Abstract
Infection of human erythrocytes with the malaria parasite Plasmodium falciparum induces many morphological and biochemical changes in the host cell. Host serine/threonine protein kinases could be involved in some of these processes. The aim of this study was to determine the effect of infection on red blood cell protein kinase C (PKC) and establish the importance of this enzyme in parasite growth and sexual stage differentiation. Phorbol myristate acetate (PMA)-induced translocation of erythrocyte PKC activity is impaired in erythrocytes enriched for mature asexual stage infected cells. Western blotting shows that this is due to a relative reduction in membrane PKC protein levels rather than inhibition of enzyme activity and analysis of PKC activity isolated from whole cell lysates by DE52 chromatography suggests that total activatable PKC levels are lower in infected erythrocytes. A reduction in PMA-induced activation is also observed in PKC assays performed in situ. Downregulation of erythrocyte PKC by overnight incubation with PMA before infection causes a significant decrease in the rate of the asexual growth, suggesting that the enzyme, although lost later in infection, may be important in the earlier development of the parasite. By contrast, the lack of PKC had no effect on the production of sexual stage parasites.
THE PHOSPHOLIPID-DEPENDENT protein kinase C (PKC) family enzymes are involved in many signal transduction systems, regulating responses to diverse external agents, including many hormones, growth factors, and cytokines.1,2 The classical isoenzymes require both Ca2+ and diacylglycerol (DAG) for full activity, while the novel and atypical PKCs depend, respectively, on DAG alone or neither.1,2 Activation of classical PKCs by DAG or the phorbol esters, such as phorbol myristate acetate (PMA) is accompanied by translocation from the cytosol to the membrane.3 The activated PKC phosphorylates specific substrates and initiates a cascade of kinase activation, culminating in a cellular response, such as cell division or differentiation.1 2
Erythrocytes cannot respond to external factors by altered protein synthesis or cell division, but they are sensitive to their environment, altering cell volume and rigidity following exposure to changes in osmotic pressure or shear stress.4,5 PKC has been implicated in both these responses6,7 and also in the process of red blood cell (RBC) senescence.8,9 Activated PKC phosphorylates many erythrocyte membrane and cytoskeletal proteins.7,10-18 Specific substrates identified include bands 4.9 and 4.1 and adducin.10-14 Phosphorylation of the latter affects subcellular location by altering associations with other proteins.18,19 PKC also regulates several erythrocyte membrane transport systems, including the glucose transporter,15 the transmembrane Ca2+ ATPase17 and the Na+/H+ exchanger.7
Infection of erythrocytes with the protozoan parasite, Plasmodium falciparum, is accompanied by many profound changes in the host cell. Membrane permeability to a wide range of organic and inorganic substrates, including amino acids, choline and Ca2+, is selectively increased (for review, see Elford et al20 ). Lipid composition21 and fluidity is altered and the cell loses its deformability and discoid shape.22 The external surface of the cell is also radically altered. As the infection progresses, the smooth surface of the cell becomes dotted with knob-like protusions, which are the location of expression of novel, parasite derived proteins, such as the Pfemp1 family of variant antigens,23-25 and modified host proteins, such as band 3.26 Inside the erythrocyte, several host proteins, including band 4.1, show increased phosphorylation.27-30 Parasite encoded kinases undoubtedly contribute to these changes and a number have now been identified.31-35 However, host cell kinases have been implicated in P falciparum invasion of erythrocytes36 and in the transport of parasite proteins, such as the ring-stage–infected erythrocyte surface antigen (RESA),37 38 to the surface of the RBC.
The similarity between the pattern of proteins phosphorylated during P falciparum infection and that induced by phorbol ester, as well as the involvement of PKC in erythrocyte functions known to be altered in infected cells, suggests a possible role for host PKC in the establishment of the parasite in the RBC. PMA can enhance P falciparum gametocytogenesis39 but no parasite PKC has been identified. Erythrocyte PKC may therefore regulate parasite development. The aims of this work were to investigate PKC in erythrocytes and measure the effect of infection with P falciparum on PKC activity and enzyme levels, and to determine whether this host cell enzyme is important to parasite survival and development.
MATERIALS AND METHODS
Materials.Unless otherwise stated, all materials were purchased from Sigma (Poole, UK). PKC assay reagents and all culture materials were purchased from Life Technologies (Paisley, UK). MC5 anti-PKC monoclonal antibody was purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). All other antibodies against PKC family members were obtained in a PKC Sampler Kit from Transduction Laboratories (Lexington, KY). Horse rabbit peroxidase conjugated goat antimouse antibody came from Biorad (Hemel Hempstead, UK). Enhanced chemiluminescence reagents and [γ-32P] adenosine triphosphate (ATP) were purchased from Amersham International (Amersham, UK).
Parasite culture.The 3D7 strain of P falciparum was cultured according to standard methods,40 in human A+ red blood cells in RPMI supplemented with 10% A+ human serum, 25 mmol/L HEPES, 2 mg/mL glucose, 50 mg/L hypoxanthine, 1 mol/L sodium bicarbonate. For each experiment, control uninfected RBCs were cultured under the same conditions. Parasites were grown to a parasitemia of 5% to 10% and trophozoite and schizont infected RBCs were enriched to 50% to 90% by Plasmagel (Bellon, Neuilly Sur Seine, France) flotation.41
Membrane translocation.Uninfected or enriched, infected RBC (5 × 108) were washed in RPMI and incubated at 37°C with 500 nmol/L PMA in RPMI for 15 minutes. Cells were fractionated by a modification of the method of Palfrey and Waseem.10 Briefly, cells were spun down at 4°C and washed once in phosphate-buffered saline (PBS), then lysed in Buffer A (1 mL 20 mmol/L Tris pH 7.5, 0.5 mmol/L EGTA, 0.5 mmol/L EDTA, 0.04% saponin, 25 mg/mL leupeptin, 25 mg/mL aprotinin, 2 mmol/L β-mercaptoethanol). The parasite pellet was removed by centrifugation for 10 minutes at 1,200g. A crude RBC membrane fraction was prepared by spinning at 18,000 rpm for 15 minutes. The pellet was washed three times in Buffer B (20 mm Tris pH 7.5, 0.5 mmol/L EGTA, 0.5 mmol/L EDTA, 2 mmol/L β-mercaptoethanol) and resuspended to a concentration of 1 to 2 mg/mL protein (concentration determined by the Bradford method) in Buffer B and assayed for kinase activity. No further purification is required for measurement of erythrocyte PKC activity prepared in this way.10 Parasite membrane and cytosol fractions were prepared as described.27
Total cell PKC preparation.RBC (2 × 108) were washed in PBS as described above, then lysed in 1 mL 20 mmol/L Tris pH 7.5, 0.5 mmol/L EGTA, 0.5 mmol/L EDTA, 0.5% Triton-X-100, 25 mg/mL leupeptin, 25 mg/mL aprotinin, 2 mmol/Lβ-mercaptoethanol. Insoluble material was removed by centrifugation at 13,000g for 10 minutes at 4°C. The supernatant was added to a 0.25-mL DE52 column and washed with Buffer B. Bound material was eluted with Buffer B including 200 mmol/L NaCl and assayed for kinase activity.
PKC assay.PKC activity was assayed using the specific substrate AcMBP.42 Samples (100 to 200 μg membrane protein or 10 μL DE52 eluate) were incubated with PMA/phosphatidylserine/Triton-X-100 mixed micelles for 20 minutes at room temperature. Negative controls contained no PMA/lipid mix and included the pseudosubstrate PKC inhibitor peptide PK19-36 at a concentration of 20 μmol/L. At this concentration, PKC19-36 is a highly specific inhibitor of classical PKC activity.41-43 Substrate solution (20 mmol/L Tris pH 7.5, 25 μmol/L AcMB,4-14 20 μmol/L [γ32P] ATP, 1 mmol/L CaCl2 , 20 mmol/L MgCl2 ) was added and the reaction stopped after 10 minutes at 30°C by spotting onto P81 paper. The paper was washed with 1% phosphoric acid and 32P incorporation determined by scintillation counting.
In situ PKC assay.Infected cells enriched as described above and uninfected cells were washed once with RPMI and resuspended in RPMI to a concentration of 1 × 108 cells/mL. Cells (2 × 107/well) were added to a 96-well ‘v’-bottomed microwell plate (Life Technologies, Paisley, UK). After stimulation with PMA, cells were spun at 1,200g for 3 minutes, washed once in PBS, and assayed immediately for PKC activity by the addition of 50 μL of 0.137 mol/L NaCl, 5.4 mmol/L KCl, 0.3 mmol/L Na2HPO4 , 0.4 mmol/L K2HPO4 , 1 mg/mL glucose, 20 mmol/L HEPES, 10 mmol/L MgCl2 , 25 mmol/L βglycerophosphate, 50 μg/mL digitonin, 100 μmol/L [γ32P]ATP (1 to 2,000 cpm/pmol), 5 mmol/L EGTA, 2.5 mmol/L CaCl2 , and 4 μmol/L substrate peptide, [Ser25]PK.19-31 [Ser25]PK19-31 is based on the PKC pseudosubstrate sequence, but is substituted with a phosphorylatable serine residue. As such, the peptide is a highly selective substrate for PKC.43 Negative controls contained no CaCl2 and 50 μmol/L PKC19-31 (also based on the pseudosubstrate sequence of PKC, but a more potent inhibitor than PKC.19-36,43 44 After a 15-minute incubation at 30°C, 25 μL was removed from each reaction and spotted onto P81 phosphocellulose discs, which were washed and counted as described above.
Acetylcholinesterase activity.Acetylcholinesterase (AchE) activity was determined as previously described.45 Briefly, 0.1 to 10 μg membrane protein was incubated in a 200-μL reaction mix containing 0.6 mmol/L thioacetylcholine chloride, 0.5 mmol/L 5,5′ dithiobis-2–nitrobenzoic acid, 100 mmol/L NaPO4 , pH 7.0 and absorbance at 415 nm was measured over time.
Western blotting.Whole cell lysates and membrane fraction proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% acrylamide gel and blotted onto nitrocellulose. Blots were blocked with PBS containing 5% milk and incubated for 1 hour with the anti-PKC antibody in PBS containing 5% milk, 0.1% Tween 20. After washing in PBS containing 0.1% Tween 20, blots were incubated with horseradish peroxidase-conjugated goat antimouse Ig in PBS containing 5% milk,0.1% Tween 20. Blots were washed in PBS containing 0.1% Tween 20 and developed by enhanced chemiluminescence according to manufacturers' instructions.
PKC downregulation.Fresh, washed RBC were incubated for 24 hours at 37°C in RPMI containing 2 μmol/L PMA. This is sufficient to cause complete downregulation of the erythrocyte PKC,9 but does not affect viability. Control cells were incubated with dimethyl sulfoxide (DMSO) only. Before infection, cells were washed four times in 50 vol of RPMI, then resuspended in parasite culture medium.
Infection and gametocytogenesis.Asexual parasites were enriched to 50% to 90% parasitemia and seeded into 24-well plates containing either DMSO-treated or PMA-treated RBC, at a final hematocrit of 2% and parasitemia of 0.5%. The plates were incubated at 37°C in a gassed chamber.40 To assay growth rate, aliquots of cells were removed at daily intervals, smeared, fixed in methanol, and stained with Giemsa. Asexual stage parasites were counted at 1,000× magnification. At least 5,000 cells were counted for each well. Results are given as the mean of triplicate counts.
For gametocyte development assays, parasites were seeded as described above and grown for up to 8 days with daily feeding. Aliquots were removed daily for counting and assessed for the number of developing gametocytes present. At least 100 fields were counted for each well, and the results represent the mean of triplicate counts.
RESULTS
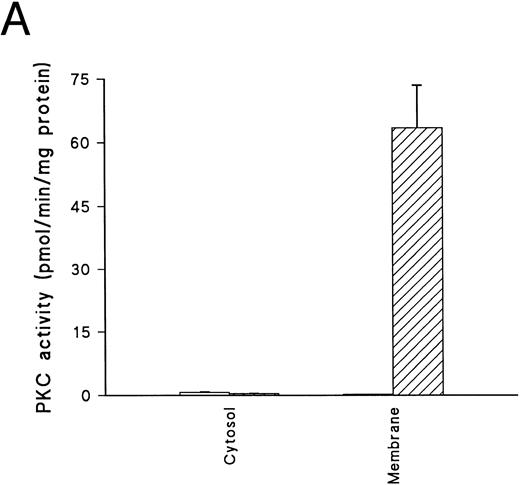
The addition of PMA to normal, uninfected human erythrocytes causes rapid membrane translocation and activation of PKC (Fig 1A). The majority of the translocated activity is inhibited by PKC19-36 (a peptide based on the pseudosubstrate sequence of PKCα, which selectively inhibits PKC activity)43 44 and has the characteristics of a classical PKC enzyme. Erythrocyte PKC activity declined gradually with storage and also during culture (data not shown). Therefore, parasitized cells were always compared with uninfected RBCs taken at the same time from the same donor, and cultured for the same period of time under identical conditions. Blotting of RBC lysates confirmed that the predominant PKC family member present in uninfected cells is the classical enzyme PKCγ, although low levels of PKCα, PKCθ, and PKCι are also present (Fig 1B). At this level of expression, these last three are unlikely to have contributed significantly to the enzyme activity detected.
PKC in erythrocytes. (A) Translocation of PKC activity from cytosol to membrane in uninfected erythrocytes exposed to DMSO (□) or 500 nmol/L PMA (▨) for 15 minutes. PKC activity given is the total kinase activity minus activity measured in the absence of lipid activators (PMA/phosphatidyl-serine/Triton-X–100 mixed micelles) and the presence of 20 μmol/L PKC.19-36 Activities represent the mean of duplicate assays + range. This result is representative of six separate experiments. (B) Western blot of whole cell lysates from uninfected cells, prepared and separated on a 10% acrylamide gel (107 cell equivalents/lane) as described in Materials and Methods. Blots were incubated with monoclonal antibodies as follows: lane 1, anti-PKCα at 0.05 μg/mL; lane 2, anti-PKCβ at 0.16 μg/mL; lane 3, anti-PKCγ at 0.25 μg/mL; lane 4, anti-PKCε at 0.5 μg/mL; lane 5, anti-PKCθ at 1 μg/mL; lane 6, anti-PKC ι at 1.6 μg/mL and lane 7, anti-PKCλ at 1.6 μg/mL. No bands were detected in parallel blots incubated with control antibodies or second layer only (not shown).
PKC in erythrocytes. (A) Translocation of PKC activity from cytosol to membrane in uninfected erythrocytes exposed to DMSO (□) or 500 nmol/L PMA (▨) for 15 minutes. PKC activity given is the total kinase activity minus activity measured in the absence of lipid activators (PMA/phosphatidyl-serine/Triton-X–100 mixed micelles) and the presence of 20 μmol/L PKC.19-36 Activities represent the mean of duplicate assays + range. This result is representative of six separate experiments. (B) Western blot of whole cell lysates from uninfected cells, prepared and separated on a 10% acrylamide gel (107 cell equivalents/lane) as described in Materials and Methods. Blots were incubated with monoclonal antibodies as follows: lane 1, anti-PKCα at 0.05 μg/mL; lane 2, anti-PKCβ at 0.16 μg/mL; lane 3, anti-PKCγ at 0.25 μg/mL; lane 4, anti-PKCε at 0.5 μg/mL; lane 5, anti-PKCθ at 1 μg/mL; lane 6, anti-PKC ι at 1.6 μg/mL and lane 7, anti-PKCλ at 1.6 μg/mL. No bands were detected in parallel blots incubated with control antibodies or second layer only (not shown).
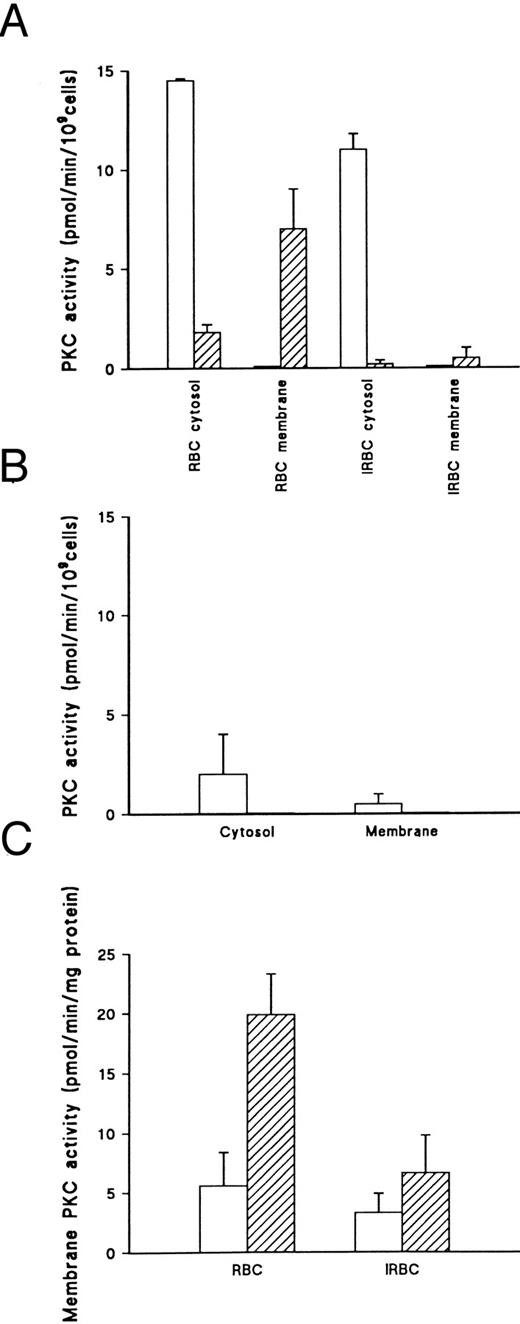
The ability of PMA to induce translocation of PKC from the cytosol to the membrane was markedly impaired in erythrocytes infected with mature asexual stage P falciparum parasites of the 3D7 strain (Fig 2A). Data are presented in terms of activity per cell rather than per unit protein because the degradation of hemoglobin in infected cells causes a substantial reduction in protein content in cytosolic fractions, such that specific activity is not directly comparable between infected and uninfected erythrocytes. The loss of cytosolic PKC activity in PMA treated infected red blood cells (IRBC) (Fig 2A) without a concomitant increase in membrane activity suggests that inhibition of membrane activity occurs in infected cells or that some PKC activity translocates to the parasite membrane. However, isolated parasites contain only low levels of PKC-like activity (Fig 2B), which probably represents contaminating PKC from uninfected RBCs in the parasite preparation rather than genuine parasite PKC, as it is difficult to remove all RBC membranes from the parasite fraction. Nevertheless, the activity detected in the parasite fraction is too low to account for the loss of PKC translocation to the infected erythrocyte membrane. The possibility that contaminating parasite membrane components in the erythrocyte membrane fraction interfere with detection of PKC activity cannot be ruled out. In addition, cytosolic activity determined by the methods used tends to give an underestimate of total cellular PKC levels10 (data not shown).
PKC activity in uninfected and P falciparum–infected erythrocytes. (A) PKC activity determined in cytosol and membrane fractions from uninfected erythrocytes (RBC) and enriched infected erythrocytes (IRBC) (60% parasitemia) incubated with DMSO (□) or 500 nmol/L PMA (▨) for 15 minutes. PKC activity given is the total kinase activity minus activity measured in the absence of lipid activators (PMA/Phosphatidyl-serine/Triton-X–100 mixed micelles) and the presence of 20 μmol/L PKC.19-36 Activities represent the mean of duplicate assays + range. This result is representative of six separate experiments. (B) Kinase activity in isolated parasites. IRBC (2.5 × 107) were incubated with DMSO (□) or 500 nmol/L PMA (▨) for 15 minutes at 37°C. Parasites were prepared by saponin lysis, then fractionated as described.28 PKC activity was determined as described above. Values represent the mean of duplicate assays + range. This result is representative of three separate experiments. (C) Membrane PKC activity in RBC and IRBC (60% parasitemia) incubated with DMSO (□) or 500 nmol/L PMA (▨) for 15 minutes. PKC activity was determined as described above. Values represent the mean of triplicate assays + standard error of mean (SEM). This result is representative of six separate experiments.
PKC activity in uninfected and P falciparum–infected erythrocytes. (A) PKC activity determined in cytosol and membrane fractions from uninfected erythrocytes (RBC) and enriched infected erythrocytes (IRBC) (60% parasitemia) incubated with DMSO (□) or 500 nmol/L PMA (▨) for 15 minutes. PKC activity given is the total kinase activity minus activity measured in the absence of lipid activators (PMA/Phosphatidyl-serine/Triton-X–100 mixed micelles) and the presence of 20 μmol/L PKC.19-36 Activities represent the mean of duplicate assays + range. This result is representative of six separate experiments. (B) Kinase activity in isolated parasites. IRBC (2.5 × 107) were incubated with DMSO (□) or 500 nmol/L PMA (▨) for 15 minutes at 37°C. Parasites were prepared by saponin lysis, then fractionated as described.28 PKC activity was determined as described above. Values represent the mean of duplicate assays + range. This result is representative of three separate experiments. (C) Membrane PKC activity in RBC and IRBC (60% parasitemia) incubated with DMSO (□) or 500 nmol/L PMA (▨) for 15 minutes. PKC activity was determined as described above. Values represent the mean of triplicate assays + standard error of mean (SEM). This result is representative of six separate experiments.
The recovery of membrane fractions from infected cells was poor compared with uninfected cells. However, membrane-associated PKC activity in PMA-treated infected cells was still significantly lower than in normal erythrocytes when expressed in terms of activity per mg membrane protein (P < .05) (Fig 2C). In the experiment shown, PKC activity in membranes from PMA-treated cells was reduced by 65% relative to uninfected cells for a parasite preparation of 60% parasitemia.
The reduction in PKC membrane translocation in infected cells was consistently observed in multiple experiments with 3D7A and also other P falciparum strains. The change was not an artefact of parasite enrichment procedure, because Percoll purification of parasites yielded the same results. The reduced translocation was also independent of fractionation method, being readily observable when cells were disrupted by hypotonic rather than saponin lysis (data not shown). Similar results were also obtained whether the specific peptide AcMB4-14 (which contains a PKC-specific phosphorylation site42 or whole myelin basic protein was used as a substrate (data not shown).
To ensure that the lowered membrane PKC activity in P falciparum–infected erythrocytes treated with PMA was a specific effect, the activity of AChE, an erythrocyte membrane-specific enzyme45 was measured (Table 1). Addition of PMA to uninfected cells did not alter membrane AChE activity, neither did infection cause any significant change. Non-PKC kinase activity (lipid-independent activity, which was not inhibited by the pseudosubstrate peptide) was increased in membranes isolated from infected cells, and showed no significant change after addition of PMA. PKC activity was significantly increased only in PMA-treated, uninfected cells (P < .05, Student's t-test), while infected cells showed a nonsignificant reduction in membrane activity in response to PMA. The lack of significance of this particular result is confirmed by the fact that no such reduction was observed in similar experiments (for example, see Fig 2A). This suggests that the loss of PMA responsive kinase activity in infected cells was specific.
Specificity of Reduction in PKC Activity
| Treatment . | AChE . | PKC . | Non-PKC Kinase (pmol/min/mg) . |
|---|---|---|---|
| . | ( μmol/min/mg) . | (pmol/min/mg) . | . |
| Control | 3.76 ± 0.16 | 21.21 ± 0.36 | 1.86 ± 0.30 |
| RBC + PMA | 3.80 ± 0.38 | 71.90 ± 8.63 | 4.85 ± 3.22 |
| Control IRBC | 4.56 ± 0.24 | 21.52 ± 6.85 | 23.7 ± 5.53 |
| IRBC + PMA | 4.11 ± 0.17 | 4.34 ± 2.33 | 31.6 ± 1.86 |
| Treatment . | AChE . | PKC . | Non-PKC Kinase (pmol/min/mg) . |
|---|---|---|---|
| . | ( μmol/min/mg) . | (pmol/min/mg) . | . |
| Control | 3.76 ± 0.16 | 21.21 ± 0.36 | 1.86 ± 0.30 |
| RBC + PMA | 3.80 ± 0.38 | 71.90 ± 8.63 | 4.85 ± 3.22 |
| Control IRBC | 4.56 ± 0.24 | 21.52 ± 6.85 | 23.7 ± 5.53 |
| IRBC + PMA | 4.11 ± 0.17 | 4.34 ± 2.33 | 31.6 ± 1.86 |
AChE activity and kinase activity were measured in membrane fractions from infected (IRBC) (55%) and uninfected RBC with and without PMA treatment (500 nmol/L for 15 minutes at 37°C). AChE activity represents the mean of triplicate values ± SEM. The difference in activity between parasitized and uninfected cells is not significant. PKC activity represents activity in the presence of lipid activators, while non-PKC activity was measured in absence of lipid activators and the presence of excess pseudosubtrate peptide (as described in Materials and Methods). Values represent the mean of triplicate values ± SEM.
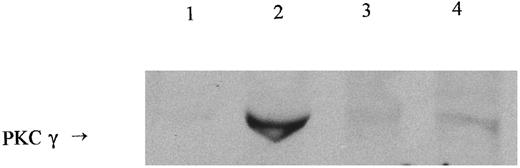
To determine whether the reduced PKC activity in membranes isolated from PMA exposed IRBC was due to a reduction in translocation or inhibition of enzyme activity, membranes were prepared from infected and uninfected cells, with and without PMA treatment. The proteins were separated by SDS-PAGE, blotted onto nitrocellulose, and detected with the anti-PKC antibody MC5 (Fig 3). PKC protein levels were very low in untreated, uninfected RBC membrane preparations (Fig 3, lane 1) and markedly increased 15 minutes after PMA treatment (Fig 3, lane 2), whereas membranes from infected cells contained a slightly higher background level of PKC (Fig 3, lane 3), but showed little increase in response to PMA (Fig 3, lane 4). This shows that the reduced enzyme activity correlates to a reduced level of PKC protein in the membrane fraction of infected cells. The effect of infection on total PKC protein levels was difficult to assess because of nonspecific background bands in lysates from infected cells (not shown).
Western blotting of membrane PKC in PMA-treated IRBC 5 × 108 RBC or IRBC (70% parasitized) were incubated with or without PMA for 15 minutes and membranes prepared as described in Materials and Methods. Membrane proteins (150 μg/lane) were separated on a 10% acrylamide gel. Lane 1, untreated RBC; lane 2, RBC + PMA; lane 3, untreated IRBC; lane 4, IRBC + PMA. Arrow indicates a band cross-reacting with the PKC-specific antibody MC5. The identity of this band was confirmed by reprobing with specific anti-PKCγ antibody (not shown).
Western blotting of membrane PKC in PMA-treated IRBC 5 × 108 RBC or IRBC (70% parasitized) were incubated with or without PMA for 15 minutes and membranes prepared as described in Materials and Methods. Membrane proteins (150 μg/lane) were separated on a 10% acrylamide gel. Lane 1, untreated RBC; lane 2, RBC + PMA; lane 3, untreated IRBC; lane 4, IRBC + PMA. Arrow indicates a band cross-reacting with the PKC-specific antibody MC5. The identity of this band was confirmed by reprobing with specific anti-PKCγ antibody (not shown).
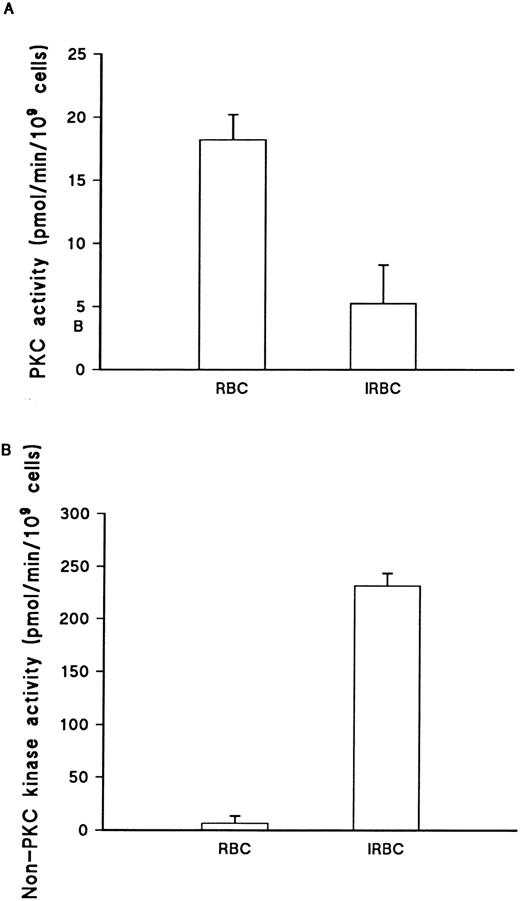
The observed changes in PKC activation following infection with P falciparum could reflect impaired translocation in infected cells, due to inhibition of PKC transfer to the membrane. Alternatively, the infected cells might contain lower levels of activatable PKC. To distinguish between these possibilities, total cellular PKC was isolated by lysis in Triton-X-100, followed by partial purification DE52 cellulose. The PKC activity recovered was consistently higher in uninfected cell lysates (Fig 4A). Over a number of experiments, the estimated total PKC activity in uninfected cells was 24.1 ± 2.21 pmol/min/109 cells, while that in infected cells (with parasitemias ranging from 60% to 90%) was significantly lower, 5.0 ± 3.3 pmol/min/109 cells (P < .001, Student's t-test). By contrast, non-PKC kinase activity showed a marked increase infected cells (Fig 4B). This indicates that the loss of PMA responsiveness in infected cells was due to a reduction in total erythrocyte PKC activity as opposed to interference with translocation mechanisms.
Total cellular PKC activity in uninfected and infected erythrocytes. (A) PKC activity in 0.2 mol/L NaCl eluate from DE52 column assayed as described in Materials and Methods. Parasites were enriched to 50% by Plasmagel flotation before lysis. PKC activity is the total activity measured, minus activity in the absence of lipid activators (PMA/Phosphatidyl-serine/Triton-X–100 mixed micelles) and presence of 20 μmol/L PKC.19-36 Values represent the mean of duplicate assays + range. This result is representative of three separate experiments. (B) Non-PKC activity, as measured in the absence of lipid activators (PMA/Phosphatidyl-serine/Triton-X–100 mixed micelles) and presence of 20 μmol/L PKC,19-36 in the same RBC and IRBC lysates. Values represent the mean of duplicate values + range. This result is representative of three separate experiments.
Total cellular PKC activity in uninfected and infected erythrocytes. (A) PKC activity in 0.2 mol/L NaCl eluate from DE52 column assayed as described in Materials and Methods. Parasites were enriched to 50% by Plasmagel flotation before lysis. PKC activity is the total activity measured, minus activity in the absence of lipid activators (PMA/Phosphatidyl-serine/Triton-X–100 mixed micelles) and presence of 20 μmol/L PKC.19-36 Values represent the mean of duplicate assays + range. This result is representative of three separate experiments. (B) Non-PKC activity, as measured in the absence of lipid activators (PMA/Phosphatidyl-serine/Triton-X–100 mixed micelles) and presence of 20 μmol/L PKC,19-36 in the same RBC and IRBC lysates. Values represent the mean of duplicate values + range. This result is representative of three separate experiments.
To rule out the possibility that differences in detection of PKC in infected cells compared with normal erythrocytes might be an artefact of fractionation procedures, an assay was developed to measure PKC activation in situ. Such assays, using digitonin to permeabilise cells, have been used to measure PKC activation in a variety of cell types.46-48 Because parasite membranes are resistant to digitonin lysis, interference by parasite kinases is minimized. To increase specificity, [Ser25]PK,19-31 a high affinity peptide based on the PKC pseudosubstrate sequence,49 was used as a substrate in these assays. Under the conditions used, PMA caused a time and dose-dependent increase in kinase activity in uninfected cells (Fig 5A and B). Background activity, which was not inhibited by the pseudosubstrate peptide inhibitor, showed little change. Consistent with the results from whole cell lysates, infection appeared to reduce the maximum PKC activity in infected cells rather than altering the kinetics of activation. The level of PKC activity detected in PMA-treated cells decreased with increasing parasitemia (Fig 5C), indicating that the reduction in activity was predominantly in infected cells. The reduced PMA responsive kinase activity detected in situ supports the view that the loss of PKC is due to a genuine downmodulation of the enzyme in infected cells.
In situ PKC activity in uninfected and infected erythrocytes. (A) Dose-dependent PKC activation by PMA. PKC activity in uninfected erythrocytes (▪) and 65% infected erythrocytes (▴) prepared as described in Materials and Methods section and incubated for 15 minutes at 37°C with various concentrations of PMA. Results represent the mean of triplicate values ± SEM. Non-PKC activity in uninfected (□) and infected (▵) cells, as measured in the absence of Ca2+ and the presence of 50 μmol/L PKC,19-31 represents the mean of duplicate values. These results are representative of three separate experiments. (B) Time course of PKC activation by PMA. PKC activity in uninfected erythrocytes (▪) and 55% infected erythrocytes (▴) prepared as described in Materials and Methods and incubated with 500 nmol/L PMA at 37°C for various times. Results represent the mean of triplicate values ± SEM. Non-PKC activity in uninfected (□) and infected (▵) cells, as measured in the absence of Ca2+ and the presence of 50 μmol/L PKC,19-31 represents the mean of duplicate values. These results are representative of three separate experiments. (C) Effect of parasitemia on PKC activity. Plasmagel-enriched parasites were diluted to various parasitemias with cells from the same culture before enrichment and incubated for 15 minutes with 500 nmol/L PMA. Results given represent the mean of triplicate assays ± SEM.
In situ PKC activity in uninfected and infected erythrocytes. (A) Dose-dependent PKC activation by PMA. PKC activity in uninfected erythrocytes (▪) and 65% infected erythrocytes (▴) prepared as described in Materials and Methods section and incubated for 15 minutes at 37°C with various concentrations of PMA. Results represent the mean of triplicate values ± SEM. Non-PKC activity in uninfected (□) and infected (▵) cells, as measured in the absence of Ca2+ and the presence of 50 μmol/L PKC,19-31 represents the mean of duplicate values. These results are representative of three separate experiments. (B) Time course of PKC activation by PMA. PKC activity in uninfected erythrocytes (▪) and 55% infected erythrocytes (▴) prepared as described in Materials and Methods and incubated with 500 nmol/L PMA at 37°C for various times. Results represent the mean of triplicate values ± SEM. Non-PKC activity in uninfected (□) and infected (▵) cells, as measured in the absence of Ca2+ and the presence of 50 μmol/L PKC,19-31 represents the mean of duplicate values. These results are representative of three separate experiments. (C) Effect of parasitemia on PKC activity. Plasmagel-enriched parasites were diluted to various parasitemias with cells from the same culture before enrichment and incubated for 15 minutes with 500 nmol/L PMA. Results given represent the mean of triplicate assays ± SEM.
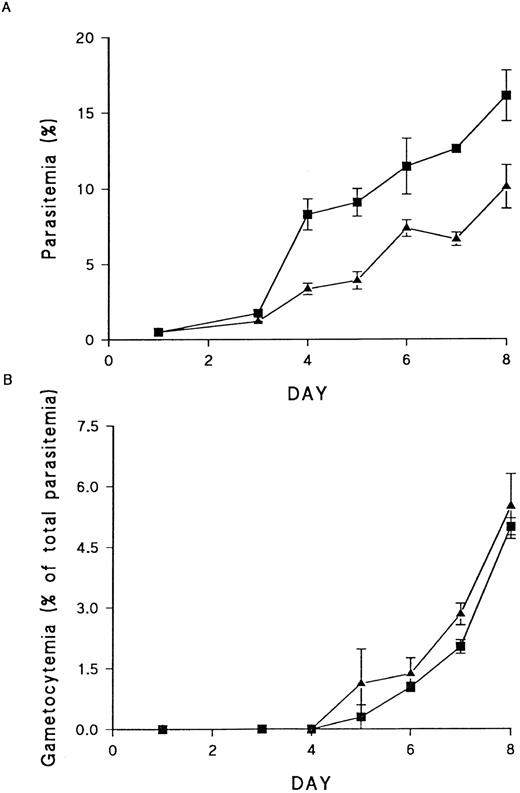
In most systems, PKC downregulation is preceded by activation.49-51 The long-term downmodulation of PKC activity in cells infected with P falciparum could therefore be an indication that the enzyme is activated early on in infection and this may be of functional significance in parasite survival. To determine whether PKC was required for parasite growth and development, erythrocytes were incubated overnight with 2 μmol/L PMA. As reported by others,9 this was sufficient to cause complete downregulation of PKC (data not shown). The loss of PKC activity is due to PMA-induced activation of calpain. This protease converts the enzyme to the smaller PKM, which is rapidly degraded, such that no detectable PKC protein is present after 24 hours of exposure.9 Erythrocytes treated in this way remained fully viable, but asexual parasite growth in PKC-deficient cells was significantly reduced compared with growth in control erythrocytes (Fig 6A). The effect was greatest at the first round of invasion, with a reduction of up to 50% in ring stage parasites observed in pretreated cells. However, no effect was seen on gametocytogenesis (Fig 6B), demonstrating that PKC is not required for sexual stage development. The poor growth of asexual parasites in cells pretreated with PMA suggests a role for PKC in the asexual parasite life cycle. However, the experiments do not distinguish between the possibility that PKC is required in the early stages of infection and the alternative interpretation that PMA treatment induces changes in the RBC, which interfere with invasion.
Effect of PKC downregulation on asexual and sexual stage parasite growth. (A) Asexual stage growth in normal (▪) and PKC-deficient (▴) RBCs. Each point represents the mean of triplicate counts ± SEM. This result is representative of four separate experiments. (B) Gametocyte production in normal (▪) and PKC-deficient (▴) cells. Gametocytemia is presented as a percentage of the total parasitemia, to allow for the effects of PKC downregulation on asexual stage growth. Each point represents the mean of triplicate counts ± SEM. This result is representative of four separate experiments.
Effect of PKC downregulation on asexual and sexual stage parasite growth. (A) Asexual stage growth in normal (▪) and PKC-deficient (▴) RBCs. Each point represents the mean of triplicate counts ± SEM. This result is representative of four separate experiments. (B) Gametocyte production in normal (▪) and PKC-deficient (▴) cells. Gametocytemia is presented as a percentage of the total parasitemia, to allow for the effects of PKC downregulation on asexual stage growth. Each point represents the mean of triplicate counts ± SEM. This result is representative of four separate experiments.
DISCUSSION
This is the first report of a specific reduction in PKC activity in P falciparum infected erythrocytes. Other investigators noted the failure of PMA to stimulate protein phosphorylation in infected cells,31 but no direct measurement of enzyme activity was made. Modulation of host cell PKC activity has been observed in a number of parasitic and microbial infections, including Leishmania donovani52 53 and Legionella pneumophilia.54 In these cases, however, the infected cell is a macrophage and the benefit of interference with activation mechanisms by the invading organism is obvious. In the case of the malaria parasite, the infected cell has no defense mechanisms, which need to be inactivated, and the importance of alterations in host cell signal transduction is unclear. However, the reduced growth rate of parasites in PKC-deficient erythrocytes suggests that the enzyme may indeed play a role in parasite survival. The potential involvement of host enzymes in parasite growth needs to be considered in the development of antimalarial drugs.
The mechanism of PKC downregulation by P falciparum is not known. Changes in the lipid composition of the infected erythrocyte membrane are known to occur.21 In particular, the reported reduction in phosphatidylserine levels in infected cells could interfere with translocation. However, such changes are unlikely to affect total cellular PKC activity, which is measured as partially purified enzyme activity in the presence of excess phosphatidylserine and is therefore independent of endogenous membrane lipids. Parasite-derived glycosylphozohotidylinositols (GPIs) and pigment, both present in parasite culture supernatants, have been implicated in the activation and subsequent downregulation of PKC in monocytes.55-58 However, we could detect no evidence of direct activation of RBC PKC by parasite exoantigens in the short-term and no loss of activity in uninfected cells exposed to parasite supernatants for 24 or 48 hours (data not shown).
It may be that PKC activation occurs at the time of invasion, with downregulation following as the parasite infection progresses. PKC could be activated secondary to stimulation of inositol lipid turnover, or by an indirect mechanism, such as the stimulation of phosphatidylcholine-specific phospholipases C and D. This could be induced by the increased intracellular calcium present in infected cells, leading to release of DAG and activation of PKC, even in the absence of inositol lipid turnover.59-61 Oxidative stress is another route of PKC activation and downregulation62 and infection with the malaria parasite is known to induce such stress in the erythrocyte.63 Finally, the influx of calcium on invasion alone may be sufficient to cause degradation of PKC, by activation of calcium dependent proteases. Digestion by calpain has been shown to be a major cause of PKC downregulation in PMA-treated cells.9,50 64 Investigation of PKC levels, localization and activity at earlier stages of RBC infection, and the use of specific inhibitors may distinguish between these possibilities.
The stimulation P falciparum gametocytogenesis by PMA,39 and the preferential development of gametocytes in reticulocytes,65 which are believed to have different patterns of PKC activity from mature erythrocytes,8 serve to implicate PKC in triggering sexual stage development. However, the lack of a specific effect of PKC downregulation on gametocyte production rules out any major role for RBC PKC in this process. Since no PMA sensitive kinase activity has been detected in the parasite itself, the mechanism by which the phorbol ester acts remains to be resolved. It may be that PKC-like enzymes are, in fact, present in P falciparum, but that their substrate specificity, cofactor requirements and sensitivity to inhibitors are sufficiently distinct from those of mammalian classical PKC family members to render them undetectable under the conditions used.
The role of erythrocyte PKC in asexual parasite growth remains to be explored. Both erythrocyte and parasite-derived kinases are thought to be important in the invasion of erythrocytes by P falciparum merozoites.29,36,66 Although administration of the kinase inhibitor staurosporine to erythrocytes before invasion does not inhibit parasite entry,66 PKC could still be involved in implementing some of the early parasite-induced changes in the erythrocyte, including increased permeability and phosphorylation of the parasite protein, RESA, and host proteins such as band 4.1. The latter appears to be important to parasite survival, since parasite growth is impaired in erythrocytes from patients suffering from hereditary elliptocytosis, which are deficient in band 4.1.67 Band 4.1 is required for association of the mature-parasite-infected erythrocyte surface antigen with the plasma membrane,67 and phosphorylation may influence interaction between the two proteins. The kinase responsible for the phosphorylation of band 4.1 has not been identified. A parasite-derived calcium-dependent kinase has been shown to phosphorylate the protein in vitro,68 but it remains to be established that this phosphorylation also occurs within the infected erythrocyte, since many protein kinases are more promiscuous in their substrate specificity in vitro than whole cells. Also, increase in band 4.1 phosphorylation is seen only 2 hours after invasion and it is not known whether the kinase is expressed and localized in the RBC plasma membrane at this early stage.68 Band 4.1 phosphorylation coincides with an increase in intracellular calcium concentration,29 which, as discussed above, could lead an increase in PKC activity early in infection.61 The later downmodulation of PKC in mature stage infected cells could also be directly responsible for changes in RBC behavior, such as loss of deformability. A depletion in cytosolic PKC is thought to contribute to the increased rigidity of senescent erythrocytes.8 9 The use of PKC-deficient erythrocytes allows direct assessment of the role of PKC in these various parasite-induced changes in RBC function and behavior.
ACKNOWLEDGMENT
We thank Dr Paul Kaye for useful discussion and Dr Chris Engwerda, Dr Martin Goodier, and Prof Alan Fairlamb for comments on the manuscript.
Supported by a Wellcome Trust Programme Grant to G.A.T.T.
Address reprint requests to Belinda S. Hall, PhD, Department of Medical Parasitology, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, UK.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal