Abstract
The response of a B cell to antigen is dependent on the surface expression of a clonotypic B-cell receptor complex (BCR) consisting of membrane-bound Ig and disulfide-linked heterodimers of Igα/β. Studies of Igα or Igβ have shown that the immunoreceptor tyrosine-based activation motif (ITAM) found in each cytoplasmic tail is capable of inducing most receptor signaling events. However, Igα, Igβ, and most of the other receptor chains that contain ITAMs, including CD3ε, CD3γ, TCRζ, and FcεRIγ, are found as components of multimeric and heterogenous complexes. In such a complex it is possible that cooperativity between individual chains imparts functional capacities to the intact receptor that are not predicted from the properties of its constituents. Therefore, we developed a novel system in which we could form and then aggregate dimers, representative of partial receptor complexes, which contained either Igα alone, Igβ alone, or the two chains together and then examine their ability to induce apoptosis in the immature B-cell line, WEHI-231. Here we present evidence that heterodimers of Igα and Igβ efficiently induced apoptosis while homodimers of either chain did not. Apoptosis was associated with the inductive tyrosine phosphorylation of a very restricted set of proteins including the tyrosine kinase Syk. These findings may provide insight into the mechanisms by which the BCR, and other such multimeric receptor complexes, initiate both apoptotic and proliferative responses to antigen.
B-CELL DEVELOPMENT is a sequential and orderly process that ensures the production of a functional antigen receptor (BCR) which is not reactive with self.1 The initiation of Ig rearrangement, with the formation of Dμ, appears to be an intrinsic property of those cells within the bone marrow (BM) or fetal liver which have committed to the B-cell lineage. However, subsequent events, including pre-B cell transition,2-5 allelic exclusion,1 and the deletion of autoreactive clones,6,7 are dependent on the expression of an antigen receptor complex containing the heavy chain of membrane-bound Ig (mIg) and disulfide-linked heterodimers of Igα and Igβ. This latter structure translates receptor engagement into the biochemical signals that drive cellular responses.8-15
Within each of the cytoplasmic tails of Igα and Igβ is embedded a consensus sequence, the immunoreceptor tyrosine-based activation motif (ITAM),16-19 which is common to other multichain immune recognition receptor (MIRR)18 subunits, including CD3ε, TCRζ, FcγRIIIγ, and FcεRIγ. The motif contains two tyrosines, both of which are critical for initiating tyrosine kinase activation.20,21 It has been postulated that phosphorylation of these tyrosines serves to form a scaffold on which tyrosine kinases can assemble, thereby facilitating their activation and the subsequent recruitment of secondary effector molecules.22-26
The presence of multiple ITAMs within each MIRR has led some to suggest that such heterologous signaling chains as Igα and Igβ may be redundant and that their multiplicity serves only to amplify a single signal.27,28 Indeed, although each has very different biochemical properties in vitro,22,29 it has been difficult to show reproducible functional differences in vivo.4,30 However, these functional studies have used chimeras in which the individual cytoplasmic domains to be studied were expressed as a fusion to an irrelevant extracellular and transmembrane domain. This approach assumes that functions ascribable to an isolated chain accurately reflect the function of that chain within the context of the intact receptor complex. Recently, we have shown that this assumption may not be valid and that significant cross-talk occurs between the cytoplasmic domains of Igα and Igβ.31 In particular, although Igβ can generate signals independently, when heterodimerized with Igα its primary function may be to enhance that chains' phosphorylation. The Igα/β heterodimer can induce the phosphorylation of a wider range of substrates at a lower threshold of stimulation than homodimers of either molecule.
Herein we report that Igα and Igβ can synergize, not only in initiating signal transduction events but in mediating biological responses. When we expressed chimeric receptors in which the extracellular and transmembrane domains of platelet-derived growth factor receptor (PDGFR)α or β were fused to the cytoplasmic domains of either Igα or Igβ in the immature B-cell line WEHI-231,32 33 we found that heterodimers of Igα and Igβ could induce apoptosis while homodimers of either chain could not. The induction of apoptosis was associated with the strong and selective induction of Syk tyrosine phosphorylation.
MATERIALS AND METHODS
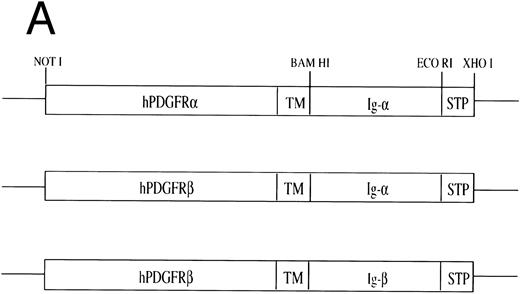
Construction of PDGFR chimeras.cDNAs encoding PDGFRα and β chains were provided by A. Kazlauskas (National Jewish Center, Denver, CO). A BamHI restriction site was introduced at bp 1867 of the hPDGFRα by knit polymerase chain reaction (PCR) using the oligonucleotides GCTCAAAATGAAGATGCTGTGAAGAGC, AAACAGAAACCGAGGATCCAAATTCTAGAGGGTCATTGAATCAAT, GACCCTCCAGCGAATTCGGATCCTCGGTTTCAGTTT, GACCCGACCAAGCACT AGTCC, and at bp 2044 of the hPDGFRβ chain using site directed mutagenesis (Promega, Madison, WI) with the oligonucleotide AAGCCACGTTACGGGATCCGATGGAAG. The resulting Not I/BamHI fragment encoding the extracellular and transmembrane domains of the hPDGFRα or hPDGFRβ chain was assembled in pSK+ with a cDNA encoding the cytoplasmic tail of either Igα or Igβ22 and an EcoRI/Xho I linker containing stop codons in all frames. The assembled Not I/Xho I chimeric construct was ligated into the expression vector pCB6+/muTk (H. Singh, University of Chicago, Chicago, IL) which contains a neo resistance gene and an IgM/thymidine kinase enhancer/promoter. The cDNAs encoding the chimeric constructs hPDGFRβ/Ig-α and hPDGFRβ/Ig-β were transfected separately, and both hPDGFRα/Ig-α, hPDGFRβ/Ig-β together into WEHI-231 by electroporation. Clones were derived by selection in complete Iscove's Modified Dulbecco's Medium (IMDM; Sigma, St Louis, MO) supplemented with G-418 (1.2 mg/mL). Clones were stained with chain specific anti-PDGFRα and anti-PDGFRβ antibodies (Genzyme, Cambridge, MA), fluoroscein isothiocyanate (FITC)-conjugated anti-IgG1 (Zymed, San Francisco, CA), and analyzed by flow cytometry (Becton Dickinson, Bedford, MA).
Assays of cell death.1 × 105 cells/mL of WEHI-231 wild-type (wt), single, or dual-expressing clones were stimulated for 24 hours at 37°C. Cells were stimulated through the endogenous receptors using a polyclonal anti-IgM (Jackson Immunoresearch, West Grove, PA) at 20 μg/mL or an anti-Fas antibody (5 μg/mL; Pharmingen, San Diego, CA). To stimulate through the chimera, cells were incubated with 100 ng/mL PDGF-BB ligand (Sigma, St Louis, MO) for 5 minutes, followed by 5 μg/mL anti-PDGFRβ antibody for 3 minutes and then 5 μg/mL anti-IgG1 antibody (Jackson Immunoresearch). After 24 hours, cells were pelleted, resuspended in 3% bovine serum albumin/phosphate-buffered saline (BSA/PBS) with 5 μg/mL of propidium iodide (PI), and analyzed by flow cytometry using LYSIS II (Becton Dickinson).
Photomicroscopy of nuclei.Nucleoids were prepared by lysing 2 × 106 cells in buffer containing 0.15% NP40 and 1 mmol phenylmethylsulfonyl fluoride (PMSF ) for 30 minutes at 4°C. Nuclei were pelleted, then resuspended in 0.2 mol/L HCl, 0.4 mmol CaCl2 , and rotated for 18 hours at 4°C. Nucleoids were pelleted, then resuspended in 50 μL 100 mmol TRIS, 5 mmol NaCl, 0.5 mmol CaCl2 , pH 8.3. The suspension was mixed 1:1 with 10 μg/mL acridine orange in PBS and analyzed by scanning confocal microscopy (488/ex/515/em ) (Zeiss, Oberkochen, Germany).
Phosphotyrosine and Syk immunoblotting.Aliquots (107 cells/sample) were either left unstimulated (unstim), stimulated with 20 μg/mL of anti-IgM antibody for 5 minutes (Ig), or stimulated through the chimeric receptors (Ch) as above except that cells were lysed after 2 minutes of stimulation with anti-IgG1 . Cells were lysed in buffer containing 1% NP40, and lysates were immunoprecipitated with the antiphosphotyrosine monoclonal antibody (MoAb) FB2 (American Type Culture Collection [ATCC], Rockville, MD). Precipitates were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) transferred to a nylon membrane, and probed with the antiphosphotyrosine MoAb Ab-2 (Oncogene Science, Uniondale, NY). The immunoblot was visualized with enhanced chemiluminescence (ECL) using a horseradish peroxidase (HRP)-conjugated polyclonal antibody against murine Ig (Amersham, Arlington Heights, IL). The immunoblot from Fig 4 was stripped and reprobed with a polyclonal antibody against Syk (gift of E. Clark, University of Washington, Seattle). The Syk immunoblot was also visualized with ECL using an HRP-conjugated polyclonal antibody against rabbit Ig (Amersham).
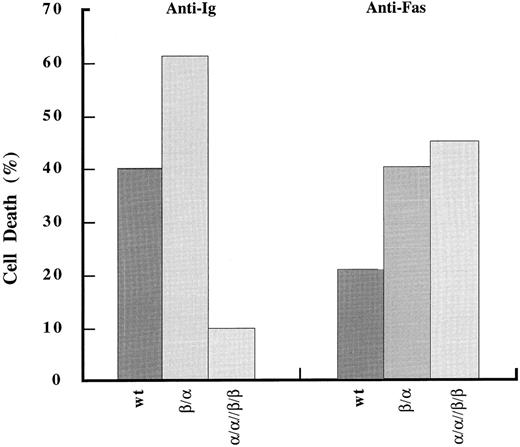
Fas-mediated apoptosis is comparable between wild-type and chimera-expressing cells. Analysis of cell death induced upon stimulation through Fas antigen was done as in Fig 2.
Fas-mediated apoptosis is comparable between wild-type and chimera-expressing cells. Analysis of cell death induced upon stimulation through Fas antigen was done as in Fig 2.
RESULTS
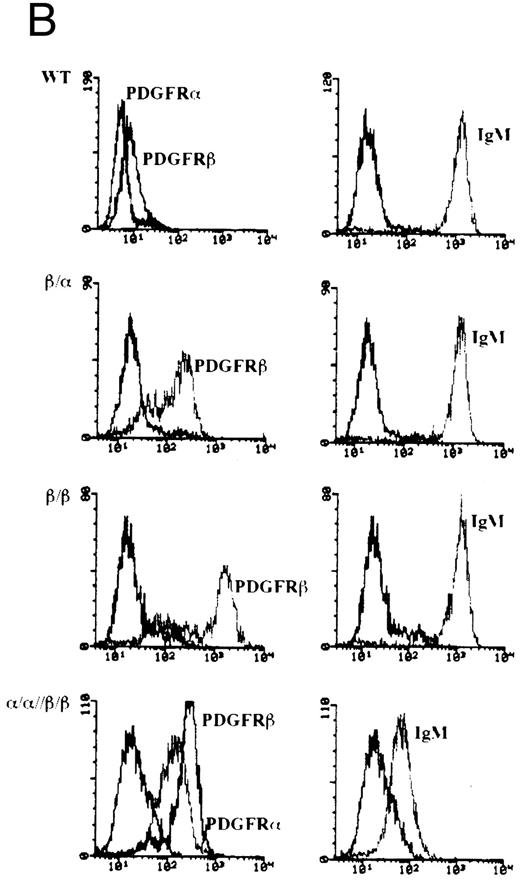
Construction, expression, and stimulation of PDGFR chimeras in WEHI-231 cells.Recently, we have shown that the cytoplasmic tails of Igα and Igβ can cooperate synergistically to initiate signal transduction pathways.31 We next asked whether this cooperativity in signaling translated into qualitative differences in mediating biologically relevant responses. To this end, we used cDNAs encoding chimeras in which the cytoplasmic tails of Igα or Igβ were fused with the extracellular and transmembrane domains of either human PDGFR α or β (Fig 1A). In WEHI-231 cells, an immature B-lymphoma line which undergoes apoptosis upon crosslinking the BCR, the PDGFR chimeras were expressed singly or in combination to establish lines expressing the following: PDGFRβ/Igα (β/α), PDGFRβ/Igβ (β/β), or PDGFRα/Igα and PDGFRβ/Igβ (α/α//β/β) (Fig 1B). As can be seen in Fig 1B, the level of expression of the PDGFRβ chimeras on β/α and β/β was equal to or greater to that seen on the doubly transfected cell line α/α//β/β. In particular, although chimera expression levels on β/α were comparable with those on α/α//β/β, the expression levels on β/β were 5- to 10-fold higher. Previously we have shown that the fluorescence intensity obtained using either anti-PDGFRα or β antibodies was approximately equal for a given amount of expressed protein.31 Therefore, approximately half as much PDGFRα/Igα is expressed on the surface of α/α//β/β as PDGFRβ/Igα.
Construction of chimeric cDNAs and expression in WEHI-231. (A) Diagram of chimeric cDNAs. Extracellular and transmembrane (TM) domains encoding cDNA fragments derived from the hPDGFRs were ligated to cDNA fragments encoding the cytoplasmic domains of murine Igα/β. (B) Surface expression of chimeric proteins and IgM on transfectants. To further characterize, the above cDNAs were also expressed in the B-cell lymphoma A20. Proteins corresponding to the predicted size of the chimeric molecules were immunoprecipitated by monoclonal antibodies to the PDGFR (Genzyme) and immunoblotted with specific antibodies against Igα or Igβ.31
Construction of chimeric cDNAs and expression in WEHI-231. (A) Diagram of chimeric cDNAs. Extracellular and transmembrane (TM) domains encoding cDNA fragments derived from the hPDGFRs were ligated to cDNA fragments encoding the cytoplasmic domains of murine Igα/β. (B) Surface expression of chimeric proteins and IgM on transfectants. To further characterize, the above cDNAs were also expressed in the B-cell lymphoma A20. Proteins corresponding to the predicted size of the chimeric molecules were immunoprecipitated by monoclonal antibodies to the PDGFR (Genzyme) and immunoblotted with specific antibodies against Igα or Igβ.31
Interestingly, when both chimeric chains were coexpressed, the surface expression of IgM was diminished by up to 100-fold. This effect was quite reproducible in that nine different clones derived from two separate transfections had a similar phenotype. Although the mechanism of decreased IgM surface expression is unclear, it is apparently not due to a decrease in the synthesis of BCR components in that immunoblotting of lysates from wild-type, singly, or doubly transfected cell lines showed no differences in either Igα or Igβ expression (data not shown).
As both PDGFRs bind their naturally occurring ligand, PDGF-BB, with equal affinity,25 homodimers or predominately heterodimers can be formed in singly or doubly transfected cells, respectively. The addition of PDGF-BB serves to form dimers that are representative of the resting partial receptor complex. This dimerization of chimeric receptors does not result in signal transduction as addition of PDGF-BB alone does not induce tyrosine kinase activation31 (and data not shown). To “activate” these homodimeric or heterodimeric complexes, they are first aggregated with anti-PDGFRβ antibodies (IgG1; Genzyme) followed by goat anti-mouse IgG1.
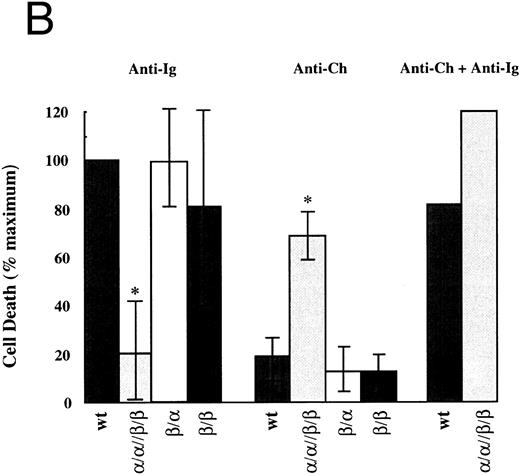
Apoptosis occurs only upon stimulation of heterochimeric complexes that contain both Igα and Igβ.Using the system described above we assayed the ability of the cytoplasmic tails of Igα, Igβ, or the two together to induce cell death. Cells were stimulated through the chimeras for 24 hours and then assayed for cell death by PI exclusion and change in forward scatter (FSC) (Fig 2). Results were compared with cell death induced by the BCR on transfectant and wild-type cells. As seen in Fig 2A, stimulation of the BCR on both wild-type and singly transfected cells readily induced cell death. In contrast, BCR-induced cell death in cells expressing α/α//β/β was diminished. The decreased response was probably due, at least in part, to the reduced BCR surface expression. Analysis of cell death induced by the chimeras gave a different result. In both β/α and β/β cell lines, stimulation of the chimeras was unable to induce cell death whereas stimulation of chimeric complexes on α/α//β/β cells did (n = 3). This induction of cell death was not due to higher expression levels of the chimeric receptors in our double transfectants as the chimeras in the β/β line were expressed at much higher levels.
Both cytoplasmic domains of Igα and Igβ are necessary to induce cell death. (A) Flow cytometric analysis of cell death induced in wild-type WEHI-231 (WT) and an α/α//β/β transfected clone. Shown are dot plots of forward scatter (FSC) versus propidium iodide (PI) staining for a typical experiment. (B) Analysis of cell death in all transfectants. The mean and standard deviation derived from three experiments is shown. For each experiment, the percent change in cells stained with PI upon stimulation was determined by subtracting the percentage of PIhigh (as gated in A) cells in unstimulated populations cells from that in the corresponding stimulated populations. This number was then expressed as a percentage of the cell death obtained from stimulating the wt BCR (% maximum). “Ig” refers to BCR and “Ch” refers to the chmeras. *Indicates values that were significantly different (P < .01, two-sided t-test) from those obtained from wild-type cells.
Both cytoplasmic domains of Igα and Igβ are necessary to induce cell death. (A) Flow cytometric analysis of cell death induced in wild-type WEHI-231 (WT) and an α/α//β/β transfected clone. Shown are dot plots of forward scatter (FSC) versus propidium iodide (PI) staining for a typical experiment. (B) Analysis of cell death in all transfectants. The mean and standard deviation derived from three experiments is shown. For each experiment, the percent change in cells stained with PI upon stimulation was determined by subtracting the percentage of PIhigh (as gated in A) cells in unstimulated populations cells from that in the corresponding stimulated populations. This number was then expressed as a percentage of the cell death obtained from stimulating the wt BCR (% maximum). “Ig” refers to BCR and “Ch” refers to the chmeras. *Indicates values that were significantly different (P < .01, two-sided t-test) from those obtained from wild-type cells.
It was possible that the phenotype of the α/α//β/β cell lines was not due to the coligation of the cytoplasmic domains of Igα and Igβ but due to the coligation of the extracellular and transmembrane domains of PDGFRα with those of PDGFRβ. Therefore, we coexpressed PDGFRβ/Igα and PDGFRβ/Igβ to generate β/α//β/β. Expression of each chimera was confirmed by immunoblotting with Igα and Igβ specific antibodies31 (data not shown). These cells expressed the same phenotype as α/α//β/β lines with decreased surface expression of IgM and impaired but detectable and equal killing induced through the BCR and the chimeras (data not shown). These data show that the induction of apoptosis and the downregulation of mIgM is dependent on the coexpression of Igα and Igβ.
The downmodulation of mIgM in α/α//β/β certainly contributed to its resistance to antigen receptor–mediated apoptosis. However, the observation that the induction of apoptosis through both the BCR and the chimeras was suboptimal suggests that either each receptor complex, or the cytoplasmic effector molecules each uses, were limiting. To examine these possibilities further, we stimulated both receptor complexes simultaneously and assayed for the induction of cell death. As expected, death induced by the coengagement of each receptor complex was additive and equaled the death induced by the BCR on wild-type and singly transfected clones (Fig 2B). These results are consistent with either possibility. However, another observation indicates that competition for secondary effector molecules is occurring. Over time, one of the α/α//β/β clones lost detectable expression of surface IgM. This was accompanied by an enhancement of killing inducible by the chimeras on these cells (data not shown).
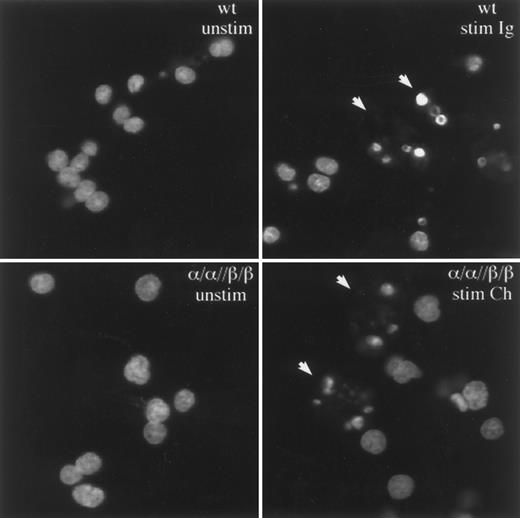
To confirm that the WEHI-231 cells were dying by apoptosis and not necrosis, cell nuclei isolated from unstimulated and stimulated cells were stained with acridine orange and visualized by confocal microscopy (Fig 3). As shown, the induction of cell death in both wild-type and α/α//β/β transfected cells was accompanied by the characteristic chromatin condensation and nuclear fragmentation of apoptosis.4 34 From these results it is clear that receptor complexes which contain the cytoplasmic tails of both Igα and Igβ can induce apoptosis and that this function is enhanced by coengagement of the BCR.
Igα and Igβ induce death by apoptosis. Stimulation of α/α//β/β or the BCR on wt cells induces nuclear changes characteristic of apoptosis. The nuclei from nonapoptotic cells stain diffusely with acridine orange, whereas nuclei from apoptotic cells stain intensely for condensed chromatin bodies (denoted by arrows).
Igα and Igβ induce death by apoptosis. Stimulation of α/α//β/β or the BCR on wt cells induces nuclear changes characteristic of apoptosis. The nuclei from nonapoptotic cells stain diffusely with acridine orange, whereas nuclei from apoptotic cells stain intensely for condensed chromatin bodies (denoted by arrows).
Fas-mediated apoptosis is comparable between wild-type and transfected cells.We next asked if the coexpression of PDGFRβ/Igβ and PDGFRα/Igα inhibited other pathways mediating apoptosis. Therefore, we examined the surface receptor Fas which, on both immature and peripherally activated B lymphocytes, can induce apoptosis independently of tyrosine kinase activation.35-37 Surface staining of Fas on wild-type and transfected WEHI-231 cells showed comparable expression levels between all cell lines (data not shown). Incubation of wild-type WEHI-231 cells with anti-Fas antibody for 24 hours induced cell death as measured by PI exclusion (Fig 4). When either β/α or α/α//β/β chimera–expressing cells were stimulated through Fas, comparable, if not enhanced, cell death was observed (Fig 4). These data suggest that while the expression of the Igα/β-containing chimeras inhibited the induction of apoptosis by the BCR they did not induce a generally unresponsive or resistant phenotype.
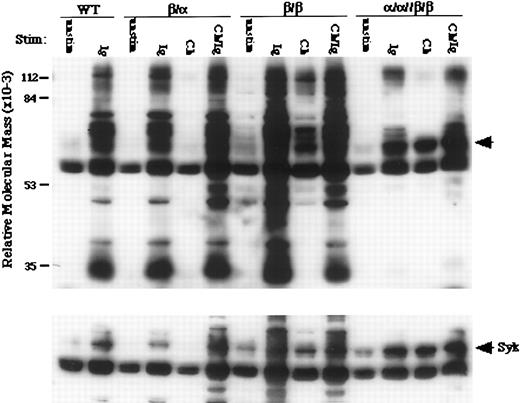
The selective activation of Syk is induced by stimulating heterodimers containing Igα and Igβ.To begin to understand the signaling events underlying heterochimeric-induced apoptosis, we examined the ability of each complex to induce tyrosine phosphorylation of cellular proteins after aggregation. Immunoprecipitated tyrosine-phosphorylated proteins from stimulated wild-type and transfected cells were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine antibodies. Shown in Fig 5 are the results from a typical experiment (n = 3). Stimulation of the chimeras on β/β and β/α cells induced little or no phosphorylation of cellular substrates, respectively. In contrast, stimulation of α/α//β/β cells induced the strong phosphorylation of a small subset of proteins, including a band of 65 to 70 kD, which were also induced by the endogenous BCR on wild-type and singly transfected cells (top panel). Stripping and reprobing the membrane showed that the 65 to 70-kD band contained the tyrosine kinase Syk (bottom panel). Although weak phosphorylation of Syk is induced by the highly expressed β/β chimera, this degree of activation, by itself, is not sufficient to induce apoptosis. Because BCR-induced apoptosis is dependent on tyrosine kinase activation,9,38,39 our results indicate that Igα and Igβ cooperate to facilitate the tyrosine phosphorylation of a subset of substrates essential for the induction of apoptosis. Recently, in parallel experiments performed in the B-cell lymphoma A20, we have observed that the coexpression of chimeras containing Igα and Igβ also lowers the threshold for tyrosine kinase activation.31
Selected phosphorylated substrates are required for apoptosis in WEHI-231. Induction of tyrosine phosphorylation in WEHI-231 expressing Igα-, Igβ-, or Igα and Igβ-containing chimeric receptors (top). Wt and transfected cells were stimulated through chimeric proteins (Ch), endogenous IgM (Ig), or both (Ch/Ig). Phosphorylation of Syk (arrow) is preferentially induced in cells undergoing apoptosis (bottom).
Selected phosphorylated substrates are required for apoptosis in WEHI-231. Induction of tyrosine phosphorylation in WEHI-231 expressing Igα-, Igβ-, or Igα and Igβ-containing chimeric receptors (top). Wt and transfected cells were stimulated through chimeric proteins (Ch), endogenous IgM (Ig), or both (Ch/Ig). Phosphorylation of Syk (arrow) is preferentially induced in cells undergoing apoptosis (bottom).
DISCUSSION
All of the receptors that recognize antigen have a structure in which multiple heterologous chains, separate from the antigen-binding substructure, form one or more signal transduction complexes. All of these signal transduction molecules contain a conserved motif, the ITAM whose amino acids are necessary for kinase activation and recruitment.9,18,23,26 Numerous studies have shown that individual chains containing a single ITAM are sufficient to initiate tyrosine kinase activation.40-42 Therefore, if one chain can mediate signaling, why does each receptor contain two or more? Herein we provide evidence that Igα and Igβ, two heterologous chains of the BCR which contain ITAMs, can function synergistically to induce the phosphorylation of specific substrates and to induce apoptosis. These data, and data we have presented elsewhere,31 show that each chain may have specialized, complementary, and nonredundant functions within the context of the receptor complex.
Our results apparently contradict the observations of Yao et al30 and of Papavasiliou et al,4 in which the cytoplasmic tails of Igα or Igβ alone were sufficient to induce apoptosis and to mediate negative selection, respectively. However, in both of these experimental models we would argue that what is being tested is the functional capacity of these single domains under optimal circumstances in which large numbers of Igα or Igβ chimeras are being engaged. Our own data, presented here31 and elsewhere, support the contention that either Igα or Igβ can, under some circumstances, activate tyrosine kinases. What is not addressed in these studies is the efficiency with which these processes are mediated. Our data in aggregate argue that the functional capacities of Igα and Igβ combine synergistically to lower the threshold of kinase activation and that at lower expression levels, or at lower rates of receptor engagement, this synergy translates into absolute differences in the ability of the heterodimer to mediate biological processes. The importance of this synergy has been recently illustrated by the work of Torres et al.5 They generated, using homologous recombination, mice in which Igα was lacking its cytoplasmic domain. In these deficient mice, B-cell development was very inefficient at all stages despite the presence of an intact Igβ chain within the receptor complex. Apparently this was insufficient, at the avidity at which the pre-B cell antigen receptor is engaged in the bone marrow, to signal progression to more mature stages. It is not known at this time whether the cytoplasmic tail of Igα is needed for negative selection.
Interestingly, although few substrates were inducibly tyrosine phosphorylated by the heterodimerized chimeras, Syk was strongly phosphorylated after stimulation. This suggests that Syk would be the preferred substrate of the Igα/β heterodimer, which is consistent with the proven importance of Syk in BCR-mediated signal transduction.9,43-46 Loss of Syk in B-cell lines sensitive to BCR-mediated apoptosis renders them resistant.38 Furthermore, B-cell development in Syk-deficient mice is arrested at the pre-B stage.47,48 Upon tyrosine phosphorylation of Igα, Syk binds via its SH2 domains49 and this binding enhances the activity of the tyrosine kinase.25 Although the downstream effectors of Syk are not known, the phosphorylation and activation of phospholipase C-γ (PLC) has been shown to depend on Syk.46,49 This is in agreement with the recently demonstrated importance of PLC in inducing apoptosis.38 However, it is doubtful that PLC activation is necessary for the induction of apoptosis in WEHI-231 in that ligation of the BCR in our cells failed to induce changes in intracellular calcium (data not shown).
One of the unexpected findings of our studies was that coexpression of the cytoplasmic domains of Igα and Igβ suppressed surface expression of the endogenous BCR in WEHI-231. This phenotype was not observed when similar heterochimeras were expressed in the more mature B-cell line A20 IIA1.6. Therefore, we do not know if the inhibition of BCR surface expression is a property of immature cells or just of WEHI-231 in particular. However, such a phenotype, whether a general or specific phenomenon, could be caused by several mechanisms including decreased synthesis, decreased transport, or increased clearance from the cell surface. It is unlikely that the decrease seen was caused by decreased synthesis in that the Igα and Igβ proteins were present in normal amounts. It seems probable that the heterochimeras are effectively competing with the BCR for transport molecules or mechanisms that are found in limiting amounts within these cells. Studies are currently in progress to discern between these possibilities.
Our data also suggest that coexpression of PDGFRα/Igα and PDGFRβ/Igβ, not only suppresses BCR expression, but permits the formation of a receptor complex which effectively competes with the BCR for secondary signal transduction molecules. This competition contributes to the decreased cell death observed upon stimulation through the endogenous BCR in our double transfectants. It is possible that the cytoplasmic tails of Igα and Igβ combine to make a unique binding site for a necessary and limiting secondary effector. Alternatively, the cytoplasmic tails of each chain could bind distinct effectors,22 each of which is redundant but which together are necessary for effective signal initiation. This seems more likely since, in previous studies using GST Igα and Igβ fusion proteins, we have shown that no new molecules bind to combinations of the fusion proteins that do not bind to one or the other individually.29
Previous studies of independently expressed Igα and Igβ have shown that each chain, despite containing a common motif, has distinct biochemical and signal transducing capacities.21,22,29,42 The results presented here indicate that these capacities combine synergistically to efficiently mediate necessary developmental functions such as the central deletion of autoreactive B-cell clones.50
ACKNOWLEDGMENT
We thank Craig Thompson, Diane DeNagel, and Shara Kabak for critical reading of this manuscript and comments.
J.T. and B.J.E. contributed equally to this work.
Supported by National Institutes of Health Grant No. GM52736 to M.R.C.. M.R.C. is also supported by a grant from the Arthritis Foundation. J.T. is supported by a William B. Graham Fellowship from the Baxter Foundation.
Address reprint requests to Marcus R. Clark, MD, Department of Medicine, University of Chicago, 5841 S Maryland Ave, MC0930, Chicago, IL, 60637.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal