Abstract
In mantle cell lymphoma, the t(11; 14)(q13; q32) and its molecular counterpart, bcl-1 rearrangement, are consistent features and lead to cyclin D1 (bcl-1, PRAD1) proto-oncogene overexpression. In order to detect cyclin D1 overexpression, we developed a simple assay involving a reverse transcription followed by competitive polymerase chain reaction (PCR). A single upstream primer was derived from a homologous region between cyclin D1 and the other D-type cyclins, cyclins D2 and D3, while three downstream primers were specific to their respective D-type cyclins. Because the upstream primer was shared in PCR amplification of the three sequences, each PCR product served as a competitor and the quantification of the target was made by comparison of the intensity of the three products. With this assay we analyzed 45 hematopoietic cell lines and 40 clinical specimens. Cyclin D1 was rarely expressed in lymphoid cell lines except in t(11; 14)(q13; q32)-bearing B-cell malignancies and/or mantle cell lymphoma, which expressed cyclin D1 predominantly. In myeloid cell lines, the levels of cyclin D1 expression varied and never exceeded the sum of cyclin D2 and D3 levels. Cyclin D3 was ubiquitously expressed while cyclins D1 and D2 were differentially used. The observations suggest that human cyclin D3 may play a fundamental role in hematopoiesis and that cyclins D1 and D2 may have different lineage- or differentiation-dependent functions. With this assay, small aliquots of clinical specimens such as 100 μL peripheral blood were enough to detect cyclin D1 overexpression without a well-controlled standard. The technique was validated as highly comparable with Northern analysis. This rapid and reliable detection of cyclin D1 overexpression may have practical clinical utility in the analysis and management of B-cell malignancies.
RECENTLY, mantle cell lymphoma (MCL) has been defined as a subtype of non-Hodgkin's lymphoma by histopathology, immunophenotyping, and chromosome translocation t(11; 14)(q13; q32).1-3 The t(11; 14) causes a rearrangement of the immunoglobulin heavy chain gene with the bcl-1 locus.4 At the bcl-1 locus lies the bcl-1/PRAD1 oncogene,5-7 whose expression is upregulated presumably by the regulatory segment of the juxtaposed immunoglobulin gene. t(11; 14)(q13; q32) and its molecular counterpart, bcl-1 rearrangement, are frequently observed in MCL and are highly diagnostic for distinguishing MCL from the other types of malignant lymphoma.5 8-10
The bcl-1/PRAD1 oncogene encodes cyclin D1, which is an important cell cycle regulator and a member of the D-type cyclin family along with cyclins D2 and D3.11-13 Cyclin D1 binds to and activates cyclin-dependent kinase 4 (or 6) and the activated kinase phosphorylates the Rb protein and facilitates G1 progression toward the S phase.14 Experimentally, forced expression of cyclin D1 in rodent cells played a role in oncogenic transformation in vitro15-18 and in vivo.19-21 Overexpression of the cyclin D1 gene is thus recognized to be a key step in the development of t(11; 14)(q13; q32)-bearing B-cell malignancies.
Because of a low proliferative rate, chromosome analysis is not always informative in order to detect specific chromosome abnormalities in indolent lymphomas which should be distinguished from MCL. Furthermore, detection of bcl-1 rearrangement is also hampered by the large area within the bcl-1 locus in which rearrangement breakpoints can be located.7,10,22,23 Reliable diagnostic procedures for t(11; 14) (q13; q32)-bearing malignancies are needed for their clinical analysis and management. A variety of fluorescence in situ hybridization techniques could be applied to detection of t(11; 14)(q13; q32) and some of them have been reported.24,25 Recent development of immunohistochemistry for cyclin D1 protein detection appears to provide a highly diagnostic procedure for MCL.26-28 As a complement to them, we have developed a simple assay to detect cyclin D1 overexpression associated with t(11; 14). The assay involves reverse transcription (RT) followed by competitive polymerase chain reaction (PCR) and makes it possible to detect cyclin D1 overexpression using small aliquots such as 100 μL peripheral blood.
MATERIALS AND METHODS
Human hematopoietic cells and RNA preparation.Cell lines used in this study are shown in Table 1. These cell lines, except FLAM-76 and SP-49, were passaged in RPMI 1640 medium (GIBCO-BRL Life Technologies, Inc, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; BioWhittaker, Walkersville, MD) and 60 μg/mL kanamycin (Meiji Seika Kaisha, Ltd, Tokyo, Japan) at 37°C in a humidified atmosphere with 5% CO2 . Cultures for FLAM-76 and SP-49 cells required additional interleukin-6 (10 ng/mL) and 5% FBS, respectively.32,33 RNAs of these cell lines were extracted from exponentially growing cells by the acid guanidinium thiocyanate-phenol-chloroform (AGPC) method described by Chomczynski and Sacchi,34 boiled for 1 minute, measured by optic density, and stored at −80°C.
Cell Lines Used in This Study
| . | Cell Line . | Source . |
|---|---|---|
| Lymphoid cells | ||
| Non-B non-T precursor cells | Reh | A |
| Pre-B cells | Nalm-6 | B |
| SMS-SB | B | |
| LBW-2 | B | |
| Mature B cells | BALL-1 | C |
| Burkitt cells | Namalwa | D |
| Ramos | B | |
| B-lymphoid cells | HA | E |
| Myeloma or plasma cells | IM-9 | E |
| HS-sultan | F | |
| t(11; 14)(q13; q32)-bearing myeloma cells | SP-49 | G |
| FLAM-76 | G | |
| T cells (I)* | P30/Ohkubo | F |
| T cells (II) | CEM | C |
| RPMI8402 | H | |
| HPB-ALL | E | |
| T cells (III) | KOPT-K1 | H |
| MOLT-3 | C | |
| Jurkat | C | |
| MOLT-4 | I | |
| MOLT-16 | E | |
| T cells (IV) | PEER | H |
| SKW-3 | E | |
| A3/Kawakami | E | |
| HTLV-1–infected T cells (V) | MT-1 | C |
| MT-2 | C | |
| HUT102 | C | |
| Myeloid cells | ||
| Myeloblast cells | KG-1 | F |
| K562 | F | |
| KCL-22 | D | |
| Promyelocytic cells | HL-60 | F |
| Monocytic cells | JOSK-K | E |
| JOSK-S | E | |
| JOSK-I | E | |
| THP-1 | F | |
| Histiocytic cells | J111 | E |
| U937 | D | |
| Erythroid cells | JK-1 | I |
| HEL | F | |
| Megakaryoblastic cells | MEG01s | J |
| MEG01 | J | |
| CMK29 | K | |
| CMK11-5 | K | |
| Meg-J30 | L | |
| MOLM-1 | E |
| . | Cell Line . | Source . |
|---|---|---|
| Lymphoid cells | ||
| Non-B non-T precursor cells | Reh | A |
| Pre-B cells | Nalm-6 | B |
| SMS-SB | B | |
| LBW-2 | B | |
| Mature B cells | BALL-1 | C |
| Burkitt cells | Namalwa | D |
| Ramos | B | |
| B-lymphoid cells | HA | E |
| Myeloma or plasma cells | IM-9 | E |
| HS-sultan | F | |
| t(11; 14)(q13; q32)-bearing myeloma cells | SP-49 | G |
| FLAM-76 | G | |
| T cells (I)* | P30/Ohkubo | F |
| T cells (II) | CEM | C |
| RPMI8402 | H | |
| HPB-ALL | E | |
| T cells (III) | KOPT-K1 | H |
| MOLT-3 | C | |
| Jurkat | C | |
| MOLT-4 | I | |
| MOLT-16 | E | |
| T cells (IV) | PEER | H |
| SKW-3 | E | |
| A3/Kawakami | E | |
| HTLV-1–infected T cells (V) | MT-1 | C |
| MT-2 | C | |
| HUT102 | C | |
| Myeloid cells | ||
| Myeloblast cells | KG-1 | F |
| K562 | F | |
| KCL-22 | D | |
| Promyelocytic cells | HL-60 | F |
| Monocytic cells | JOSK-K | E |
| JOSK-S | E | |
| JOSK-I | E | |
| THP-1 | F | |
| Histiocytic cells | J111 | E |
| U937 | D | |
| Erythroid cells | JK-1 | I |
| HEL | F | |
| Megakaryoblastic cells | MEG01s | J |
| MEG01 | J | |
| CMK29 | K | |
| CMK11-5 | K | |
| Meg-J30 | L | |
| MOLM-1 | E |
Differentiation stage of T cells defined by Dr J. Minowada31 is shown in parentheses.
The sources of cell lines were as follows: (A) Dr M. Higashihara, First Department of Internal Medicine, University of Tokyo; (B) Dr T. Nakamura, First Department of Internal Medicine, University of Tokyo; (C) Dr M. Yoshida, Institute of Medical Science, University of Tokyo; (D) our lab; (E) Drs S. Ogawa and H. Hirai, Third Department of Internal Medicine, University of Tokyo; (F) Japanese Cancer Research Resource Bank; (G) Drs I. Kubonishi and I. Miyoshi, Department of Medicine, Kochi Medical School; (H) Dr Y. Hayashi, Department of Pediatrics, University of Tokyo; (I) Dr K. Tani, Institute of Medical Science, University of Tokyo; (J) Dr M. Ogura, Aichi Cancer Hospital and Dr H. Saito, First Department of Internal Medicine, Nagoya University; (K) Dr T. Sato, Department of Pediatrics, Chiba University; and (L) Drs M. Teramura and H. Mizoguchi, Department of Hematology, Tokyo Women's Medical School.
Mononuclear cells in patients with B-cell malignancies were separated from peripheral blood or bone marrow by standard Ficoll-Paque (Pharmacia, Piscataway, NJ) density-gradient centrifugation as described by the manufacturer. Lymph node cells were prepared by sieving minced lymph nodes. These cells were also subjected to the AGPC method for RNA preparation. In order to examine D-type cyclins expression in peripheral blood, we used whole blood drawn with ethylenediaminetetraacetic acid dipotassium salt (final 0.12%; Terumo, Tokyo, Japan) for complete blood counts in the outpatient clinic of hematology in the Branch Hospital, University of Tokyo, School of Medicine. An aliquot (100 μL) of the whole blood was centrifuged at 5,000 rpm for 1 minute in a microtube and the pellet was directly subjected to the AGPC method with 20 μg yeast tRNA (GIBCO-BRL) as a carrier. RNA was suspended in 30 μL diethyl pyrocarbonate (Sigma, St Louis, MO)-treated distilled water, boiled for 1 minute, and stored at −80°C until analysis. RNAs of bone marrow cells were prepared from aliquots (50 μL each) of bone marrow aspirates with ethylenediaminetetraacetic acid dipotassium salt as were those of peripheral blood. Clinical specimens used in this study were obtained with written informed consent.
D-type cyclin cDNAs and Southern and Northern analyses.The cDNAs used in this study were as follows: cyclin D1, the EcoRI 1.4-kilobase (kb) insert of pP1-8/λP1-46; cyclin D2, the 1.3-kb cDNA generously provided by Dr Gordon Peters35; cyclin D3, the EcoRI 2-kb insert of pA7R.13 They were 32P-radiolabeled with a random primer DNA labeling kit (Takara, Kyoto, Japan). An aliquot (5 μL) of RT-PCR reaction products was separated on 1.2% agarose gel, denatured in NaOH 0.2 N and NaCl 0.6 mol/L for 1 hour, neutralized in Tris-HCl (pH 8.0) 1 mol/L and NaCl 0.6 mol/L for 1 hour, and blotted onto nitrocellulose membrane. An aliquot (10 μg/lane) of each RNA as described above was separated on a formaldehyde-agarose gel, blotted onto nitrocellulose membrane. Both membranes were hybridized with each labeled cDNA probe (1 × 105 cpm/mL for Southern and 3.5 × 105 cpm/mL for Northern) as described.36 The membranes were washed in high stringency of 0.1 × SSC, 0.1% SDS, at 60°C for Southern and at 70°C for Northern, and autoradiographed at −80°C with intensifying screen.
Competitive RT-PCR.The shared upstream primer, D1S385, was 5′-CTGGCCATGAACTACCTGGA-3′ corresponding to 385 to 404 nucleotide (nt) in the sequence of cyclin D1,6 403 to 422 nt in cyclin D3,13 and 256 to 275 nt in cyclin D212 with one mismatch, respectively. The downstream primer specific to cyclin D1, D1AS867, was 5′-GTCACACTTGATCACTCTGG-3′ corresponding to 867 to 848 nt in the sequence of cyclin D1.6 The primer specific to cyclin D2, D2AS609, was 5′-CATGGCAAACTTAAAGTCGG-3′ corresponding to 609 to 590 nt in cyclin D2.12 The primer specific to cyclin D3, D3AS649, was 5′-CCAGGAAATCATGTGCAATC-3′ corresponding to 649 to 630 nt in cyclin D3.13 Their relative location is depicted in Fig 1A. The primers were synthesized by Greiner Japan (Tokyo, Japan). An aliquot (2 μg) of total RNA was placed in 20 μL of 1 × RT buffer (10 mmol/L Tris-HCl, 50 mmol/L KCl, 4 mmol/L MgCl2 , 10 mmol/L dithiothreitol, pH 8.3) with 250 μmol/L each deoxyribonucleoside triphosphate, 1 μmol/L oligo(dT)15 (Boehringer Mannheim), 20 U rRNasin ribonuclease inhibitor (Promega Co, Madison, WI), 200 U M-MLV reverse transcriptase (GIBCO-BRL). The reaction mixture was incubated at 37°C for 30 minutes and diluted with 80 μL of water, an aliquot (5 μL) of which was placed in 100 μL of 1 × PCR buffer (10 mmol/L Tris-HCl, 50 mmol/L KCl, 1.5 mmol/L MgCl2 , pH 8.3) with 200 μmol/L each deoxyribonucleoside triphosphate, 0.2 μmol/L each primer, and 2.5 U recombinant Taq DNA polymerase (Takara). Reaction mixtures were overlaid with mineral oil (Sigma) and 30 cycles, unless otherwise described, of PCR amplification was started by placing the capped tubes on the block in the Perkin Elmer DNA thermal cycler, which was already heated at higher than 90°C. Each cycle constituted denaturation (1 minute at 94°C, first cycle 5 minutes), annealing (2 minutes at 50°C), and extension (3 minutes at 72°C, last cycle 10 minutes). Ten microliters of the reaction products were electrophoresed on 1.2% agarose gel containing ethidium bromide. The photograph of the gel was scanned with ScanTouch (Nikon, Japan) and densitometrical analysis was performed with NIH image software. [α-32P]dCTP incorporation was performed by adding [α-32P]dCTP 1 μCi/PCR reaction. After the indicated cycle, an aliquot (5 μL) was harvested, separated on 4.5% polyacrylamide gel, and autoradiographed at −80°C with intensifying screen. The gel pieces corresponding to the signals were cut out and counted in scintillant for [α-32P]dCTP incorporation. One sixth of RNA derived from 100 μL peripheral blood or from 50 μL of a bone marrow aspirate was subjected to RT in 20 μL and 5 μL of the reaction products was subjected to PCR in 50 μL. Therefore, the signal on the gel was derived from less than 1 μL of peripheral blood or 0.5 μL of bone marrow.
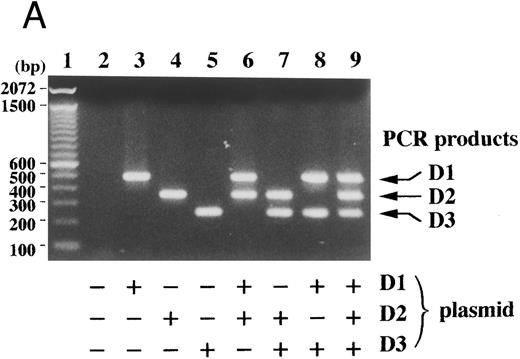
Competitive RT-PCR for D-type cyclins. (A) Schematic presentation of primer locations on the D-type cyclin cDNA sequences is shown, where thick lines indicate the coding regions and thin lines represent truncated non-coding regions. Thick arrows indicate primers used in the competitive RT-PCR. The upstream primer (D1S) is shared in amplification of the three D-type cyclin sequences, while the downstream primers, D1AS, D2AS, and D3AS, are specific to their respective D-type cyclin sequences. D1S is derived from the identical region between cyclin D1, D2, and D3 sequences except for one mismatch in the cyclin D2 sequence (B and C) RNA extracted from MEG01s cells was subjected to RT-PCR in the presence (+) and/or absence (−) of the indicated primer with (B) or without (C) [α-32P]dCTP. Thin arrows denote PCR products corresponding to cyclins D1, D2, and D3, which were visualized by autoradiography (B) or by ethidium bromide staining (upper part in C). PCR products were analyzed by Southern analysis with cDNA probes for the indicated D-type cyclins (middle part in C). The sizes of 100-bp DNA ladders (lane M) are shown on the left.
Competitive RT-PCR for D-type cyclins. (A) Schematic presentation of primer locations on the D-type cyclin cDNA sequences is shown, where thick lines indicate the coding regions and thin lines represent truncated non-coding regions. Thick arrows indicate primers used in the competitive RT-PCR. The upstream primer (D1S) is shared in amplification of the three D-type cyclin sequences, while the downstream primers, D1AS, D2AS, and D3AS, are specific to their respective D-type cyclin sequences. D1S is derived from the identical region between cyclin D1, D2, and D3 sequences except for one mismatch in the cyclin D2 sequence (B and C) RNA extracted from MEG01s cells was subjected to RT-PCR in the presence (+) and/or absence (−) of the indicated primer with (B) or without (C) [α-32P]dCTP. Thin arrows denote PCR products corresponding to cyclins D1, D2, and D3, which were visualized by autoradiography (B) or by ethidium bromide staining (upper part in C). PCR products were analyzed by Southern analysis with cDNA probes for the indicated D-type cyclins (middle part in C). The sizes of 100-bp DNA ladders (lane M) are shown on the left.
RESULTS AND DISCUSSION
The single upstream primer was derived from a region of homologous sequence within human cyclin D1 and its related genes, cyclins D2 and D3, while three downstream primers were specific to their respective D-type cyclins (Fig 1A). RNA extracted from MEG01s cells,37 which express three D-type cyclins (see below), was subjected to the RT-PCR with different combinations of these primers. As shown in Fig 1B and C (lane 9), only 4 primers were needed to amplify the three D-type cyclin sequences by PCR. Each downstream primer was used only for amplification of its respective product, which was each of the expected size (cyclin D1, 483 bp; cyclin D2, 354 bp; cyclin D3, 247 bp). The shared upstream primer, D1S, was needed for amplification of any D-type cyclin sequence (Fig 1B and C, lane 5). The sequence of each amplified product was proven by Southern blot hybridization with each cDNA probe for D-type cyclins (Fig 1C). The kinetics for the PCR, determined by incorporation of [α-32P]dCTP, revealed that amplification proceeded logarithmically until 24 to 27 cycles and relative amounts of PCR products remained fairly equal even after plateaus were reached, probably because of the shared upstream primer (Fig 2). In SP-49 cells with t(11; 14)(q13; q32), the ratio of cyclin D1 over cyclin D3 products remained greater than 10. Taking maximum sensitivity and specificity into account, we used 30 cycles of PCR in all subsequent studies.
Kinetics of the competitive RT-PCR. RNAs extracted from MEG01s and SP-49 cells were subjected to competitive RT-PCR in the presence of [α-32P]dCTP. PCR products were harvested after the indicated cycle of the PCR, separated on a polyacrylamide gel, and visualized by autoradiography (upper part). Arrows indicate the specific PCR products corresponding to cyclins D1, D2, and D3. Gel pieces corresponding to the PCR products of cyclins D1 (•), D2 (○), and D3 (▵), were excised and the radioactivity was counted. [α-32P]dCTP incorporation was plotted on a logarithmic scale over number of cycles of PCR (lower part).
Kinetics of the competitive RT-PCR. RNAs extracted from MEG01s and SP-49 cells were subjected to competitive RT-PCR in the presence of [α-32P]dCTP. PCR products were harvested after the indicated cycle of the PCR, separated on a polyacrylamide gel, and visualized by autoradiography (upper part). Arrows indicate the specific PCR products corresponding to cyclins D1, D2, and D3. Gel pieces corresponding to the PCR products of cyclins D1 (•), D2 (○), and D3 (▵), were excised and the radioactivity was counted. [α-32P]dCTP incorporation was plotted on a logarithmic scale over number of cycles of PCR (lower part).
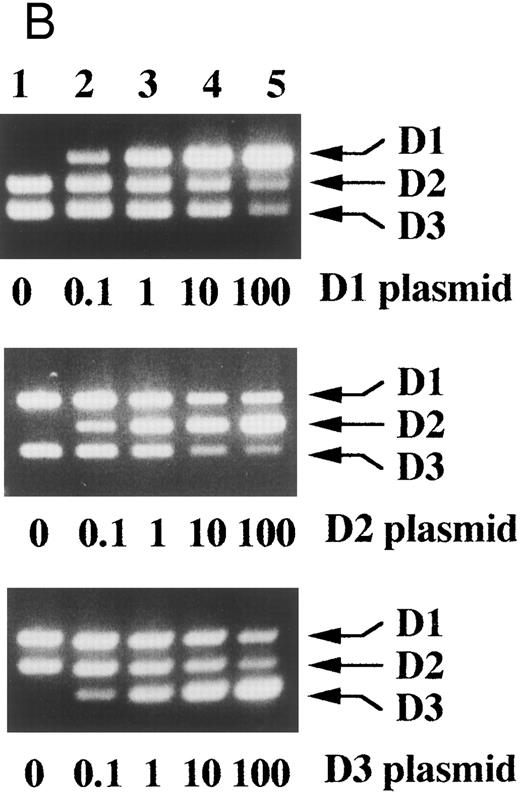
With cDNA plasmids for each D-type cyclin as templates, specific amplification of each D-type cyclin sequence was again confirmed (Fig 3A). Once the initial amount of any one of the cDNAs increased by 10-fold, PCR products of the other D-type cyclins decreased obviously even on ethidium bromide-stained agarose gels, which indicated that each PCR product served as a competitor (Fig 3B, lanes 4 and 5).
Competitive PCR amplified each D-type cyclin sequence specifically and each PCR product served as a competitor. (A) Cyclin D1, D2, and D3 cDNA plasmids (+, 1 × 106 molecules per reaction; −, none) were subjected to the competitive PCR. The sizes of 100 bp DNA ladders (lane 1) are shown on the left. (B) The amount of the indicated plasmid was changed as indicated ratios over the other two and the competitive PCR was performed. Arrows indicate PCR products corresponding to cyclins D1, D2, and D3.
Competitive PCR amplified each D-type cyclin sequence specifically and each PCR product served as a competitor. (A) Cyclin D1, D2, and D3 cDNA plasmids (+, 1 × 106 molecules per reaction; −, none) were subjected to the competitive PCR. The sizes of 100 bp DNA ladders (lane 1) are shown on the left. (B) The amount of the indicated plasmid was changed as indicated ratios over the other two and the competitive PCR was performed. Arrows indicate PCR products corresponding to cyclins D1, D2, and D3.
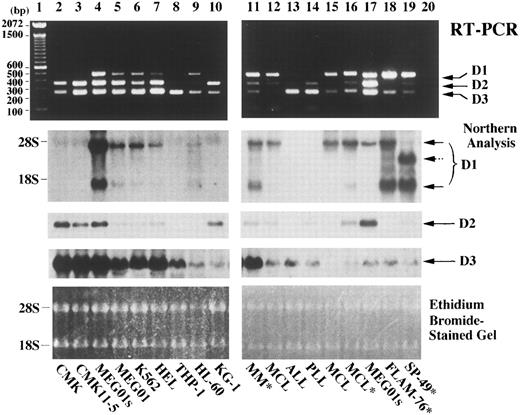
We compared the competitive RT-PCR assay with Northern analysis by using RNAs extracted from cell lines and clinical specimens. As shown in Fig 4, the two methods revealed similar results with the RT-PCR showing slightly better sensitivity on these samples. By Northern analysis, MEG01s cells expressed cyclin D1 mRNA more abundantly than the other myeloid cell lines (Fig 4, lane 4 vs 5-7, and 9) and appeared to express cyclin D1 at a level similar to those of t(11; 14)-bearing tumor and/or MCL cells (Fig 4, lane 17 vs 11, 12, 15, 16, 18, and 19). However, by competitive RT-PCR, expression levels of cyclin D1 relative to the other D-type cyclins were clearly different between MEG01s cells, where cyclin D2 products were rather abundant, and t(11; 14)-bearing tumor and/or MCL cells, where cyclin D1 products were predominant (Fig 4, lane 17 v 11, 12, 15, 16, 18, and 19). Therefore, the competitive RT-PCR was more reliable than Northern analysis to address cyclin D1 overexpression associated with t(11; 14)(q13; q32) and/or MCL. Furthermore, because of its rapidity and greater tolerance for RNA quality differences, this assay should be more practical than Northern analysis and could complement the utility of immunohistochemistry for cyclin D1 protein detection, which has recently been developed.26-28
Comparison between competitive RT-PCR and Northern analysis. RNAs extracted from the indicated cells on each lane were subjected to the competitive RT-PCR (upper part) and to Northern analysis (middle part). Cell lines are on lanes 2 through 10, 17, 18, and 19. Mononuclear cells from patients with multiple myeloma (MM),38 mantle cell lymphoma (MCL), B-cell acute lymphoblastic leukemia (ALL), or B-cell prolymphocytic leukemia (PLL) are on lanes 11 through 16. Mononuclear cells were obtained from bone marrow (lanes 11-13) or peripheral blood (lanes 14-16). Asterisks (*) denote that t(11; 14)(q13; q32) were documented. RNA extraction, the competitive RT-PCR, and Northern analysis were performed as described in Materials and Methods. Negative control of PCR product in the absence of any template is in lane 20. Arrows indicate PCR products corresponding to cyclins D1, D2, and D3 in the upper part, and cyclin D1, D2, and D3 transcript signals in the middle part. A dashed arrow indicates an aberrant cyclin D1 transcript observed in SP-49 cells.39 The sizes of 100-bp DNA ladders (lane 1) and ribosomal RNAs are shown on the left. RNA loading in each lane is shown in the photograph of the ethidium bromide-stained gel at the bottom.
Comparison between competitive RT-PCR and Northern analysis. RNAs extracted from the indicated cells on each lane were subjected to the competitive RT-PCR (upper part) and to Northern analysis (middle part). Cell lines are on lanes 2 through 10, 17, 18, and 19. Mononuclear cells from patients with multiple myeloma (MM),38 mantle cell lymphoma (MCL), B-cell acute lymphoblastic leukemia (ALL), or B-cell prolymphocytic leukemia (PLL) are on lanes 11 through 16. Mononuclear cells were obtained from bone marrow (lanes 11-13) or peripheral blood (lanes 14-16). Asterisks (*) denote that t(11; 14)(q13; q32) were documented. RNA extraction, the competitive RT-PCR, and Northern analysis were performed as described in Materials and Methods. Negative control of PCR product in the absence of any template is in lane 20. Arrows indicate PCR products corresponding to cyclins D1, D2, and D3 in the upper part, and cyclin D1, D2, and D3 transcript signals in the middle part. A dashed arrow indicates an aberrant cyclin D1 transcript observed in SP-49 cells.39 The sizes of 100-bp DNA ladders (lane 1) and ribosomal RNAs are shown on the left. RNA loading in each lane is shown in the photograph of the ethidium bromide-stained gel at the bottom.
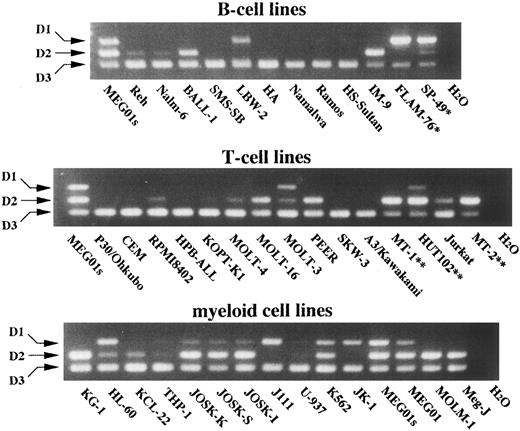
Using this RT-PCR assay, we analyzed hematopoietic cell lines for expression of D-type cyclins. As shown in Fig 5, all the cells within any lineage expressed cyclin D3, suggesting its lineage-independent role in hematopoiesis. On the other hand, cyclins D1 and D2 were expressed differentially by hematopoietic cells. Cyclin D1 expression was faintly detected in few lymphoid cell lines of either B- or T-cell lineage, except t(11; 14)-bearing cell lines in which expression was high. Interestingly, human T-cell lymphotropic virus type 1 (HTLV-1)-infected cell lines expressed cyclin D2 predominantly, an observation that has been noted by others and might be due to induction by Tax1 of HTLV-140 or to their fully differentiated stage. In myeloid cell lines, different combinations of D-type cyclins were detected. Their preferential usage of D-type cyclins was not obviously associated with specific cell lineages and might be related to oncogenesis. Further analysis of normal counterparts for regulation of D-type cyclins is needed for understanding the significance of apparently promiscuous usage of D-type cyclins by these cell lines.
Competitive RT-PCR analysis of hematopoietic cell lines for differential expression of D-type cyclins. The indicated B-cell lines (upper part), T-cell lines (middle part), and myeloid cell lines (lower part) at log phase were subjected to RNA extraction and competitive RT-PCR. PCR products were separated on an agarose gel containing ethidium bromide and photographed. Arrows indicate PCR products corresponding to cyclins D1, D2, and D3. MEG01s cells served as size markers. *t ( 11; 14 ) ( q13; q32 ) - bearing cell lines; **HTLV-1–infected cell lines.
Competitive RT-PCR analysis of hematopoietic cell lines for differential expression of D-type cyclins. The indicated B-cell lines (upper part), T-cell lines (middle part), and myeloid cell lines (lower part) at log phase were subjected to RNA extraction and competitive RT-PCR. PCR products were separated on an agarose gel containing ethidium bromide and photographed. Arrows indicate PCR products corresponding to cyclins D1, D2, and D3. MEG01s cells served as size markers. *t ( 11; 14 ) ( q13; q32 ) - bearing cell lines; **HTLV-1–infected cell lines.
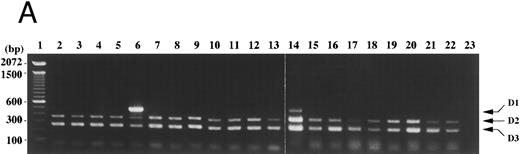
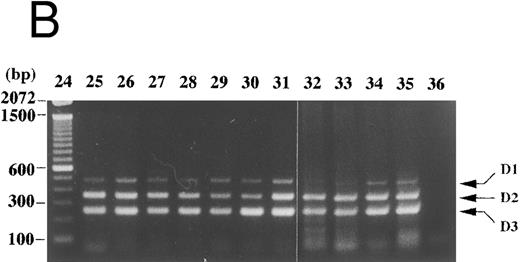
In peripheral blood of a normal volunteer and of patients with benign diseases (pernicious anemia, aplastic anemia, megaloblastic anemia, etc), cyclin D1 expression was not detected with the competitive RT-PCR (Fig 6A, lanes 2-5, 9, 11, 15, 17, 21, and 22, and Fig 7, lane 2). In one of two patients with chronic myeloid leukemia in the accelerated phase, cyclin D1 expression was detected at a relatively low level and may reflect the appearance of immature myeloid cells or cell cycling of tumor cells (Fig 6A, lane 14). On the contrary, in a patient with lymphoplasmacytic lymphoma, the cyclin D1 signal was predominant (Fig 6A, lane 6). Her white blood count was normal with plasmacytoid cells and atypical lymphocytes. Southern and Northern analyses of her bone marrow mononuclear cells revealed cyclin D1 overexpression and bcl-1 rearrangement (data not shown). In bone marrow of lymphoma patients without bone marrow involvement, cyclin D1 expression was faintly detected (Fig 6B, lanes 32-35). Weak cyclin D1 signals were also detected in lymph node cells of patients with non-Hodgkin's lymphoma other than mantle cell lymphoma and of a patient with necrotizing lymphadenitis (Fig 6B, lanes 25-31). In these clinical specimens, cyclins D2 and D3 were dominent and cyclin D1 overexpression was not observed with this assay. In case of cutaneous or mucosal infiltration of lymphoid cells, contaminated epithelial cells may lead to a false positive result because of cyclin D1 expression in these cells. We have not tested such clinical specimens, therefore interpretation of results derived from such specimens needs control examinations.
Cyclin D1 expression in peripheral blood, bone marrow, and lymph nodes. (A) Peripheral blood (100 μL each, lanes 2-22) of patients in the hematology outpatient clinic were subjected to RNA extraction and the competitive RT-PCR as described in Materials and Methods. (B) Lymph node cells of a patient with necrotizing lymphadenitis (lane 25) and of lymphoma patients (lanes 26-31) and bone marrow aspirates (50 μL each, lanes 32-35) of lymphoma patients without bone marrow involvement were also subjected to RNA extraction and the competitive RT-PCR. Diseases affecting these patients were as follows: lanes 2, 11, and 22, pernicious anemia; lanes 3 and 5, collagen disease; lane 4, idiopathic thrombocytopenic purpura; lane 6, lymphoplasmacytic lymphoma with bcl-1 rearrangement and cyclin D1 overexpression; lane 7, acute myeloid leukemia in complete remission; lanes 8 and 26-28, follicular lymphoma; lane 9, pulmonary infiltrate with eosinophilia; lanes 10 and 29-31, diffuse lymphoma; lanes 12, 13, 18, and 19, non-Hodgkin's lymphoma in complete remission; lanes 14 and 20, chronic myeloid leukemia in the accelerated phase; lane 15, aplastic anemia; lane 16, myelodysplastic syndrome; lane 17, autoimmune hemolytic anemia; lane 21, iron-deficiency anemia. Negative controls of PCR without added template are in lanes 23 and 36. The sizes (bp) of 100 bp DNA ladders (lanes 1 and 24) are shown on the left.
Cyclin D1 expression in peripheral blood, bone marrow, and lymph nodes. (A) Peripheral blood (100 μL each, lanes 2-22) of patients in the hematology outpatient clinic were subjected to RNA extraction and the competitive RT-PCR as described in Materials and Methods. (B) Lymph node cells of a patient with necrotizing lymphadenitis (lane 25) and of lymphoma patients (lanes 26-31) and bone marrow aspirates (50 μL each, lanes 32-35) of lymphoma patients without bone marrow involvement were also subjected to RNA extraction and the competitive RT-PCR. Diseases affecting these patients were as follows: lanes 2, 11, and 22, pernicious anemia; lanes 3 and 5, collagen disease; lane 4, idiopathic thrombocytopenic purpura; lane 6, lymphoplasmacytic lymphoma with bcl-1 rearrangement and cyclin D1 overexpression; lane 7, acute myeloid leukemia in complete remission; lanes 8 and 26-28, follicular lymphoma; lane 9, pulmonary infiltrate with eosinophilia; lanes 10 and 29-31, diffuse lymphoma; lanes 12, 13, 18, and 19, non-Hodgkin's lymphoma in complete remission; lanes 14 and 20, chronic myeloid leukemia in the accelerated phase; lane 15, aplastic anemia; lane 16, myelodysplastic syndrome; lane 17, autoimmune hemolytic anemia; lane 21, iron-deficiency anemia. Negative controls of PCR without added template are in lanes 23 and 36. The sizes (bp) of 100 bp DNA ladders (lanes 1 and 24) are shown on the left.
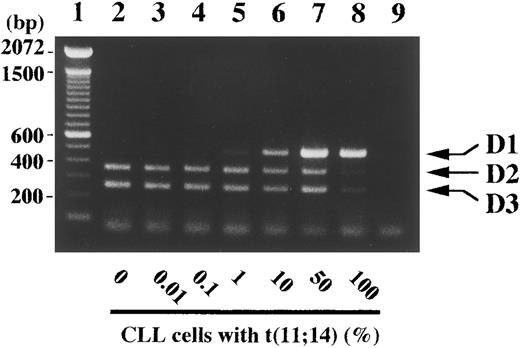
Detection sensitivity of 1% tumor cells with t(11; 14)(q13; q32) within peripheral blood by competitive RT-PCR. Peripheral blood (100 μL) of a normal volunteer was mixed as an indicated percentage with mononuclear cells from a patient with chronic lymphocytic leukemia (CLL) with t(11; 14)(q13; q32) and subjected to RNA extraction and the competitive RT-PCR as described in Materials and Methods. Arrows indicate PCR products corresponding to cyclins D1, D2, and D3. Negative control of PCR without added template is in lane 9. The sizes (bp) of 100 bp DNA ladders (lane 1) are shown on the left.
Detection sensitivity of 1% tumor cells with t(11; 14)(q13; q32) within peripheral blood by competitive RT-PCR. Peripheral blood (100 μL) of a normal volunteer was mixed as an indicated percentage with mononuclear cells from a patient with chronic lymphocytic leukemia (CLL) with t(11; 14)(q13; q32) and subjected to RNA extraction and the competitive RT-PCR as described in Materials and Methods. Arrows indicate PCR products corresponding to cyclins D1, D2, and D3. Negative control of PCR without added template is in lane 9. The sizes (bp) of 100 bp DNA ladders (lane 1) are shown on the left.
Using mononuclear cells in peripheral blood from a patient with t(11; 14)(q13; q32)-bearing chronic lymphocytic leukemia (CLL), we examined the sensitivity of the competitive RT-PCR assay. One percent CLL cells were sufficient for the detection of cyclin D1 expression, which was not detected in peripheral blood of patients with benign diseases and of normal volunteers with the current RT-PCR method (Fig 6A and 7, data not shown), and CLL cells had to comprise more than 10% of total cells in order for a dominant cyclin D1 signal to be observed. Obviously, this RT-PCR approach is not appropriate for detection of minimal residual disease, which would require a disease-specific sequence, and positive results should require more than 10% cells of interest in specimens.
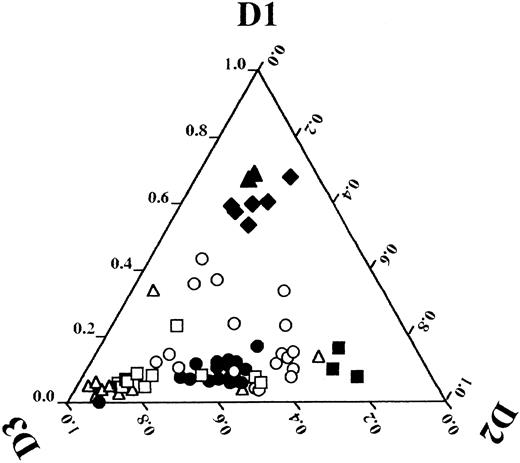
Detection of overexpression of a certain gene in tumor cells requires an appropriate control such as a normal counterpart. Regarding detection of cyclin D1 overexpression in MCL, a normal lymphoid tissue and/or lymphoma cells of another type5,8,9 were used as controls because the normal counterparts of MCL cells are speculated to be CD5-positive B lymphocytes in the mantle zone1 and are difficult to obtain in appropriate quantities. In the current method, other D-type cyclins serve as internal controls. In t(11; 14)-bearing tumors and/or MCL, the levels of cyclin D1 expression clearly exceeded those of cyclins D2 and D3. Data from densitometrical analysis of ethidium bromide-stained gels were plotted in the diagram of D-type cyclins shown in Fig 8, and revealed that t(11; 14)-bearing tumors and/or MCLs (mean ± SD of the relative intensity of cyclin D1, 0.62 ± 0.05, n = 8) were clearly distinguishable from cell lines of the other types (other lymphoid cells: 0.08 ± 0.07, n = 25; myeloid cells: 0.18 ± 0.12, n = 18). Among cell lines in the myeloid series, cyclin D1 may be expressed at a level similar to that in MCL, judged by Northern analysis (Fig 4, lane 17). However, the levels of cyclin D1 in myeloid cells never exceeded the sum of cyclin D2 and D3 levels. Therefore, cyclin D1 overexpression detected by this RT-PCR approach, which is defined as relative intensity of cyclin D1 of more than or equal to the mean minus 2 SDs (0.52 = 0.62 − 2 × 0.05), is highly specific to t(11; 14)-bearing tumors and/or MCL.
Relative expression levels of D-type cyclin plotted in a diagram. After densitometrical analysis of photographs of ethidium bromide-stained PCR products shown in Fig 4, 5, 6A, and 7, a relative expression level of each D-type cyclin was calculated by use of the following formula: cyclin D1, a/(a + b + c); cyclin D2, b/(a + b + c); cyclin D3, c/(a + b + c); where a is a density of cyclin D1 PCR product, b is that of cyclin D2, and c is that of cyclin D3. Relative expression levels of D-type cyclins are plotted for each cell line or clinical specimen as follows: 0-1.0 cyclin D1 from base to apex, 0-1.0 cyclin D2 from left side to right vertex, and 0-1.0 cyclin D3 from right side to left vertex. Each symbol denotes the following: open triangle (▵), B-cell lines; closed triangle (▴), t(11; 14)(q13; q32)-bearing B-cell lines; open square (□), T-cell lines; closed square (▪), HTLV-1–infected T-cell lines; open circle (○), myeloid cell lines; closed diamond (♦), peripheral blood or bone marrow cells of patients with t(11; 14)(q13; q32)-bearing B-cell neoplasms and/or MCL; closed circle (•), peripheral blood or bone marrow cells of a normal volunteer and patients with other disorders described as in Figs 4 and 6A.
Relative expression levels of D-type cyclin plotted in a diagram. After densitometrical analysis of photographs of ethidium bromide-stained PCR products shown in Fig 4, 5, 6A, and 7, a relative expression level of each D-type cyclin was calculated by use of the following formula: cyclin D1, a/(a + b + c); cyclin D2, b/(a + b + c); cyclin D3, c/(a + b + c); where a is a density of cyclin D1 PCR product, b is that of cyclin D2, and c is that of cyclin D3. Relative expression levels of D-type cyclins are plotted for each cell line or clinical specimen as follows: 0-1.0 cyclin D1 from base to apex, 0-1.0 cyclin D2 from left side to right vertex, and 0-1.0 cyclin D3 from right side to left vertex. Each symbol denotes the following: open triangle (▵), B-cell lines; closed triangle (▴), t(11; 14)(q13; q32)-bearing B-cell lines; open square (□), T-cell lines; closed square (▪), HTLV-1–infected T-cell lines; open circle (○), myeloid cell lines; closed diamond (♦), peripheral blood or bone marrow cells of patients with t(11; 14)(q13; q32)-bearing B-cell neoplasms and/or MCL; closed circle (•), peripheral blood or bone marrow cells of a normal volunteer and patients with other disorders described as in Figs 4 and 6A.
To date, PCR has been used for detection of bcl-1 rearrangement41-43 or cyclin D1 overexpression.44 However, even within a group of known MCL, detectability of bcl-1 rearrangement was limited to 50% to 70%, probably because of the large target region for possible rearrangement breakpoints.7,10,23,42 In order to detect cyclin D1 overexpression by a simple RT-PCR with limited cycles, good quality of RNA and well-controlled conditions were required.45 In the current RT-PCR, the signals from the internal control confirm the technical quality of the procedures, and the result is unequivocal. The wide range of tissue and RNA quality encountered in typical clinical specimens should not, therefore, be an obstacle to obtaining reliable results with this assay. In the diagram shown in Fig 8, the relative levels of cyclin D2 in HTLV-1–infected T-cell lines were more than 0.6 and distinguishable from those of the other cell lines. In addition to cyclin D1 overexpression associated with t(11; 14), this RT-PCR assay may be useful for detecting a subset of hematopoietic malignancies, such as adult T-cell leukemia, that preferentially express cyclin D2.40 Also with this technique, expression of D-type cyclins in normal hematopoiesis could be examined with the potential outcome of clarifying the significance of differential expression of D-type cyclins observed in such cell types.
In MCL, leukemic presentation or bone marrow infiltration is frequent.46,47 In addition to MCL, t(11; 14)(q13; q32) and its resultant cyclin D1 overexpression have been documented in other types of lymphoid malignancies including multiple myeloma,23,38,48 splenic lymphoma with villous lymphocytes,49-51 CLL,52,53 prolymphocytic leukemia,23,54,55 and others.56 In all these malignancies, tumor cells are usually obtainable from peripheral blood or bone marrow and the RT-PCR described here would be easily applicable. The rapid and reliable detection of cyclin D1 overexpression should provide guidance for the clinical analysis and management of these tumors.
ACKNOWLEDGMENT
We thank Drs M. Higashihara and T. Nakamura, First Department of Internal Medicine, Drs S. Ogawa and H. Hirai, Third Department of Internal Medicine, and Dr Y. Hayashi, Department of Pediatrics, University of Tokyo; Drs K. Tani and M. Yoshida, Institute of Medical Science, University of Tokyo; Drs M. Teramura and H. Mizoguchi, Department of Hematology, Tokyo Women's Medical School; Dr M. Ogura, Aichi Cancer Hospital; Dr H. Saito, First Department of Internal Medicine, Nagoya University; Dr T. Sato, Department of Pediatrics, Chiba University; Drs I. Kubonishi and I. Miyoshi, Department of Medicine, Kochi Medical School for human cell lines; and Dr Gordon Peters, Imperial Cancer Research Fund Laboratories, for human cyclin D2 cDNA. We also thank Dr H. Kobayashi, Nagano Red Cross Hospital, and Dr H. Yamauchi, Kurobe City Hospital, for clinical specimens of B-cell malignancies.
Supported in part by grants from the Ministry of Education, Science, and Culture of Japan, an Araki Grant (T.M.), Grant No. CA55909 from the National Institutes of Health, and an American Cancer Society Faculty Research Award (A.A.). K.U. and T.T. contributed to the work equally.
Address reprint requests to Toru Motokura, MD, Fourth Department of Internal Medicine, University of Tokyo, School of Medicine, 3-28-6 Mejirodai, Bunkyo-ku, Tokyo 112, Japan.

![Fig. 1. Competitive RT-PCR for D-type cyclins. (A) Schematic presentation of primer locations on the D-type cyclin cDNA sequences is shown, where thick lines indicate the coding regions and thin lines represent truncated non-coding regions. Thick arrows indicate primers used in the competitive RT-PCR. The upstream primer (D1S) is shared in amplification of the three D-type cyclin sequences, while the downstream primers, D1AS, D2AS, and D3AS, are specific to their respective D-type cyclin sequences. D1S is derived from the identical region between cyclin D1, D2, and D3 sequences except for one mismatch in the cyclin D2 sequence (B and C) RNA extracted from MEG01s cells was subjected to RT-PCR in the presence (+) and/or absence (−) of the indicated primer with (B) or without (C) [α-32P]dCTP. Thin arrows denote PCR products corresponding to cyclins D1, D2, and D3, which were visualized by autoradiography (B) or by ethidium bromide staining (upper part in C). PCR products were analyzed by Southern analysis with cDNA probes for the indicated D-type cyclins (middle part in C). The sizes of 100-bp DNA ladders (lane M) are shown on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/3/10.1182_blood.v89.3.965/8/m_bl_0001f1a.jpeg?Expires=1767767141&Signature=1g6yqL3S5Lx8fA8N6iJtRrOfyakENj9Q8W2K6lUl2a8783eyxvW7zOjJYvuTQVYx9Qxp5dVLWlziJTQLDwRam2PF86goR6pTlqu854IBX~UuivKcxgMhSFMIpaY1uj2AILbm-HcaVdj2GPg6pevAXuLCNqxwX7OJPpjcfLF-px8FjsqMcTyGg2Wjcq87w6HXqisRUQfxvWioHYKlqpds-L-Yhm2gXHMWOVZpz0TaFzrTAemklqk4U6SL9-x7UF6y6qs~v4FHXIKpwKbFG6Hp8oypz1q~u6nn0vDwEExlFOkankTX~B7EWroRjt2-5sEBRBe12z87Q0PSJCAKtvlj8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Competitive RT-PCR for D-type cyclins. (A) Schematic presentation of primer locations on the D-type cyclin cDNA sequences is shown, where thick lines indicate the coding regions and thin lines represent truncated non-coding regions. Thick arrows indicate primers used in the competitive RT-PCR. The upstream primer (D1S) is shared in amplification of the three D-type cyclin sequences, while the downstream primers, D1AS, D2AS, and D3AS, are specific to their respective D-type cyclin sequences. D1S is derived from the identical region between cyclin D1, D2, and D3 sequences except for one mismatch in the cyclin D2 sequence (B and C) RNA extracted from MEG01s cells was subjected to RT-PCR in the presence (+) and/or absence (−) of the indicated primer with (B) or without (C) [α-32P]dCTP. Thin arrows denote PCR products corresponding to cyclins D1, D2, and D3, which were visualized by autoradiography (B) or by ethidium bromide staining (upper part in C). PCR products were analyzed by Southern analysis with cDNA probes for the indicated D-type cyclins (middle part in C). The sizes of 100-bp DNA ladders (lane M) are shown on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/3/10.1182_blood.v89.3.965/8/m_bl_0001f1b.jpeg?Expires=1767767141&Signature=RDB4H5d7tadZ1sAMU8hnu-PzUrENb4yPLxuBhsGVDWLZa-VHsr6Gdo9~hm9JT1aV60yJVx3ZJSHLpKLELWk11fFHAj0w4ZpYPm0FdPR8OiZUDnr49Tmfsk3v6MhAc~IBCcWFYajToKbuxAgFoRf7Msv8rZfWr4wGB8Z0Do7E-V85dFG0MZP~l6qWjBqSK8EXBO-clcgh0AlCdNRMEeRDv3pPNYJ0sKKVTf4POk-pqA7X~7FEepwgC7WFw33bZPrqrNqFPmdUyJ3MduH-zt~QwZbWMfiIeOqiDH6NTNciY4aEJL-WKoLT8wL6grOb4oT4lcqLNZyX-67l2uxAItO7Ag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Competitive RT-PCR for D-type cyclins. (A) Schematic presentation of primer locations on the D-type cyclin cDNA sequences is shown, where thick lines indicate the coding regions and thin lines represent truncated non-coding regions. Thick arrows indicate primers used in the competitive RT-PCR. The upstream primer (D1S) is shared in amplification of the three D-type cyclin sequences, while the downstream primers, D1AS, D2AS, and D3AS, are specific to their respective D-type cyclin sequences. D1S is derived from the identical region between cyclin D1, D2, and D3 sequences except for one mismatch in the cyclin D2 sequence (B and C) RNA extracted from MEG01s cells was subjected to RT-PCR in the presence (+) and/or absence (−) of the indicated primer with (B) or without (C) [α-32P]dCTP. Thin arrows denote PCR products corresponding to cyclins D1, D2, and D3, which were visualized by autoradiography (B) or by ethidium bromide staining (upper part in C). PCR products were analyzed by Southern analysis with cDNA probes for the indicated D-type cyclins (middle part in C). The sizes of 100-bp DNA ladders (lane M) are shown on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/3/10.1182_blood.v89.3.965/8/m_bl_0001f1c.jpeg?Expires=1767767141&Signature=j9axv6pgqXUZW9IgWt10kSKWTa5sl1BcG7nHnBd6vqMZT97Dtew4TBSsPUZrZbcSegRtyhpYgVo4-~9rvi~0vBonFgXHtE5GaF7oN8dxaaquHqri1OEWnPuZSEsnydulZbsBw5lDUyaEBdp2wQKbDX0voVl1TWMVeE218EYON76ZhbqD-2lprffhGSEaaIcuybSmP6-n1F-2oifPbpsXs8aX7UO7kf41aLB86rjIm8f0~hvFagkI-A3QqbJVew4JlUcRoMGANIJJLNIATGJK2jWUN8dBm6hN0V5y1OS9hXKNj3Eyp1o2wGvXkNIgyz9Z-gmCZf8hR1ihvrwDOrhQtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Kinetics of the competitive RT-PCR. RNAs extracted from MEG01s and SP-49 cells were subjected to competitive RT-PCR in the presence of [α-32P]dCTP. PCR products were harvested after the indicated cycle of the PCR, separated on a polyacrylamide gel, and visualized by autoradiography (upper part). Arrows indicate the specific PCR products corresponding to cyclins D1, D2, and D3. Gel pieces corresponding to the PCR products of cyclins D1 (•), D2 (○), and D3 (▵), were excised and the radioactivity was counted. [α-32P]dCTP incorporation was plotted on a logarithmic scale over number of cycles of PCR (lower part).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/3/10.1182_blood.v89.3.965/8/m_bl_0001f2.jpeg?Expires=1767767141&Signature=vtc4UJl5-CALv3WAImP7lP2tlFMiCjZ8DZZFO33uQA5~bpX9nYPdvqGVKmae9tCU5l0Q6nidzdpqTFUL521hwDpUUAq0qv8CQlWrO3CO7Pe-sfiTvKj3Z4917HE74taOrvAnlQiVRLcbPptzqi~j-O4K7vDiL7nkD1l4OMr58DR92YxH6XEApxYeFTRxY7Bk-H3xlkSB7HtXo6mZLHD8cxDf04rmjZh0NPPO~K3zLy~Sli7NJq5ZoLw3xEk6uo1WDsRVKg5hdpQiMbVjGBz445~BNB4Ke3G7bA4KTPzruuEgHEjejNzTQiBPXqtcVcKS9r30w1i-853Exy--GhVKGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal