Abstract
The ob gene product, leptin, has been shown in several studies to be involved in weight control and recombinant leptin recently has entered clinical trials to treat obesity. The leptin receptor (OB-R/B219) is expressed in a variety of protein isoforms not only in the central nervous system, but also in reproductive, and hematopoietic tissues. We reported recently that the OB-R/B219 was associated with a variety of hematopoietic lineages as well as the small fraction of cells containing the long-term reconstituting hematopoietic stem cells. Herein we report that leptin significantly stimulates the proliferation and differentiation of yolk sac cells and fetal liver cells and stimulates directly hematopoietic precursors. Leptin alone can increase the number of macrophage and granulocyte colonies, and leptin plus erythropoietin act synergistically to increase erythroid development. These data show that leptin has a significant, direct effect on early hematopoietic development and can stimulate the differentiation of lineage-restricted precursors of the erythrocytic and myelopoietic lineages. These observations along with a recent report strongly support our previous hypothesis that leptin has an unanticipated important role in hematopoietic and immune system development.
MANY FACTORS have been described that control or influence adult hematopoiesis.1 The factors that control early hematopoietic differentiation are less well characterized. In an effort to identify and study the early acting factors, recently we isolated a novel cDNA (B219) from both fetal liver and yolk sac.2 The B219 gene product is a member of the hematopoietin receptor family and has been shown to be a receptor for the obese (ob) gene product, leptin. Leptin is a 16-kD protein that is involved in the regulation of body weight.3,4 The primary amino acid sequence indicated that leptin may adopt a helical cytokine structure similar to interleukin-2 (IL-2) and growth hormone, and this led to the early indication that its receptor might be a member of the hemopoietin receptor family.5 Treatment of mice with leptin results in a reduction of food consumption and an increase in the metabolic rate and a concomitant loss of weight.4 To date, four human and six murine splice variants of the leptin receptor have been identified.2,6,7 The exact significance of the various isoforms has yet to be elucidated. In some cell lines, the truncated OB-R molecules have a reduced signaling ability.8,9 More recent evidence suggests that the truncated isoforms, especially the B219.3 isoform, may be capable of transmitting a signal in certain circumstances (B. Barut, et al, unpublished observations, August 1996). Leptin treatment of hepatic cells expressing only the B219.3 isoform attenuates insulin induced activities, and alters second messenger events.10 In our previous publication we presented data detailing the expression pattern of B219/OB-R and suggested the hypothesis that leptin is involved with primitive hematopoietic precursors.2 This hypothesis is significantly strengthened by the data contained herein and by the recent publication of Bennett et al.11
MATERIALS AND METHODS
Tissue preparation and embryonic stem cells cultures.Yolk sac and fetal liver tissues were obtained from timed matings with day of plug detection as day 0. CCE embryonic stem (ES) cell cultures were maintained on Dulbecco's Modified Eagle Medium (DMEM) containing 15% fetal bovine serum (FBS) and rLIF (1,000 U/mL) and differentiated into embryoid bodies (EBs) as described.12 Briefly, 2 days before initiation of differentiation, cells were passaged into Iscove's Modified Dulbecco's Medium (IMDM) containing 15% FBS. For inducing differentiation (d0), the cells were trypsinized and resuspended in IMDM containing 15% FBS. Petri dishes (100 mm) were seeded at 4,500 cells/mL. EBs were allowed to settle and were collected on the indicated days.
Reverse transcriptase-polymerase chain reaction (RT-PCR).Total RNA was extracted using RNAzol reagent (Biotecx Laboratories, Inc, Houston, TX). Total RNA was used to generate oligo d(T) primed cDNA with RNA PCR Core Kit reagents (Perkin Elmer, Foster City, CA) and a 9600 Gene Amp thermocycler (Perkin Elmer). The integrity of the cDNA was determined by assessing the relative levels of β-actin amplification. The primers used for amplifying the OB-R (long) isoform were 5′-ATTATTTCCTCTTGTGTCCTA-3′ (forward) and 5′-GTCTGATAAAAGGAAAAATGT-3′ (reverse). Primers that amplified the murine B219.3 isoform were 5′-GGTCAGAAGATGTGGGAAA-3′ (forward) and 5′-AAGTTGGTAGATTGGGTTCA-3′ (reverse). The expected PCR products are a 582-bp product for the reported OB-R long isoform, and a 661-bp product for the B219 type 3 isoform.
Proliferation and hematopoietic colony assays.Microtiter plates were seeded with 2.5 × 104 yolk sac or fetal liver cells in 100 μL of IMDM containing 2% FBS. The plates were incubated at 37°C with 5% CO2 for 72 hours. Cell number was measured using the MTS assay (Promega). Day 13 (d13) murine fetal liver hematopoietic colony assays were performed as described.13 Murine and adult marrow colony-forming assays were performed as described.14 ES-derived hematopoietic precursors were replated into MeC as described12 with 100 ng/mL of SLF and 200 ng/mL of leptin.
Recombinant leptin.For the production of leptin, cDNA encoding murine leptin was prepared from mRNA isolated from adipose tissue of C57 mice. OB-encoding cDNA was produced by RT-PCR amplification using primers based on the published sequence3: forward primer: 5′ GGAATTCCATATGGTGCCTATCCAGAAAGT; reverse primer: 5′ GCGGATCCTCAGCATTCAGGGCTAA. cDNA fragments were cloned into the Escherichia coli expression vector pET15b (Novagen Inc, Madison, WI). Clones encoding two variants of leptin, with and without glutamine at residue 49, were confirmed by DNA sequence analysis. Leptin protein was produced from the glutamine negative form. Recombinant leptin was produced with E coli strain BL21(DE)pLysS, following the protocol recommended by the manufacturer. The recombinant protein included a sequence of six histidine residues and a thrombin cleavage site fused at the amino terminus of the mature leptin protein sequence. Inclusion bodies were collected and dissolved in 8 mol/L urea, 50 mmol/L Tris-Cl (pH 8.0), 0.5 mmol/L EDTA, 100 mmol/L dithiothreitol for 2 hours at room temperature. Recombinant protein was refolded by extended incubation at 4°C at a protein concentration of 10 μg/mL in 100 mmol/L Tris-Cl (pH 8.0), 100 mmol/L (NH4 )2SO4 , 100 mmol/L Triton X100, 2 mmol/L reduced glutathione, and 0.4 mmol/L oxidized glutathione. Recombinant leptin was purified directly from the refolding reaction with His-Bind resin according to the manufacturer's protocol (Novagen Inc). Purified leptin was dialyzed into Dulbecco's phosphate-buffered saline (GIBCO-BRL, Gaithersburg, MD) and stored at 4°C. Similar results were obtained with a commercially available HPLC purified recombinant murine leptin (PeproTech Inc, Rock Hill, NJ).
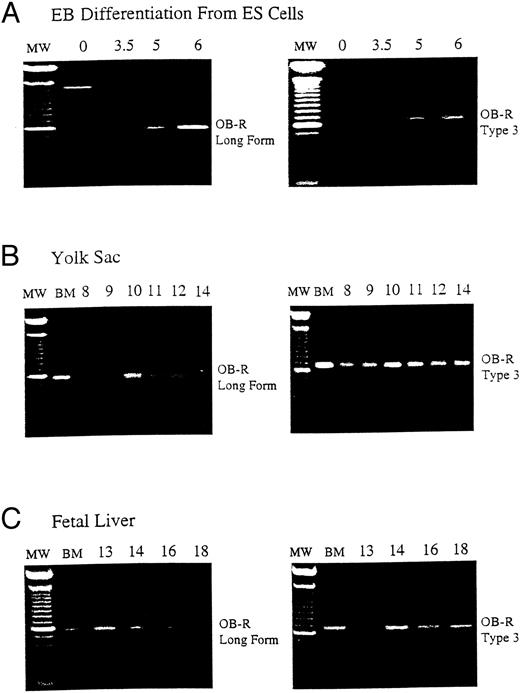
B219/OB-R long isoform is expressed early in hematopoietic tissue. B219/OB-R long form is the full-length6 and type 3 isoform receptor molecule2 are evaluated by PCR in samples from (A) murine ES cells from 0 to 6 days of differentiation, (B) yolk sac (YS) from d8 to d18 murine embryos, and (C) fetal liver (FL) from d13 to d18 murine embryos. MW, 100-bp molecular-weight markers; BM, adult bone marrow.
B219/OB-R long isoform is expressed early in hematopoietic tissue. B219/OB-R long form is the full-length6 and type 3 isoform receptor molecule2 are evaluated by PCR in samples from (A) murine ES cells from 0 to 6 days of differentiation, (B) yolk sac (YS) from d8 to d18 murine embryos, and (C) fetal liver (FL) from d13 to d18 murine embryos. MW, 100-bp molecular-weight markers; BM, adult bone marrow.
RESULTS
Murine embryonic stem (ES) cell differentiation cultures offer a powerful in vitro model of hematopoietic development from nonhematopoietic precursors.14,15 As ES cells differentiate into EB, the first erythroid precursors appear at day 4 (d4) of differentiation and increase in frequency until d6.12 The expression of B219/OB-R isoforms was evaluated during ES cell differentiation to examine the correlation of leptin receptor expression with hematopoietic differentiation. The long OB-R isoform variant is first detected on d3.5 and gradually increases until d6 of differentiation (Fig 1A). This expression parallels the appearance of committed hematopoietic precursors developing in the EB.12 In vivo, the first hematopoietic precursors appear in the yolk sac at 7 to 8 days of development.16 By d12 of gestation in the mouse the fetal liver becomes the primary site of erythropoiesis.17 18 This progression continues through d18 when hematopoietic activity migrates to the bone marrow. RT-PCR shows that both the long OB-R and short B219.32 isoforms are expressed in yolk sac and fetal liver at levels that reflect this hematopoietic development (Fig 1B and C).
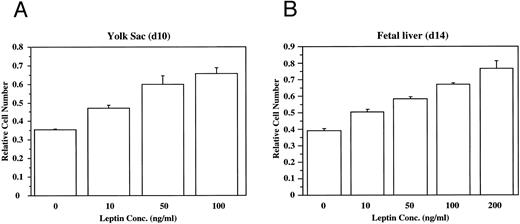
To evaluate directly the role of leptin in hematopoietic development, bacterial recombinant leptin was produced and bioassayed with an engineered leptin sensitive cell line (manuscript in preparation). It is clear that leptin stimulates proliferation of both yolk sac and fetal liver cells in a dose-dependent fashion (Fig 2). At d13 of development, 80% to 90% of the fetal liver cells express TER119, a marker for late-stage erythroid lineage.19 Sorting analyses indicated that essentially all of the leptin-responsive cells at this stage are in the TER119-negative subpopulation (data not shown). After a 4-day culture of TER119-negative d13 fetal liver cells with leptin, there is no significant increase of TER119 expression over controls, but there is a fourfold increase in the frequency of cells that express the macrophage/monocyte markers Mac-1 (CD11b) and F4/80 (data not shown). This suggests that leptin has a significant stimulatory effect on early hematopoietic cell populations and can induce differentiation of at least the myeloid lineage.
Leptin stimulates the proliferation of yolk sac (YS) and fetal liver (FL) cells. The dose-dependent effects of leptin on the proliferation of freshly isolated cells from (A) d10 murine yolk sac cells, and (B) d14 murine fetal liver cells was evaluated. These data are typical of many experiments.
Leptin stimulates the proliferation of yolk sac (YS) and fetal liver (FL) cells. The dose-dependent effects of leptin on the proliferation of freshly isolated cells from (A) d10 murine yolk sac cells, and (B) d14 murine fetal liver cells was evaluated. These data are typical of many experiments.
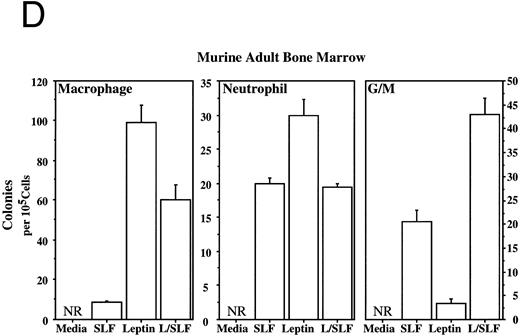
Methylcellulose colony assays were used to evaluate further the hematopoietic lineages affected by leptin. Leptin stimulates the committed colony-forming cells (CFCs) of the macrophage lineage found in EBs developing in ES differentiation cultures. In this case, steel factor (SLF ) synergizes with leptin (Fig 3A). Leptin also stimulates the CFCs of the macrophage and neutrophil lineages from both the murine fetal liver and murine adult bone marrow (Fig 3B and D). This response does not require additional cytokines and there is little or no increased response in combination with SLF; however, SLF does increase the colony size.
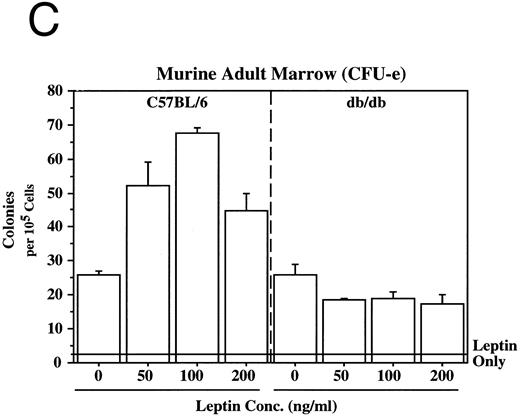
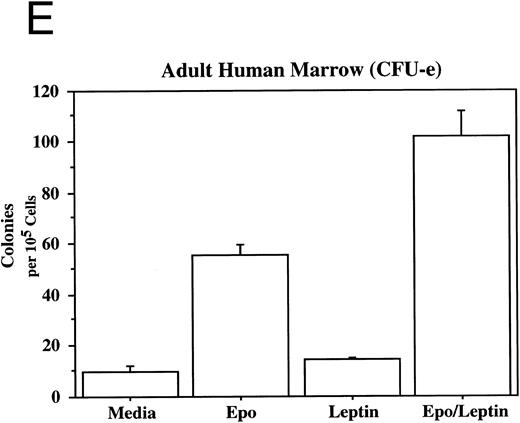
Leptin stimulates erythroid and myeloid colonies. Hematopoietic cells from (A) ES-derived embryoid bodies (d6), (B) murine d13 fetal liver, (C) adult bone marrow from normal and db mutant mice, (D) adult murine bone marrow, or (E) adult human bone marrow are cultured in methylcellulose colony assays containing leptin (L) and/or steel factor (SLF ). For adult marrow assays all groups contained saturating levels of rhEPO (2 U/mL). Sex- and age-matched marrow from normal and C57BL/6J db/db mice were used for the experiments in (C). Each graph is representative of data from three experiments. NR, no response.
Leptin stimulates erythroid and myeloid colonies. Hematopoietic cells from (A) ES-derived embryoid bodies (d6), (B) murine d13 fetal liver, (C) adult bone marrow from normal and db mutant mice, (D) adult murine bone marrow, or (E) adult human bone marrow are cultured in methylcellulose colony assays containing leptin (L) and/or steel factor (SLF ). For adult marrow assays all groups contained saturating levels of rhEPO (2 U/mL). Sex- and age-matched marrow from normal and C57BL/6J db/db mice were used for the experiments in (C). Each graph is representative of data from three experiments. NR, no response.
In addition to the other myeloid lineages, leptin stimulates the end-stage colony-forming-unit-erythroid (CFU-e) in the mouse (Fig 3C) and the human (Fig 3E). Although the CFU-e effect was EPO-dependent, the number of colonies doubled over that observed with saturating levels of EPO alone. The nonresponsiveness of marrow to leptin from db/db mice (Fig 3C), whose mutation results in dramatically reduced expression of full-length leptin receptor isoform,7,20 demonstrates the direct involvement of the leptin receptor and indicates that the effect is leptin-specific. The fact that the CFU-e counts are linearly related to the input cell number (data not shown) suggests, but does not prove, that the leptin stimulation is a direct effect on the CFU-e. The fact that the leptin-responsive fetal liver cell is TER119-negative (data not shown) is consistent with the results in Fig 3 because erythroid CFCs are TER119-negative.19 Although leptin increases the EPO-dependent CFU-e response from fetal liver, this effect has not been as dramatic as that seen with adult bone marrow. For example, in one experiment with a background of 28 colonies, EPO stimulated 486 ± 123 and leptin/EPO stimulated 646 ± 79 CFU-e colonies from d13 fetal liver cells. We are investigating whether the quantitative difference in the CFU-e responses between adult bone marrow and fetal liver reflects a true difference in the frequency of leptin-responsive cells between the two populations, the presence of leptin-binding nonsignaling receptor forms, and/or a difference in sensitivity to other factors that might be present in these cultures.
Leptin stimulates an E/Mac colony that contains both erythroid and macrophage lineages from EBs (Fig 3A) and from AA4+ fetal liver (data not shown). In both cases the response requires costimulation with SLF. It is not yet clear whether this E/Mac colony is derived from a bipotential CFC or from the synergistic growth of lineage-restricted CFCs. In addition to this bipotential colony, leptin and SLF act synergistically to stimulate the granulocytic/macrophage (G/M) precursors in adult bone marrow and fetal liver (Fig 3B and D). Leptin has little effect on CFC-mast and the multilineage CFC-mixed from adult bone marrow (data not shown). These data indicate that leptin by itself stimulates the lineage-committed myeloid precursors, whereas the more immature bipotential myeloid CFCs require costimulation with SLF.
DISCUSSION
These data strongly suggest that leptin has a significant effect on hematopoietic development. It is interesting in this context that bone marrow contains many adipocytes and that the crucial stromal elements necessary for the establishment of long-term bone marrow cultures contain a high percentage of adipogenic cells.21 Furthermore, the anecdotal observation that older mice with higher fat content have a better survival rate in radiation reconstitution studies may now have a rational biologic explanation. Because adipocytes are the natural source of leptin,22 it is possible that one of the functions of these cells in the marrow or in the hematopoietic microenvironment is to produce leptin and thereby promote hematopoietic development. Furthermore, we have discovered that there is significant batch variation in the levels of biologically active leptin, neutralizable with soluble murine leptin receptor, in FBS (data not shown). This variable needs to be controlled for, and may be one of the factors that distinguishes lots of FBS for hematopoietic assays.
With the exception of reports detailing impaired immune responses23,24 and a recent report on defects in the lymphoid system,11 there have been few reports of hematologic abnormalities in db and ob homozygous animals.25 As suggested by our work and others, the impairments seen in the immune system may be a direct result of a deficit in macrophage function and lymphoid development. It is possible that peripheral blood abnormalities in these animals are attenuated by one or more of the other cytokines. This would be analogous to the lack of significant effects on basal hematopoiesis seen in the absence of GM colony-stimulating factor26 and IL-6.27
An intriguing possible explanation for the relative lack of effects on the hematopoietic system may lie in tissue specific effects of the db mutation on leptin receptor signaling. The fact that multiple isoforms are expressed and that one or more of these isoforms may have reduced signaling ability in some situations8,9 suggests the possibility for tissue-specific regulation of functional leptin receptors. A nonsignaling isoform paired with a full-length molecule would bind leptin but may not be capable of transmitting a signal. This dominant-negative effect would be controlled by the level of expression of the isoforms similar to that seen in other systems.28,29 The fact that in some circumstances the B219.3 isoform can transmit meaningful signals10 (B. Barut, et al, unpublished observations, August 1996) suggests the possibility that in some tissues there may be an as yet unidentified signaling molecule that associates with the B219 chain. This would be similar to many of the hematopoietin receptors including IL-3R, IL-6R, and GM-CSFR.30 31 These studies indicate the need for further careful evaluations of the role of leptin in the development and maintenance of the entire hematopoietic system, including inflammatory reactions, in vivo hematopoietic stem cell reconstitution, and ex vivo stem cell expansion.
Our studies with hematopoietic colony-forming assays show that leptin has a significant, direct effect on differentiation of lineage-restricted erythrocytic and myelopoietic lineages. In conjunction with our previous observations showing the expression of leptin receptor in primitive hematopoietic cells, these data support our original hypothesis that leptin plays a role in early hematopoiesis.2 The findings that leptin stimulates the proliferation of yolk sac cells and that biologically active leptin is present in yolk sac fluid (data not shown) support this hypothesis. These data strongly suggest that further work is needed to evaluate the clinical utility of leptin as a growth factor for the ex vivo expansion of hematopoietic precursors, perhaps used in conjunction with enrichment for leptin receptor-positive bone marrow cells. In addition, the in vivo use of leptin either alone or in combination with EPO to treat anemias or to increase macrophage and immune function needs further evaluation.
ACKNOWLEDGMENT
We acknowledge the contribution of Charles Wall and Marian Kennedy for their expert technical assistance to this work, to Wendy Greenwell for preparing the manuscript, and to Carrie Wick for critically reading the manuscript.
Address reprint requests to H. Ralph Snodgrass, PhD, Progenitor, Inc, 1507 Chambers Rd, Columbus, OH 43212.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal