitoxantrone (8226/MR4) resulted in an overall increase in Fas antigen mRNA expression by 37%, as compared with the parental cell line. This overall increase in mRNA is likely due to the subpopulation of 8226/MR4 cells shown by flow cytometry to display very high levels of Fas antigen on their surface. Fas antigen mRNA expression in the highly resistant 8226/MR20 is reduced by 72%, as was anticipated from the majority of the cells displaying reduced surface expression detected by flow analysis. In all cases, the level of mRNA expression correlated well with the level of Fas surface expression as measured by flow cytometry.
For the low-level doxorubicin-resistant CEM/Dox1-F5 cell line, Fas antigen mRNA was elevated by 59% compared with the sensitive parent cell line. Similar to the low-level resistant mitoxantrone and doxorubicin myeloma cell lines, this overall increase is likely attributed to the presence of a small subpopulation of cells that stain very brightly for surface protein as detected by flow cytometry analysis (Fig 4).
Removal of the selecting agent restores Fas antigen expression, but only partially restores the apoptotic response.To further determine whether the reduction in Fas antigen expression and function is a stable phenotype, we examined the expression and function of the Fas antigen in a derivative of 8226/Dox40 that was cultured in drug-free medium for 53 weeks (8226/Dox40ood53). This cell line retains 78% of P-gp expression found in the 8226/Dox40 cell line from which it was derived and is only 180-fold resistant to doxorubicin, compared with 1,350-fold resistance for 8226/Dox40. Treatment of the 8226/Dox40ood53 cell line with the apoptosis-inducing monoclonal antibody CH-11 resulted in only 18% of the cells undergoing apoptosis, a modest increase over the 12% seen in the drug-resistant parental cell line, 8226Dox40 (Table 1). Analysis of the 8226/Dox40ood53 cell line for surface expression of Fas antigen with the non–apoptosis-inducing antibody UB2 showed a highly heterogeneic population of cells with a threefold increase in the MFI compared with the drug-sensitive parent 8226/S (Fig 4). Reverse transcription-PCR (RT-PCR) analysis of the mRNA expression also indicated an overall increase of 39% over the parental cell lines. Similarly, 8226/MR20 maintained in drug-free medium for 48 weeks regained expression of Fas antigen to 60% over parental expression levels. These data suggest two possible mechanisms of resistance to Fas-mediated apoptosis associated with drug selection. Chronic selection with doxorubicin or mitoxantrone results in moderate to high levels of drug resistance and coselection of cells with reduced Fas antigen expression. In contrast, removal of the selecting agent results in partial reversion to drug sensitivity and a cell line that regains high expression of the Fas antigen; however, these cells do not fully recover their ability to undergo Fas-mediated apoptosis.
DISCUSSION
The Fas antigen is a 45-kD type I transmembrane receptor that is structurally related to the TNF-R family.15,31 Cross-linking of the Fas antigen by ligand or monoclonal antibody has been shown to initiate a signal transduction cascade leading to programmed cell death in susceptible cells. In many hematopoietic cells, expression of the Fas antigen can be rapidly induced by cytokines, antigenic activation, T-cell receptor engagement, or viral infection.32-34 These findings implicate the Fas/Fas ligand system as a mechanism of negative immune regulation. Fas-mediated apoptosis has also been shown to be one of two major effector pathways in the elimination of target cells by cytotoxic T cells.14,35,36 In transformed cells, Fas expression has shown a high degree of variability, depending on the tissue.34,37 Many lymphoid malignancies tend to demonstrate an oncofetal regulation in which the fetal cell type is Fas positive, adult normal cells are Fas negative, and tumor cells re-express Fas.37 This pattern of expression suggests that Fas-mediated apoptosis may be a promising strategy for elimination of lymphoid malignancies.
Previous studies have shown that, although many myeloma cells express Fas antigen on their surface, only some respond to an apoptotic signal when treated with the anti-Fas monoclonal antibody.26,27 Additionally, in several cell lines analyzed, Fas expression in plasma cells did not fully correlate with biologic response. For example, the myeloma cell line U266 expresses relatively high levels of the Fas antigen on its surface; however, treatment of this cell line with anti-Fas antibody does not induce the cells to undergo apoptosis (Westendorf et al26 and unpublished observations). A similar finding has been reported for freshly isolated plasma cells from myeloma patients.26,27 In a study by Westendorf et al,26 15 of 28 myeloma patients expressed Fas antigen. Shima et al27 reported cell surface Fas expression on all patient plasma cells except for one. However, not all patient samples identified as positive for Fas expression underwent apoptosis when challenged with cross-linking anti-Fas antibody.
In the present study, we used lymphoid malignant cell lines selected for resistance to anthracenes by continuous culture in the presence of these agents. We found that cell lines selected for resistance to doxorubicin or mitoxantrone were also resistant to Fas-mediated apoptosis in a dose-dependent manner. Resistance to Fas-mediated apoptosis is not related to the expression of P-gp, as shown by the resistance observed in mitoxantrone-selected cells, which have a non–P-gp mechanism of drug resistance. These observations would suggest that, as tumors develop mechanisms of drug resistance, they may also develop mechanisms of resistance to physiologic signals of apoptosis.
Friesen et al29 have recently reported that CEM and Jurkat cells selected for resistance to Fas-mediated apoptosis were also resistant to doxorubicin. In these experiments, drug cytotoxicity was associated with a rapid induction of Fas ligand expression by physiologic doses of doxorubicin in the Fas-sensitive cell line. Cell lines selected for Fas resistance by continuous exposure to cross-linking antibody showed a failure to express Fas ligand when treated with doxorubicin. These data were interpreted as indicating an autocrine or paracrine Fas/Fas ligand interaction in doxorubicin cytotoxicity, which could be blocked by inhibitors of protein synthesis. In our study, we find a similar pattern of cross-resistance between Fas-mediated apoptosis and doxorubicin cytotoxicity. However, in our drug-resistant cell lines, we find a decreased level of Fas antigen. Our data would substantiate a relationship between Fas-mediated apoptosis and drug-induced cell death. Future studies will examine the expression and function of Fas ligand as a mediator of drug resistance.
Our data suggest that two mechanisms of resistance to Fas-mediated apoptosis may be occurring under exposure to chemotherapeutic agents. When cells are chronically exposed to anthracene-type agents and become drug-resistant, we find a dose-related decrease in Fas antigen expression with a direct correlation to reduced Fas-mediated apoptosis. This regulation was found to occur at the level of mRNA transcription. In contrast, partial revertant cell lines regained Fas antigen expression, but did not respond to cross-linking with the Fas antibody. This suggests alterations in a downstream effector common to both Fas- and drug-mediated cytotoxicity. Both doxorubicin- and Fas-mediated cytotoxicity have been shown to be capable of activating the synthesis of ceramide as one mechanism of initiating an apoptotic pathway.38-40 Because ceramide represents a potential common effector in signal transduction leading to apoptosis, it is attractive to speculate that selection for resistance may involve an abrogation of the ceramide synthetic pathway. We are currently investigating the generation of ceramide in the low-level drug-resistant cell lines.
These observations may have profound clinical implications in the treatment of drug-resistant tumors. The induction of Fas-mediated apoptosis in vivo has been proposed as a potential strategy for anticancer therapy.41,42 Additionally, modulation of intrinsic cytotoxic lymphocyte (CTL) antitumor activity represents an opportunity for treatment of neoplastic diseases that have become refractory to conventional therapies.43 44 The present study suggests that, as tumors develop mechanisms of drug resistance, they also develop mechanisms of resistance to physiologic signals of apoptosis, particularly Fas-mediated apoptosis. The selective pressure applied by prolonged chemotherapeutic treatment may coselect for tumor cells that have lost the capacity to undergo apoptosis in response to a physiologic stimulus such as Fas-mediated apoptosis. Suppression of Fas antigen expression on tumor cells would render them resistant to CTL and avert any potential for an antitumor immune response. A fundamental understanding of the events contributing to resistance to apoptosis is critical to the development of effective strategies to circumvent drug resistance.
Address reprint requests to William S. Dalton, MD, PhD, H. Lee Moffit Research Center, University of South Florida, 12902 Magnolia Dr, Tampa, FL 33612.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal