Abstract
Glucocorticoid (GC) use is known to induce or enhance the growth of Kaposi's sarcoma (KS) in many clinical settings including human immunodeficiency virus infection, collagen vascular disease, lymphoproliferative disorders, and renal transplantation. Because GCs may induce immune suppression and thus tumor growth, we determined whether GCs had a direct effect on KS growth. We found that GCs directly induce the growth of KS cell lines. In examining the mechanism of action of GCs, we did not observe induction of known autocrine growth factors for KS including interleukin-1 (IL-1), IL-6, oncostatin-M, basic fibroblast growth factor (bFGF ), and vascular endothelial growth factor (VEGF ). We thus examined factor(s) that inhibit KS growth. Transforming growth factor-β (TGF-β) is produced by KS cells and has pleiotropic effects, including inhibiting the growth of hematopoietic and endothelial cells. We show that TGF-β is produced by KS cells in both the latent and active forms, and that TGF-β is an autocrine growth inhibitory factor. We then studied the effects of GCs on the regulation of TGF-β and found that GCs do not inhibit TGF-β transcription, but significantly inhibit TGF-β activation. This effect is mediated through regulation of the TGF-β activation pathway. TGF-β is activated by plasmin which is positively regulated by plasminogen activator (PA) and PA receptor (PAR), and negatively regulated by plasminogen activator inhibitor (PAI). GCs downregulated PAR and upregulated PAI. Thus, glucocorticoids enhance KS cell growth through the regulation of TGF-β activation.
KdAPOSI'S SARCOMA (KS) is the most common tumor associated with human immunodeficiency virus-1 (HIV-1) infection.1 KS is the first acquired immunodeficiency syndrome (AIDS)-defining illness in nearly 10% of all patients infected with HIV-1,2 but up to 30% of the cases with AIDS develop KS during the course of the illness. The skin is the most common site of disease; however, KS can also involve visceral organs such as the lungs and the gastrointestinal tract leading to severe morbidity and contributing to death in nearly 30% of AIDS patients.3
KS lesions are vascular structures that lack basement membranes and display slitlike vascular structures containing extravasated red blood cells and mononuclear cells.3 The lesion is characterized by the proliferation of spindle-like cells which have a similar phenotype to endothelial and vascular smooth muscle cells.4 5
In vitro isolation and long-term maintenance of KS-spindle cells and, more recently, isolation of transformed KS cell lines have allowed for the study of factors that regulate KS cell growth.6,7 Interleukin-1 (IL-1), IL-6, oncostatin-M, basic fibroblastic growth factor (bFGF ), and vascular endothelial growth factor (VEGF ) have all been shown to regulate KS cell growth.8-13 Further, HIV-1 tat protein is a mitogen for KS cells in vitro.14,15 KS-like tumors develop only in male mice transgenic for HIV-tat, analogous to the clinical findings of male predominance with male to female incidence ratio of over 15 to 1.15
Administration of glucocorticoids (GCs) is associated with the development of KS in renal transplant recipients,16,17 autoimmune diseases,18 lymphoproliferative disorders,19 and HIV-1 infection.20-22 Withdrawal of GCs is often associated with spontaneous regression of KS.16-22 GCs may exert effects on tumor cell growth directly or indirectly by regulation of immunologic parameters.23 To study the effects of GCs, we first show that KS cells, both primary tumor tissues and in vitro isolates, express GC receptors. We studied the effects of GCs on KS cell growth and found no induction of known KS growth factors (IL-1, IL-6, bFGF, VEGF ). However, we did find that transforming growth factor-β (TGF-β) is an autocrine inhibitory factor and that GCs inhibit the activation of the latent form of TGF-β, which leads to enhanced cell growth.
MATERIALS AND METHODS
Cell culture.AIDS-KS–derived spindle cell isolates and transformed cell lines were made from primary tumor tissues as described previously.6-8 Cells were cultured continuously in 75-cm2 flasks, coated with 1.5% gelatin, in KS medium consisting of RPMI 1640 (GIBCO, Grand Island, NY), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L glutamine, essential and nonessential amino acids, 10% charcoal-treated fetal calf serum (FCS; GIBCO), and 1% Nutridoma-HU (Boehringer Mannheim, Indianapolis, IN). Human umbilical vein endothelial cells (HUVEC) were also cultured on gelatin-coated plates in RPMI 1640, 10% charcoal-treated FCS, 2 mmol/L glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL), heparin (20,000 U/mL), and endothelial cell growth supplement (ECGS; 30 μg/mL) (GIBCO). Human foreskin fibroblasts were grown in Dulbecco's Modified Eagle Medium (DMEM) (GIBCO) and 10% charcoal-treated FCS. Human aortic smooth muscle cells (HASM) (Clonetics, San Diego, CA) were grown in RPMI 1640 and 10% charcoal-treated FCS.
Immunofluorescent techniques.Cells were prepared on slides with a cytospin (Shandon, Astmoor, UK) and fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 30 minutes. The slides were rinsed twice in PBS and incubated with 0.2% Triton-X100 (J.T. Baker, Phillipsburg, NJ) in PBS and 10% FCS for 20 minutes. The slides were then preincubated with 50% FCS for 30 minutes and again with rabbit anti-human GC receptor antibody (B2) with 1:50 dilution in PBS and 10% FCS for 45 minutes at 37°C.24 After washing three times, the slides were incubated with a fluoroscein isothiocyanate–conjugated goat anti-rabbit IgG antibody (Sigma) for 30 minutes at 37°C. Photographs were taken with a Zeiss III RS fluorescence microscope (Oberkochen, Germany) using Kodak Ektachrome 400 color film (Eastman Kodak, Rochester, NY).
Cellular proliferation assay.Early passage (passages 5-10) AIDS-KS spindle cell isolates (KSC-1, KSC-2, and KSC-3) were seeded at a density of 1.0 × 104 cells/well in a 24-well plate in KS medium. The cells were allowed to attach overnight and treated with varying concentrations of hydrocortisone, TGF-β1 (Amgen, Thousand Oaks, CA), and TGF-β neutralizing antibody (R&D Systems, Minneapolis, MN) all given alone or in various combinations, on day 1 and day 3. The cell count was performed on day 6 using a Coulter Particulate Counter (Hialeah, FL). Similarly, cell proliferation assays were performed on HUVEC, HASM, and human skin fibroblast.6 8
Cell-cycle analysis by flow cytometry.KS cells (KSY-1) were seeded in gelatin-coated T-75 flasks with 750,000 cells. The cells were cultured and starved in RPMI medium without serum for 24 hours. Then the cultures were washed three times with Ca2+- and Mg2+-free PBS and cultured in RPMI medium plus 0.4% FCS with or without 10−6 mol/L hydrocortisone. After 24 hours of incubation, the cells were washed three times with PBS and obtained by incubation with 0.1% trypsin at 37°C for 5 minutes. The cells were washed twice with PBS; then 1 mL of ice-cold 70% ethanol was added into the cells drop by drop. The fixed cells were placed at 4°C overnight. The cells were washed once with PBS and stained with 1 mL of propidium iodide solution (PI; 50 μg/mL) and RNAse A (5 μg/mL). The samples were incubated at room temperature for 2 hours. Fluorescence of the stained cells was measured by flow cytometry and the data were displayed as a plot of the fluorescence intensity versus the number of cells and the percent of cells in G1 , S, or G2 + M was estimated.
Northern analysis.Cells were grown to near 80% confluence in 75-cm2 culture flasks and treated with hydrocortisone for 4 to 24 hours. The cells were trypsinized and washed twice with Hanks' balanced salt solution (HBSS). Total RNA was extracted by guanidium thiocyanate method (RNAzol; Tel-Test Inc, Friendswood, TX) and separated on a 1% agarose formaldehyde gel followed by transfer onto a nylon membrane and cross-linked with UV-light (Stratagene, La Jolla, CA). The transferred RNA was prehybridized at 68°C for at least 30 minutes in 10 mL Quick Hyb hybridization solution (Stratagene, San Diego, CA) containing 100 μg of salmon sperm DNA. The filters were then hybridized with 32P-nick translated full-length cDNA probes for IL-6 (generous gift from Pravin Sehgal, New York Medical College, Valhalla), IL-1β, VEGF, bFGF (generous gift from Judith Abraham, Scios Nova, Mountain View, CA), TGF-β1 (full-length cDNA provided kindly by Anita Roberts, National Institutes of Health, Bethesda, MD), and β-actin at 68°C for 2 hours. The membrane was washed twice for 15 minutes at room temperature with 2× standard saline citrate (SSC)/0.1% sodium dodecyl sulfate (SDS) followed by a single wash at 60°C for 30 minutes with 0.1× SSC/0.01% SDS and overnight exposure onto autoradiography film. The membranes were hybridized with one probe at a time, stripped of the radiolabeled probe, and reprobed. The level of β-actin mRNA was used to normalize for the quantity of total mRNA.8
Quantitative measurement of IL-6, tPA, PAI-1, and uPAR.Supernatants were collected from an equal number of cells treated with hydrocortisone (10−6 mol/L) for 6, 24, 48, and 72 hours. The supernatants were centrifuged to remove cell debris and stored at −70°C until analysis.8 The cells were also obtained and stored at −70°C until analysis. IL-6 (R&D Systems), tissue plasminogen activator (tPA), and plasminogen activator inhibitor-1 (PAI-1) were measured by enzyme-linked immunosorbent assay (ELISA; American Diagnostica Inc, Greenwich, CT) with the procedures recommended by the manufacturers. Urokinase-type plasminogen activator receptor (uPAR) was measured from the cell lysates using the protocol recommended by the manufacturer (American Diagnostica Inc). uPAR was normalized to the total protein in the cell lysates measured by the Bradford method (Bio-Rad Protein Kit, Bio-Rad Laboratory, Hercules, CA).
TGF-β determination.Supernatants were collected from serum-free cultures at the time points indicated, centrifuged, and frozen at −70°C. Samples were tested for TGF-β activity with and without transient acidification of the supernatants to pH 1.5 by the addition of 5N HCl and neutralization with 1.4N NaOH in 0.7 mmol/L HEPES.25 Titers of TGF-β were expressed in nanograms per milliliter based on a standard curve generated by using purified porcine TGF-β1 (R&D Systems). This was performed with each set of assays.
The isoforms of TGF-β were determined by antibody neutralization. Antibody specificity, used to neutralize active TGF-β, was demonstrated by comparing their effects in the CCL64 assay. TGF-β1 and TGF-β2 were neutralized to the same extent by the antibody recognizing both TGF-β1 and TGF-β2 (12.5 μg/mL of antibody completely neutralized 1 ng/mL TGF-β1 or TGF-β2 ). This antibody also neutralizes the biologic activity of TGF-β3 . The antibody to TGF-β2 neutralizes TGF-β2 and has no effect on TGF-β1 or TGF-β3 activity. The preimmune rabbit control antibody (IgG-fraction; Sigma, St Louis, MO) had no neutralizing effect on either TGF-β1 or TGF-β2 . The rabbit antiserum to TGF-β3 was raised against a peptide from the sequence of mature TGF-β3 . It is specific for TGF-β3 and does not cross-react with either TGF-β1 or TGF-β2 .26
Regulation of TGF-β promoter.Because we were unable to transfect KS isolates and cell lines, we used HeLa cells for studies of the TGF-β1 promoter.27 Twenty-four hours before transfection, 1 × 106 Hela cells were plated in 100-mm tissue culture dishes in DMEM supplemented with 10% FCS. Three hours before transfection, medium was changed to DMEM containing 2% FCS. For every transfection, the calcium phosphate-DNA coprecipitates were prepared as follows: 10 μg of TGF-β1 reporter CAT construct phTG5-CAT DNA (kindly provided by Anita Roberts) and 1 μg of β-gal DNA construct were dissolved in 220 μL of 0.1× Tris-EDTA buffer (TE, pH 8.0) mixed with 250 μL of 2× HEPES-buffered saline (HBS, pH 7.0) and 31 μL of 2 mol/L CaCl2 . The mixture was incubated for 20 to 30 minutes at room temperature and then slowly dropped into the culture medium and incubated overnight at 37°C. The medium was removed and replaced with DMEM supplemented with 0.1% bovine serum albumin with or without hydrocortisone (HC, 10−6 mol/L) and incubated for 3 to 6 hours. TPA (12-O-tetradecanoylphorbol 13-acetate, 100 ng/mL) was then added and incubated overnight. Cells were obtained and lysed by repeated freeze and thaw to extract protein and then assay for chloramphenicol acetyltransferase (CAT) activity. Each cell extract containing 200 μg of total protein was heated to 65°C for 10 minutes and then spun at 12,000 rpm for 2 minutes. Fifty microliters of supernatant was collected and mixed with 50 μL of 1 mol/L Tris.Cl (pH 7.8), 10 μL of 14C-labeled chloramphenicol (0.1 mCi/mL), and 20 μL of acetyl coenzyme A (13.5 mg/μL). The reaction was incubated at 37°C for 2 hours and then 1 mL of ethyl acetate was added, vortexed, and centrifuged at 12,000g for 5 minutes. Nine hundred microliters of the upper phase was collected and was allowed to evaporate in a spin vacuum. The product was redissolved in 25 μL of ethyl acetate and 20 μL of the product was applied to a thin-layer chromatography (TLC) plate (Whatman, Maidstone, Kent, UK), developed in chloroform:methanol (95:5) and exposed to x-ray film.
RESULTS
AIDS-KS cells express GC receptors (GRs).The immunofluorescence studies were performed using a polyclonal antibody against the amino terminus of GRs. The antibody specificity has been confirmed previously.24 KS-isolates showed GR expression in all KS isolates examined (Fig 1). Preimmune serum did not show any reactivity.
GR expression in KS cells. GR was studied by immunofluorescence staining using (A) rabbit polyconal antibodies to synthetic peptides to the amino terminus of GR at concentrations of 1:50. (B) Preimmune rabbit serum was used at equal dilution to show the specificity of the antibodies.
GR expression in KS cells. GR was studied by immunofluorescence staining using (A) rabbit polyconal antibodies to synthetic peptides to the amino terminus of GR at concentrations of 1:50. (B) Preimmune rabbit serum was used at equal dilution to show the specificity of the antibodies.
Schema of GC regulation of plasmin, TGF-β1 , and the resulting enhancement of KS growth. The schema summarizes the inhibitory effects of GCs on the activation pathway of plasmin. GCs induce PAI-1, downregulate PAR, and thus block tPA-mediated activation of plasminogen to plasmin. Reduced levels of plasmin prevent activation of latent TGF-β to its active form and thus abolish the autocrine growth inhibitory effects on KS cells.
Schema of GC regulation of plasmin, TGF-β1 , and the resulting enhancement of KS growth. The schema summarizes the inhibitory effects of GCs on the activation pathway of plasmin. GCs induce PAI-1, downregulate PAR, and thus block tPA-mediated activation of plasminogen to plasmin. Reduced levels of plasmin prevent activation of latent TGF-β to its active form and thus abolish the autocrine growth inhibitory effects on KS cells.
KS cells show enhanced proliferation in response to GCs.The effect of GCs on KS induction and progression has been reported in diverse clinical settings; withdrawal of GCs is associated with tumor regression in many of these cases. Thus, we examined the proliferative effects of GCs on KS cell isolates. Cells were seeded at equal density and treated with various concentrations of hydrocortisone on days 1 and 3. Cell counts were performed on day 6. Hydrocortisone enhanced the KS cell proliferation in a dose-dependent manner, with significant effects observed at concentrations of 10−8 mol/L and above (Fig 2A). Hydrocortisone at 10−6 mol/L concentration almost doubled the proliferation of KS cells. An increase in cell proliferation occurs through the progression of KS cells in cell cycle from G1 to S and then to the G2 + M phase (Fig 2D and E). Subsequent experiments to study the mechanism of hydrocortisone effects were conducted at 10−6 mol/L concentrations of hydrocortisone. However, hydrocortisone did not enhance the proliferation of HUVEC or HASM (Fig 2B and C). KS cells thus display a distinct response to GCs compared with other related cell types.
Effect of GC on the growth of (A) KS cells, (B) HUVEC, and (C) AOSM and KS cell cycle (D and E). KS cells (KSC-2), HUVEC, and aortic smooth muscle cells (AOSM) were each assayed by plating the cells in 24-well plates and treating with hydrocortisone (HC) on days 1 and 3, and the cells were counted on day 6. The assays were done in triplicate; results are shown as mean ± SE. KS cell cycle analysis (D and E): Serum-starved KS cells were cultured in T-75 flasks without (D) or with (E) HC (10−6 mol/L) for 24 hours. Cells were harvested, DNA stained with propidium iodide, and cell-cycle was analyzed by flow cytometry. The results were expressed as DNA content in the different phases of the cell cycle. Channels 68-86 represent cells in G1 phase, channels 86-138 represent cells in S phase, and channels 138-160 represent cells in G2 + M phase.
Effect of GC on the growth of (A) KS cells, (B) HUVEC, and (C) AOSM and KS cell cycle (D and E). KS cells (KSC-2), HUVEC, and aortic smooth muscle cells (AOSM) were each assayed by plating the cells in 24-well plates and treating with hydrocortisone (HC) on days 1 and 3, and the cells were counted on day 6. The assays were done in triplicate; results are shown as mean ± SE. KS cell cycle analysis (D and E): Serum-starved KS cells were cultured in T-75 flasks without (D) or with (E) HC (10−6 mol/L) for 24 hours. Cells were harvested, DNA stained with propidium iodide, and cell-cycle was analyzed by flow cytometry. The results were expressed as DNA content in the different phases of the cell cycle. Channels 68-86 represent cells in G1 phase, channels 86-138 represent cells in S phase, and channels 138-160 represent cells in G2 + M phase.
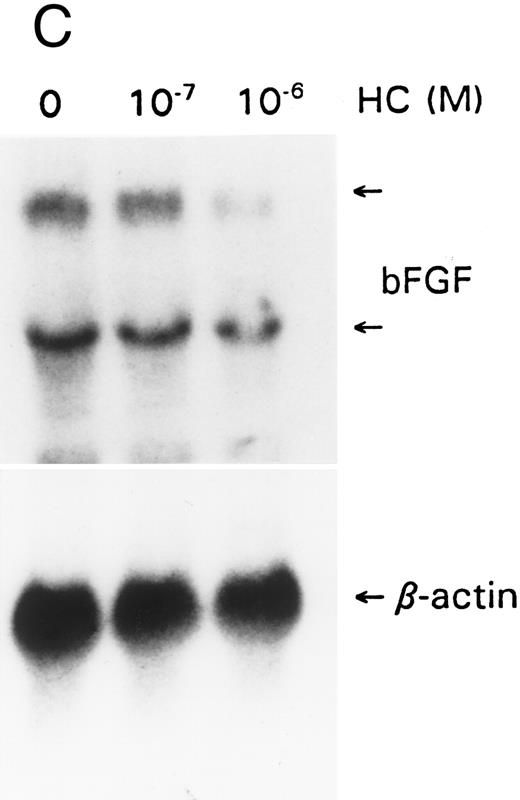
Effect of GCs on the regulation of IL-6, IL-1, bFGF, and VEGF.IL-6, IL-1, bFGF, and VEGF have been shown to be autocrine growth factors for AIDS-KS cells.7-12 bFGF and VEGF are mitogens for endothelial cells and may be responsible for the vascular proliferative lesion of KS in vivo.10 We examined the effect of hydrocortisone on the expression of these factors. IL-6 gene expression, as reported in other cell types, was downregulated within 6 hours (Fig 3A). Similarly, IL-6 levels in the supernatants decreased rapidly upon exposure to hydrocortisone (Fig 3B). Hydrocortisone is thus unlikely to enhance KS cell growth through regulatory effects on IL-6. Similarly, downregulation was observed for IL-1β, bFGF, and VEGF expression at the transcriptional level (Fig 3A, C, and D). We have examined KSY-1, a neoplastic cell line derived from a patient with AIDS-KS,7 and control cell types (HUVEC, HASM) were also studied. The results were similar and showed downregulation of IL-1, IL-6, bFGF, and VEGF (data not shown). These findings are consistent with the previously reported effects of hydrocortisone on IL-1 and bFGF.28,29 Downregulation of VEGF has not been previously reported. This effect may result from the direct regulatory effect of hydrocortisone on the VEGF promoter30 or through the downregulation of IL-1 and the active form of TGF-β1 , both of which can induce VEGF. Thus, it is unlikely that the regulatory effects of hydrocortisone on any of these factors explained the proliferative effects. We thus studied other possible mechanisms to explain hydrocortisone's effects on KS cells.
GCs downregulate (A) IL-1 and IL-6, (B) IL-6 protein, (C) bFGF, and (D) VEGF. For mRNA studies, KS cells were seeded in 75-cm2 flasks and treated with hydrocortisone (10−6 and 10−7 mol/L). The cells were obtained at 6 or 24 hours. Total RNA was extracted and assayed by the Northern method. The membranes were also probed for β-actin to normalize for the quantity of RNA. For IL-6 protein assays, equal numbers of cells were seeded in triplicate wells and treated with hydrocortisone (10−6 mol/L). Supernatants were collected at various time points, cleared of cell debris by centrifugation, and assayed by ELISA for IL-6. The results represent mean ± SE.
GCs downregulate (A) IL-1 and IL-6, (B) IL-6 protein, (C) bFGF, and (D) VEGF. For mRNA studies, KS cells were seeded in 75-cm2 flasks and treated with hydrocortisone (10−6 and 10−7 mol/L). The cells were obtained at 6 or 24 hours. Total RNA was extracted and assayed by the Northern method. The membranes were also probed for β-actin to normalize for the quantity of RNA. For IL-6 protein assays, equal numbers of cells were seeded in triplicate wells and treated with hydrocortisone (10−6 mol/L). Supernatants were collected at various time points, cleared of cell debris by centrifugation, and assayed by ELISA for IL-6. The results represent mean ± SE.
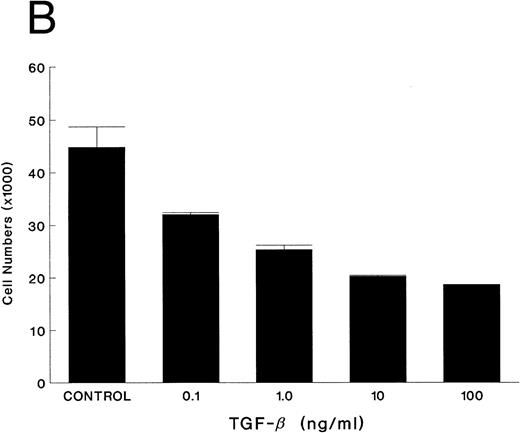
TGF-β is an autocrine inhibitory factor for KS.Although AIDS-KS has several known autocrine growth factors, no inhibitory factor has been described. TGF-β has diverse effects in different cell types, including inhibition of breast cancer cell growth27 and induction of smooth muscle cells.31 Thus, we studied the expression of TGF-β in KS cells. All examined KS cells expressed high levels of TGF-β1 mRNA (Fig 4). TGF-β2 and TGF-β3 were not expressed in any of the KS cell lines. Furthermore, the TGF-β1 protein was measured in the supernatant. The latent form (110 kD) of TGF-β undergoes enzymatic cleavage to the active form (around 25 kD) by plasmin or acidification.32 Both the latent and active forms of TGF-β were secreted by KS cells (see Fig 6). To determine if TGF-β had biologic effect on KS cell growth, the effects of TGF-β were blocked with polyclonal neutralizing antibody. KS cell growth doubled compared with the controls (Fig 5A). In other experiments, the effect of TGF-β was evaluated by adding the active form of recombinant human TGF-β1 (rhTGF-β1) (kindly provided by Amgen, Thousand Oaks, CA). Cell growth was inhibited in a dose-dependent manner (Fig 5B). KS cells thus respond to TGF-β1 and secrete both the latent and active forms of TGF-β1 and show reduced growth in response to exogenous rhTGF-β1 . Thus, TGF-β1 is an autocrine growth inhibitory factor for KS cells.
Effect of GC on TGF-β mRNA. The assays were performed as described in Fig 3. The membranes were probed with full-length TGF-β1 and β-actin cDNA.
Effect of GC on TGF-β mRNA. The assays were performed as described in Fig 3. The membranes were probed with full-length TGF-β1 and β-actin cDNA.
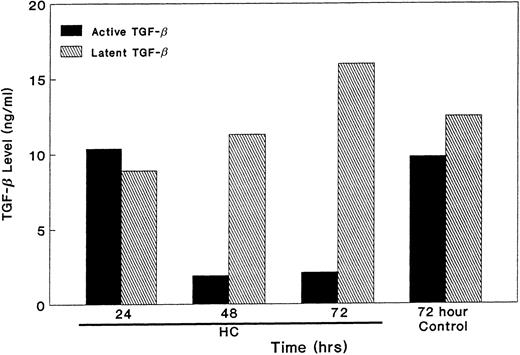
Effect of GC on the activation of TGF-β. Equal numbers of KS cells were seeded and treated with HC (10−6 mol/L). The supernatants were collected at various time points, removed of cell debris by centrifugation, and the levels of latent and active form of TGF-β were measured by the mink lung epithelial cell assay in triplicate. The results are presented as mean ± SE.
Effect of GC on the activation of TGF-β. Equal numbers of KS cells were seeded and treated with HC (10−6 mol/L). The supernatants were collected at various time points, removed of cell debris by centrifugation, and the levels of latent and active form of TGF-β were measured by the mink lung epithelial cell assay in triplicate. The results are presented as mean ± SE.
Effect of TGF-β on KS cell growth. Equal numbers of KS cells were seeded in triplicate in 24-well plates and treated with either polyclonal TGF-β neutralizing antibody (10 μg/mL) or preimmune serum (A) or recombinant human TGF-β1 at various concentrations (B) on day 1 and day 3, and the cells were counted on day 6. The results are shown as mean ± SE.
Effect of TGF-β on KS cell growth. Equal numbers of KS cells were seeded in triplicate in 24-well plates and treated with either polyclonal TGF-β neutralizing antibody (10 μg/mL) or preimmune serum (A) or recombinant human TGF-β1 at various concentrations (B) on day 1 and day 3, and the cells were counted on day 6. The results are shown as mean ± SE.
Hydrocortisone regulates TGF-β in KS cells.To understand how GCs may enhance KS cell growth, we considered that GCs may inhibit TGF-β1 production or prevent processing of TGF-β1 from the latent to the active form. We examined the effect of hydrocortisone on TGF-β1 mRNA. KS cells were treated with hydrocortisone for various time periods (4, 6, 8, and 24 hours). No significant effect was observed on the mRNA levels of TGF-β1 (Fig 4). We also examined the effect of hydrocortisone on the regulation of TGF-β1 by using a reporter CAT construct linked to the TGF-β1 promoter. No change in the maximal levels of TGF-β1 promoter activity was observed after treatment with hydrocortisone in two independent experiments (data not shown).
We then examined the effect of hydrocortisone on production of the latent and active forms of TGF-β in serum-free conditions for 24 to 72 hours. Both the latent and active forms of TGF-β were produced in KS isolates untreated or treated with hydrocortisone for a period of 24 hours. All of the activity that was present in the conditioned media was neutralized by antibody specific to TGF-β. However, the active form of TGF-β decreased precipitously after 48 hours and remained very low at 72 hours; there was, however, no effect on the latent form of TGF-β (Fig 6). These data suggest that the lack of active TGF-β leads to KS cell growth.
To further confirm this effect, we added rh TGF-β1 to KS cells treated with hydrocortisone. Exogenous TGF-β1 completely abrogated the growth-promoting effect of hydrocortisone (Fig 7). Thus, GCs enhance KS cell growth by lowering the levels of the activated form of TGF-β.
rhTGF-β abrogated the effect of GC on KS cell growth. Equal numbers of cells were seeded in triplicate in 24-well plates and treated with HC alone or in combination with TGF-β on days 1 and 3. The cell count was performed on day 6 and the results represent the mean ± SE.
rhTGF-β abrogated the effect of GC on KS cell growth. Equal numbers of cells were seeded in triplicate in 24-well plates and treated with HC alone or in combination with TGF-β on days 1 and 3. The cell count was performed on day 6 and the results represent the mean ± SE.
Effect of GC on the levels of (A) tPA, (B) PAI, and (C) uPAR. Equal number of KS cells were seeded in 75-cm2 flasks and treated with HC (10−6 mol/L). The supernatants were collected at different time points and tPA and PAI-1 levels were measured by ELISA. For the uPAR assay, protein extracts were quantitated by the Bradford method and the levels of uPAR were measured by ELISA and normalized for the total protein in each sample. The results represent the mean ± SE of triplicate experiments.
Effect of GC on the levels of (A) tPA, (B) PAI, and (C) uPAR. Equal number of KS cells were seeded in 75-cm2 flasks and treated with HC (10−6 mol/L). The supernatants were collected at different time points and tPA and PAI-1 levels were measured by ELISA. For the uPAR assay, protein extracts were quantitated by the Bradford method and the levels of uPAR were measured by ELISA and normalized for the total protein in each sample. The results represent the mean ± SE of triplicate experiments.
GCs induce PAI-1.The latent form of TGF-β is activated through proteolytic cleavage by plasmin.33 Plasminogen is activated to plasmin by plasminogen activators (PA) through binding to the cell surface plasminogen activator receptor (PAR).34,35 PA activity is thus regulated by the level of PAR and type-1 plasminogen activator inhibitor (PAI-1).36 We hypothesized that GCs may block TGF-β activation through induction of PAI-1 or downregulation of tissue-type plasminogen activator (tPA).37-44 KS cells were treated with GCs for various time intervals and tPA and PAI-1 supernatant levels were quantitated. The total amount of tPA increased during 48 hours with no change in response to hydrocortisone. However, PAI-1 also increased during 48 hours with a dramatic increase in cells treated with hydrocortisone (Fig 8B). Thus, the activation of plasminogen to plasmin may be reduced in response to hydrocortisone.
Hydrocortisone inhibits uPAR.We measured the level of uPAR in KS cell lines after exposure to hydrocortisone. uPAR levels decreased nearly 50% within 6 hours and persisted for 48 hours (Fig 8C). Induction of PAI-1 and downregulation of uPAR would thus inhibit the production of plasmin and activation of TGF-β.
DISCUSSION
GCs have been associated with the development of KS or rapid growth and dissemination of existing lesions in several disease states.16-22 We have observed a direct effect of GCs on the growth of KS cell isolates and a transformed KS cell line. The proliferative effects were dose dependent, with maximal induction observed at concentrations of 1 μmol/L. Induction of KS cell proliferation was observed in several isolates examined. This feature was also observed in KS isolates made from an HIV-negative, classic form of KS. Notably, HUVEC and vascular smooth muscle cells, which share phenotypic characteristics with KS, do not show proliferative response to GCs. This feature distinguishes KS from the other cell types.
We then examined the mechanism of GC-enhanced KS cell growth. We first studied the effects of GC on known autocrine and paracrine KS growth factors: IL-6, IL-1, bFGF, and VEGF.8-14 IL-6 and IL-1 were downregulated by hydrocortisone, consistent with previously reported effects of GCs in other cell types.28,29 GCs also downregulated bFGF and VEGF, a finding that has not been previously reported. GCs may regulate the VEGF promoter directly or indirectly through downregulation of IL-1 and the active form of TGF-β, both of which can induce VEGF.44 However, these results show that GCs do not induce KS cell growth through the regulation of these cytokines.
We then studied the effects of GC on potential KS growth inhibitors. TGF-β is known to inhibit the growth of epithelial cells and lymphocytes. The effect of exogenous TGF-β on KS cells has previously been examined and shown to minimally inhibit cell growth.45 We thus examined if KS cells produce TGF-β and found that KS cells release abundant amounts of TGF-β1 in both the latent and active forms, similar to the findings in breast cancer cells and chronic lymphocytic leukemia cells,27,46 and contrary to fibroblast lines.32 TGF-β is produced by a variety of cell types as a pro-form (latent TGF-β),32 which undergoes proteolytic digestion with the release of the active form of TGF-β (25-kD dimer). To demonstrate the biologically active form of TGF-β production, we used neutralizing antibodies to TGF-β. The growth of KS cell isolates and cell lines nearly doubled when the cells were exposed to neutralizing TGF-β antibodies. These findings show that the active form of TGF-β inhibits KS cell growth. TGF-β has also been shown to be an autocrine inhibitory factor for chronic lymphocytic leukemia and breast cancer cell lines.27,46 These findings are consistent with reports of the potential role of TGF-β in preventing tumor progression.47-49 Studies of TGF-β knock-out mice have shown hyperproliferation of basal layers of the epidermis.49 Furthermore, tumors derived from the keratinocytes of these TGF-β null mice show a more aggressive phenotype than the wild-type counterparts.
We then speculated that GCs may induce KS cell growth either by downregulation of TGF-β, or by interfering with proteolytic activation of the latent form of TGF-β. We found that hydrocortisone had no significant effect on TGF-β expression. However, hydrocortisone nearly completely inhibited activation of the latent form of TGF-β.
It is known that plasmin activates TGF-β.32 Plasmin is an activation product of plasminogen. The conversion of plasminogen to plasmin occurs in the presence of activated PA bound to its cognate cell-surface antigen (PAR). PA is a serine protease that converts plasminogen to the enzymatically active proteinase, plasmin.34 Two plasminogen activators have been identified: urokinase-type PA (molecular weight [Mr] around 50,000) and tissue-type PA (Mr around 70,000); both are the products of different genes.36,50 PA activation requires conversion from a single chain to a two-chain form. Two-chain PA binds to cell-surface PA receptor (PAR). Thus, levels of PA and PAR determine the activation of plasminogen to plasmin.35,37 This reaction is further regulated by PAI-1, which only interacts with the two-chain form of PA and inhibits PA function.36,51,52 Induction of PAI-1 thus inhibits PA and, consequently, activation of plasminogen to plasmin.53 GCs inhibit PA and thus the activation of plasminogen to plasmin in a variety of cell types.40-42 GCs did not inhibit PA in KS cell lines. Thus, other mechanisms must be operative in blocking the activation of TGF-β in KS cell lines.
GC induction of PAI-1 in KS cells which would lower plasmin levels would also reduce the activation of latent form of TGF-β. GC directly regulates PAI-1 promoter and enhances its expression.39 GC response elements are localized in two different regions, including nucleotide region −100 and +75 and between nucleotides −800 and −549.39 It is notable that PAI-1 promoter is regulated in a tissue-specific manner and has strong activity in aortic endothelial cells and fibroblasts but weak to no activity in HeLa cells. Thus, strong expression and regulation in KS cells that have phenotypic similarity to endothelial cells was not unexpected.
It has previously been shown that bFGF induces the production of PA in bovine endothelial cells. bFGF also increases the expression of urokinase receptor in vascular endothelial cells.53 GC-regulated inhibition of bFGF may thus contribute to the failure of latent TGF-β to undergo activation.
In summary, we have shown that TGF-β is an autocrine inhibitory factor for KS. Further, GCs have profound effects on various factors that regulate TGF-β activation. GCs thus enhance KS cell growth by blocking the activation of TGF-β, schematically shown in Fig 9. These findings may have implications on other disease states such as atherosclerosis and restenosis of coronary artery disease following angioplasty, in which TGF-β regulates the proliferation of vascular smooth muscle cells, which is responsible for restenosis.
Address reprint requests to Parkash Gill, MD, University of Southern California, Norris Cancer Hospital and Research Institute, 1441 Eastlake Ave, MS 34, Los Angeles, CA 90033.












This feature is available to Subscribers Only
Sign In or Create an Account Close Modal