Abstract
Interferon-γ (IFN-γ) is critical for an effective innate immune response against infection. A combination of interleukins (ILs) derived from activated T cells (IL-2) and monocytes (IL-12), or monocytes alone (IL-15 and IL-12), induces optimal production of IFN-γ from natural killer (NK) cells. The mechanism by which human NK cells downregulate their production of IFN-γ is unknown. Here we show that the same cytokines that induce human NK cell IFN-γ production subsequently induce apoptosis of the NK cells. Fas, bcl-2, or bax do not appear to be involved in this process. The mechanism of cytokine-induced apoptosis of human NK cells appears to involve NK cell production of tumor necrosis factor-α (TNF-α). Neutralization of TNF-α or inhibition of TNF-α binding to the p80 TNF-α receptor partially inhibited apoptosis. Transforming growth factor-β, which inhibits cytokine-induced NK cell production of IFN-γ and TNF-α, also decreased cytokine-induced NK cell apoptosis. Costimulation of a CD3−CD56+ NK leukemia cell line with IL-2 and IL-12 or IL-15 and IL-12 induced apoptosis in vitro, which increased when combined with a chemotherapeutic agent. In summary, costimulation of human NK cells via the IL-2 receptor and the IL-12 receptor induces significant IFN-γ production, followed by NK cell apoptosis and a decline in IFN-γ production. Hence, cytokines that activate this innate immune response may also serve to limit it via apoptosis. This novel observation may have implications for the regulation of the innate immune response during infection, the toxicity of combination cytokine therapy, and the treatment of NK cell leukemia.
NATURAL KILLER (NK) cells are large granular lymphocytes (LGL) that lack rearrangement of both T-cell receptor and immunoglobulin genes and constitutively express a number of cell surface cytokine receptors. These properties are consistent with their role as innate immune effector cells, which provide interferon-γ (IFN-γ) as an early response to infection. NK cell production of IFN-γ is pivotal for effective monocyte elimination of obligate intracellular pathogens such as Listeria monocytogenes, Toxoplasma gondii, and Leishmania major. NK cell production of IFN-γ also appears to be important in the pathogenesis of septic shock in various animal models (reviewed in Bancroft et al1 and Biron and Gazzinelli2 ).
It is clear that interleukin-12 (IL-12) is required, yet not sufficient for optimal production of IFN-γ by resting human NK cells.3-5 Several laboratories have shown that the combination of T-cell–derived IL-2 with monocyte-derived IL-12 provides a strong stimulus for IFN-γ production by NK cells.4-6 This is consistent with the fact that NK cells constitutively express receptors for IL-2 (IL-2R)7 and possibly IL-12 (IL-12R).8 IL-15 activates resting NK cells via components of the IL-2R. Both IL-15 and IL-12 are produced by lipopolysaccharide (LPS)-activated monocytes, and in combination, they also provide a potent costimulus for NK cell IFN-γ production.6 9 Thus, in both T-cell–dependent and T-cell–independent activation of NK cells, dual signaling via components of the IL-2R and the IL-12R can provide substantial NK cell IFN-γ production. The mechanism by which human NK cells can limit this response is unknown and was investigated in this report.
MATERIALS AND METHODS
Cell isolation, culture conditions, and cell lines.Human CD3−CD56+ NK cells were isolated from fresh blood of normal volunteers (American Red Cross, Buffalo, NY). Peripheral blood mononuclear cells (PBMC) were isolated using a discontinuous ficoll gradient and adhered to plastic for 12 hours in RPCI 1640 containing 10% human AB (HAB) serum (C-Six Diagnostic, Inc, Mequon, WI) and antibiotics without cytokines. Nonadherent cells were recovered and incubated in the presence of monoclonal antibodies (MoAbs) reactive against CD3, CD4, and HLA-DR, washed twice, and subjected to two consecutive immunomagnetic bead depletions within 2 hours as described.10 Nondepleted PBMC were next stained with anti-CD56–phycoerythrin (PE) (Coulter Immunology, Hialeah, FL) and sorted to greater than 97% purity on a FACstar Plus (Becton Dickinson, San Jose, CA). Unless otherwise indicated, NK cells were plated at 5 × 104 cells/well in a 96-well plate and cultured for 4 days at 37°C. Medium consisted of RPMI 1640 (Sigma, St Louis, MO) containing 10% HAB, antibiotics, recombinant human (rhu) cytokines, and MoAbs where indicated. The NK-92 LGL leukemia cell line11 was generously provided by Dr H.-G. Klingemann (University of British Columbia, Vancouver, Canada), and was maintained in RPCI 1640 medium supplemented with 20% fetal bovine serum (FBS), antibiotics (GIBCO, Grand Island, NY), and either IL-2 or IL-15 at a concentration of 10 ng/mL.
Cytokines, MoAbs, and measurement of cytokines.All assays described in this report contained rhu cytokines. IL-15 was provided by Immunex Corp (Seattle, WA) and was used at a concentration of 15 ng/mL. IL-12 (specific activity of 4.5 × 106 U/mg) was provided by Genetics Institute (Andover, MA) and was used at a concentration of 3 ng/mL. IL-2 (specific activity 1.53 × 107 U/mg) was provided by Hoffmann LaRoche (Nutley, NJ) and used at a concentration of 15 ng/mL. c-kit ligand was provided by Amgen Corp (Thousand Oaks, CA) and used at 100 ng/mL. Tumor necrosis factor-α (TNF-α; specific activity 2 × 103 U/mg) was obtained from the Asahi Chemical Corporation (Tokyo, Japan) and IFN-γ was obtained from Genentech (San Francisco, CA). Purified porcine transforming growth factor; (TGF )-β2 was used at a concentration of 2 ng/mL (R & D Systems, Minneapolis, MN). Doxorubicin (Adria Labs, Indianapolis, IN) was used at a concentration of 600 ng/mL (1 μmol/L). For the experiments with the NK-92 cells, IL-12 and IL-2 were purchased from R & D Systems. The TNF-α receptor-Fc fusion protein (TNFR-Fc) and the IL-4 receptor-Fc fusion protein (IL-4R-Fc) were provided by Immunex.12 A blocking MoAb to the p80 chain of the TNF-α receptor (TNF-αR) (M1, rat) and a blocking MoAb to the p60 chain of the TNF-αR (M50, mouse) were both provided by Immunex.13 For measurement of IFN-γ and TNF-α production in NK cell cultures, supernatants were removed from duplicate wells only once without disturbing the cell pellet and frozen at −70°C until assayed by commercial enzyme-linked immunosorbent assay (ELISA) (IFN-γ: GIBCO-BRL, Gaithersburg, MD; TNF-α: R & D) following the manufacturers' recommendation. The total IFN-γ concentration in culture supernatants was determined by ELISA. Daily production of IFN-γ was calculated as follows: total IFN-γ in supernatants from day x supernatant − mean total IFN-γ in supernatants from day (x-1).
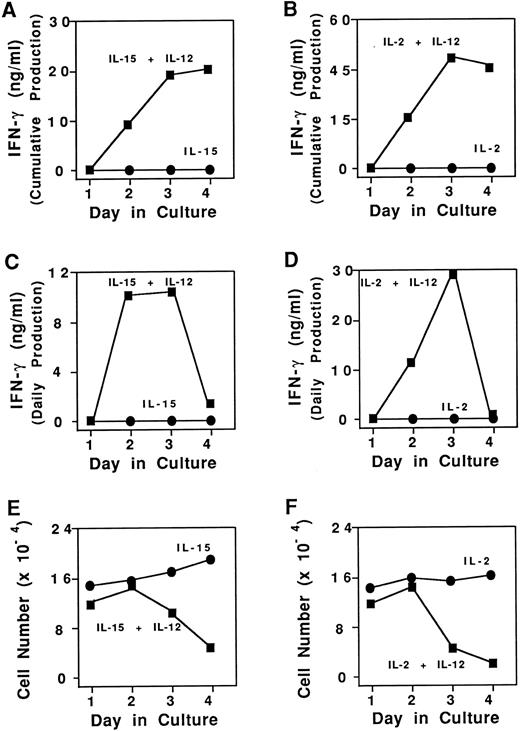
Cumulative and daily IFN-γ production and survival of purified CD3−CD56+ human NK cells. (A) Cumulative NK cell IFN-γ production in the presence of 15 ng/mL IL-15 alone (•) or IL-15 and 3 ng/mL IL-12 (▪). (B) Cumulative NK cell IFN-γ production in the presence of 15 ng/mL IL-2 alone (•) or IL-2 and IL-12 (▪). (C) NK cell daily IFN-γ production in the presence of 15 ng/mL IL-15 alone (•) or IL-15 and 3 ng/mL IL-12 (▪). (D) NK cell daily IFN-γ production in the presence of 15 ng/mL IL-2 alone (•) or IL-2 and IL-12 (▪). (E) Enumeration of viable NK cells as determined by vital dye exclusion assay in the presence of IL-15 alone (•), or IL-15 and IL-12 (▪). (F ) Enumeration of viable NK cells in the presence of IL-2 alone (•), or IL-2 and IL-12 (▪). Results represent the mean cell count for duplicate wells, with <15% standard deviation. Four independent experiments showed similar results. Results were generally more pronounced in the CD3−CD56bright + subset of NK cells.
Cumulative and daily IFN-γ production and survival of purified CD3−CD56+ human NK cells. (A) Cumulative NK cell IFN-γ production in the presence of 15 ng/mL IL-15 alone (•) or IL-15 and 3 ng/mL IL-12 (▪). (B) Cumulative NK cell IFN-γ production in the presence of 15 ng/mL IL-2 alone (•) or IL-2 and IL-12 (▪). (C) NK cell daily IFN-γ production in the presence of 15 ng/mL IL-15 alone (•) or IL-15 and 3 ng/mL IL-12 (▪). (D) NK cell daily IFN-γ production in the presence of 15 ng/mL IL-2 alone (•) or IL-2 and IL-12 (▪). (E) Enumeration of viable NK cells as determined by vital dye exclusion assay in the presence of IL-15 alone (•), or IL-15 and IL-12 (▪). (F ) Enumeration of viable NK cells in the presence of IL-2 alone (•), or IL-2 and IL-12 (▪). Results represent the mean cell count for duplicate wells, with <15% standard deviation. Four independent experiments showed similar results. Results were generally more pronounced in the CD3−CD56bright + subset of NK cells.
Assessment of viability and apoptosis.Cell number and viability were determined by vital dye exclusion using a standard hemocytometer. Methods used to detect apoptosis in cultured NK cells (ie, propidium iodide [PI] nuclear staining and ethidium bromide gel electrophoresis) have been described elsewhere.14 As these experiments were all initiated with a sorted (>97%) population of CD3−CD56+ NK cells, the fluorescence-activated cell sorting (FACS) analyses for PI fluorescence after 3 days of culture were performed without a gate. Morphologic analysis was performed using Wright-Giemsa staining on cytocentrifuge preparations.
Surface staining of TNF-αR.Flow cytometric analysis of resting NK cell TNF-αR expression was evaluated using methods described previously.10 Briefly, cells were incubated with anti-p80 TNF-αR MoAb, anti-p60 TNF-αR MoAb, or species-specific isotype control MoAb, washed, and incubated with goat antimouse fluorescein isothiocyanate (FITC) (1:100) or goat antirat FITC (1:200). Cells were washed and counterstained with NKH1-RD1, washed, fixed in 1% formalin and analyzed by FACScan (Becton Dickinson).
RESULTS
Kinetics of cumulative and daily IFN-γ production.NK cells (≥ 97% pure) were isolated from fresh blood and cultured for 4 days in the presence of two monocyte-derived cytokines, IL-15 and IL-12. Total NK cell IFN-γ production was noted to plateau by day 4 (Fig 1A). IFN-γ stability testing and culture in the presence of an anti-IFN–γ receptor MoAb showed that this plateau in NK cell production of IFN-γ was not due to protein degradation or consumption by NK cells, respectively (not shown). The amount of IFN-γ produced each day was next calculated by subtracting the IFN-γ of identical cultures on the previous day from the total amount of IFN-γ in the well on the current day. NK cell daily IFN-γ production peaked on day 3 and underwent a dramatic decline to nearly zero by day 4 (Fig 1C). In contrast, culture of NK cells in IL-15 alone yielded minimal amounts of daily IFN-γ production cumulatively or daily (Fig 1A and C). Identical results were obtained in both sets of experiments when IL-2 was substituted for IL-15 (Fig 1B and D). Culture of fresh, resting NK cells in the presence of IL-12 alone also yielded low levels of IFN-γ (<1 ng/mL).6
Cell death accounts for the decline in daily IFN-γ production.We sought to determine the reason why daily NK cell IFN-γ production decreased dramatically following 3 days of culture in the presence of excess IL-15 and IL-12 or IL-2 and IL-12. Daily cell counts were performed by vital dye exclusion and indicated a sharp decline in viable cell number by day 4 in cultures containing IL-15 and IL-12. In contrast, NK cells cultured in IL-15 alone, had no decrease in viable cell number (Fig 1E). Concentrations of IL-15 as low as 0.01 ng/mL will maintain NK cell survival for days.14a Thus, the combination of IL-15 with IL-12 appeared to interfere with the IL-15–mediated survival pathway. Identical results were obtained when IL-2 was substituted for IL-15 (Fig 1F ). To determine whether this decrease in cell number specifically required the survival signal delivered via components of the IL-2R, CD56brightNK cells were incubated with c-kit ligand, another NK cell survival factor that mediates its effect via the constitutively expressed tyrosine kinase receptor c-kit.15 Culture of NK cells with c-kit ligand plus IL-12 did not induce cell death when compared with NK cells cultured with c-kit ligand alone. Similarly, culture of NK cells in the presence of c-kit ligand and IL-12 did not induce substantial (ie, ng/mL) IFN-γ production (not shown). Thus, IL-12 interrupts NK survival mediated via components of the IL-2R, but not NK survival mediated via c-kit.
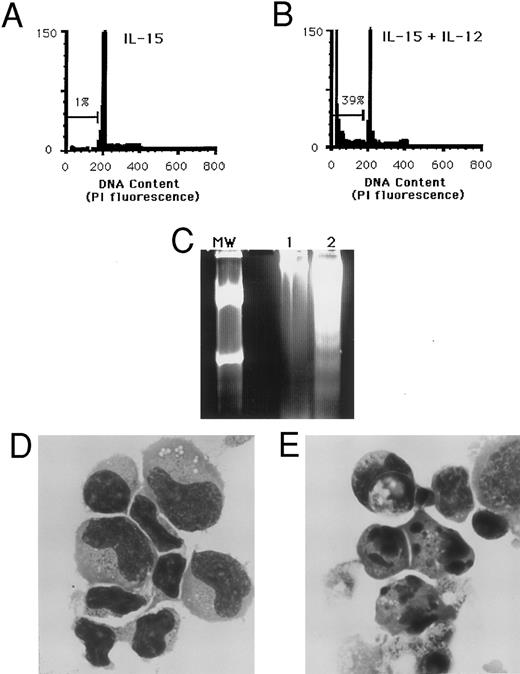
Apoptosis of NK cells following costimulation with IL-15 and IL-12. (A) Purified NK cells cultured for 3 days in medium containing 15 ng/mL IL-15 alone have only 1% of nuclei within the hypodiploid DNA fraction. (B) A parallel culture of NK cells from the same individual in the presence of IL-15 and 3 ng/mL IL-12 shows 39% of nuclei in the hypodiploid DNA region that characterizes apoptosis. (C) Agarose gel electrophoresis of total DNA from CD3−CD56+ NK cells that were cultured for 3 days in IL-15 alone (lane 1) or IL-15 and IL-12 (lane 2). Genomic DNA was isolated from an equal number of wells initially containing 1.4 × 106 sorted NK cells. MW indicates a molecular weight standard. The DNA laddering effect characteristic of apoptosis is seen in lane 2. (D) Cytospin preparation of CD3−CD56+ NK cells cultured 3 days in IL-15 alone or (E) IL-15 and IL-12, with nuclear fragmentation and membrane blebbing characteristic of apoptosis. (Wright Giemsa stain, original magnification × 330.) These results are representative of four independent experiments. Similar results were obtained when IL-2 was substituted for IL-15 (data not shown).
Apoptosis of NK cells following costimulation with IL-15 and IL-12. (A) Purified NK cells cultured for 3 days in medium containing 15 ng/mL IL-15 alone have only 1% of nuclei within the hypodiploid DNA fraction. (B) A parallel culture of NK cells from the same individual in the presence of IL-15 and 3 ng/mL IL-12 shows 39% of nuclei in the hypodiploid DNA region that characterizes apoptosis. (C) Agarose gel electrophoresis of total DNA from CD3−CD56+ NK cells that were cultured for 3 days in IL-15 alone (lane 1) or IL-15 and IL-12 (lane 2). Genomic DNA was isolated from an equal number of wells initially containing 1.4 × 106 sorted NK cells. MW indicates a molecular weight standard. The DNA laddering effect characteristic of apoptosis is seen in lane 2. (D) Cytospin preparation of CD3−CD56+ NK cells cultured 3 days in IL-15 alone or (E) IL-15 and IL-12, with nuclear fragmentation and membrane blebbing characteristic of apoptosis. (Wright Giemsa stain, original magnification × 330.) These results are representative of four independent experiments. Similar results were obtained when IL-2 was substituted for IL-15 (data not shown).
The reproducible decrease in NK cell numbers seen following costimulation via the IL-2R and the IL-12R suggested that the mechanism of cell death might involve activation-induced apoptosis. Staining of cellular DNA with PI provided a quantitative measurement of apoptosis by detecting the fluorescence of individual nuclei using flow cytometry.16 PI fluorescence of cells with the normal diploid DNA content falls within a characteristic peak of constant intensity (set here at 200). Apoptotic nuclei undergoing endonucleolytic cleavage of DNA appear within the hypodiploid region to the left of the diploid peak in the DNA histogram. Incubation of fresh human NK cells with the combination of IL-15 and IL-12 or IL-2 and IL-12 consistently resulted in the induction of DNA fragmentation, as determined by nuclear staining with PI (Fig 2A and B). Electrophoretic analysis of DNA from NK cells cultured in IL-15 and IL-12 showed bands in multiples of 200 bp, a “DNA ladder” characteristic of apoptosis. No DNA ladder was present in cells cultured in IL-15 alone (Fig 2C). Similarly, morphologic evaluation of NK cells cultured in IL-15 and IL-12 showed a significant percentage of cells exhibiting the classical characteristics of apoptosis: nuclear fragmentation, chromatin condensation, and membrane blebbing (Fig 2E), whereas cultures in IL-15 alone contained few cells undergoing apoptosis (Fig 2D).
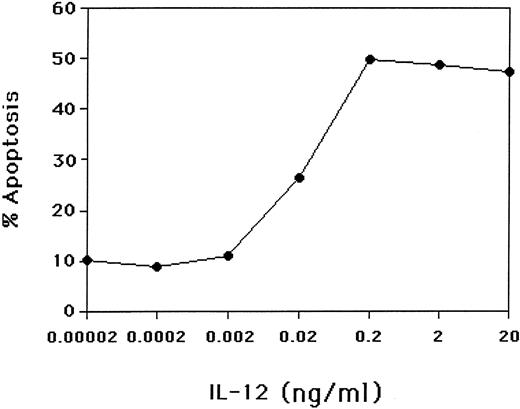
The percentages of NK cells undergoing apoptosis when cultured in IL-2, IL-2 plus IL-12, IL-15, or IL-15 plus IL-12 are shown for 18 random normal donors in Table 1. Initiation of the process of apoptosis as determined by PI staining began within 48 hours of culture and required at least 18 hours of exposure to IL-12 in the presence of IL-15 or IL-2. We performed a dose-response curve to determine how much IL-12 was required to interrupt the IL-15–mediated NK survival. Increases in NK cell apoptosis were seen at IL-12 concentrations as low as 0.02 ng/mL and achieved a plateau at 0.2 ng/mL (Fig 3). Tenfold decreases in the number of NK cells per well or NK cell density resulted in only modest (10% to 15%) reductions in cytokine-induced apoptosis (data not shown).
Percent Apoptosis of Human NK Cells
| Donor No. . | IL-2 . | IL-2 + IL-12* . | Donor No. . | IL-15 . | IL-15 + IL-12* . |
|---|---|---|---|---|---|
| 1 | 5 | 36 | 10 | 1 | 39 |
| 2 | 8 | 63 | 11 | 10 | 50 |
| 3 | 19 | 40 | 12 | 10 | 63 |
| 4 | 20 | 74 | 13 | 10 | 70 |
| 5 | 23 | 58 | 14 | 18 | 62 |
| 6 | 16 | 69 | 15 | 8 | 67 |
| 7 | 18 | 53 | 16 | 19 | 52 |
| 8 | 21 | 48 | 17 | 20 | 49 |
| 9 | 28 | 63 | 18 | 23 | 64 |
| Donor No. . | IL-2 . | IL-2 + IL-12* . | Donor No. . | IL-15 . | IL-15 + IL-12* . |
|---|---|---|---|---|---|
| 1 | 5 | 36 | 10 | 1 | 39 |
| 2 | 8 | 63 | 11 | 10 | 50 |
| 3 | 19 | 40 | 12 | 10 | 63 |
| 4 | 20 | 74 | 13 | 10 | 70 |
| 5 | 23 | 58 | 14 | 18 | 62 |
| 6 | 16 | 69 | 15 | 8 | 67 |
| 7 | 18 | 53 | 16 | 19 | 52 |
| 8 | 21 | 48 | 17 | 20 | 49 |
| 9 | 28 | 63 | 18 | 23 | 64 |
Purified NK cells were cultured for 3 days with IL-2 (15 ng/mL) or IL-2 plus IL-12 (3 ng/mL); IL-15 (15 ng/mL) or IL-15 plus IL-12. The percentage of cells undergoing apoptosis was determined by nuclear staining with PI using flow cytometry. Results from 20 individual donors are shown. The standard deviation of the mean for experiments done in triplicate (donors 6 to 9 and 15 to 18) was <8%.
P = .004 as calculated by the Exact Wilcoxon Signed Rank Test.
The effect of IL-12 concentration on induction of apoptosis of NK cells. Purified CD3−CD56+ human NK cells were cultured for 3 days in the presence of 15 ng/mL IL-15 and varying concentrations of IL-12. The percent of NK cells undergoing apoptosis was determined by PI-stained nuclei using flow cytometry. Results are representative of three experiments.
The effect of IL-12 concentration on induction of apoptosis of NK cells. Purified CD3−CD56+ human NK cells were cultured for 3 days in the presence of 15 ng/mL IL-15 and varying concentrations of IL-12. The percent of NK cells undergoing apoptosis was determined by PI-stained nuclei using flow cytometry. Results are representative of three experiments.
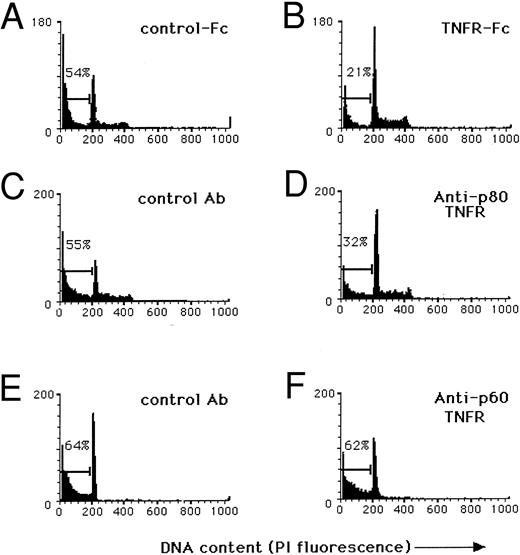
Cytokine-induced apoptosis is mediated in part by an autocrine TNF/TNFR pathway.We next tried to determine the mechanism by which the combination of IL-15 and IL-12 or IL-2 and IL-12 could trigger programmed cell death in human NK cells. In data not shown, incubation of NK cells in IL-2 and IL-12 did not alter the expression of bcl-2 or bax protein. Cytokine-induced apoptosis was not inhibited by blocking Fas, a member of the TNF-αR family known to induce apoptosis following stimulation by its natural ligand.17 The TNF-α–mediated program of apoptosis can be delayed for days following receptor activation,18 raising the possibility that this death pathway might be involved. Indeed, costimulation of NK cells by IL-15 and IL-12 or IL-2 and IL-12 is a potent inducer of TNF-α production, compared with stimulation by either cytokine alone.6 In fact, daily NK cell TNF-α production parallels that of IFN-γ, with a peak by day 3 and rapid decline on day 4 (data not shown). The costimulation experiments were, therefore, repeated in the presence of soluble TNFR-Fc, which neutralizes TNF-α,12 in an attempt to block the autocrine contribution of TNF-α production to the cytokine-induced apoptosis. As shown in Fig 4A and B, the addition of soluble TNFR-Fc resulted in a greater than 50% reduction of NK cell programmed cell death following costimulation with IL-15 and IL-12, which correlated with a reduction in TNF-α protein detected in culture supernatants. Combination of anti-Fas MoAb and soluble TNFR-Fc did not further inhibit apoptosis (data not shown).
Autocrine production of TNF-α contributes to cytokine-induced apoptosis of human NK cells via the p80 TNF-αR. In each condition described below, purified CD3−CD56bright+ NK cells were isolated from fresh blood10 and cultured at 1 × 105 cells/well for 3 days in medium containing 15 ng/mL IL-15 and 3 ng/mL IL-12, after which nuclei were stained with PI to obtain a quantitative measurement of apoptosis. (A) Culture in the presence of a nonreactive receptor fusion protein (IL-4R-Fc , 500 μg/mL) shows 54% of nuclei in the hypodiploid peak, characteristic of apoptosis, while (B) culture in the presence of a TNFR-Fc fusion protein (500 μg/mL) partially neutralizes TNF-α and shows only 21% of nuclei in the hypodiploid peak. (C) Cultures containing a nonreactive isotype control MoAb (50 μg/mL) show 55% of nuclei in the hypodiploid fraction of DNA while (D) incubation with a blocking MoAb to the p80 TNF-αR (M1, 50 μg/mL) shows 32% of nuclei in the hypodiploid DNA fraction. (E) Cultures containing a nonreactive isotype control MoAb (50 μg/mL) show 64% of NK cell nuclei in the hypodiploid fraction of DNA and (F ) incubation with a blocking MoAb to the p60 TNF-αR (M50, 50 μg/mL) shows no measurable effect compared with control. Results were obtained with NK cells from a single donor and are representative of three experiments using different donors.
Autocrine production of TNF-α contributes to cytokine-induced apoptosis of human NK cells via the p80 TNF-αR. In each condition described below, purified CD3−CD56bright+ NK cells were isolated from fresh blood10 and cultured at 1 × 105 cells/well for 3 days in medium containing 15 ng/mL IL-15 and 3 ng/mL IL-12, after which nuclei were stained with PI to obtain a quantitative measurement of apoptosis. (A) Culture in the presence of a nonreactive receptor fusion protein (IL-4R-Fc , 500 μg/mL) shows 54% of nuclei in the hypodiploid peak, characteristic of apoptosis, while (B) culture in the presence of a TNFR-Fc fusion protein (500 μg/mL) partially neutralizes TNF-α and shows only 21% of nuclei in the hypodiploid peak. (C) Cultures containing a nonreactive isotype control MoAb (50 μg/mL) show 55% of nuclei in the hypodiploid fraction of DNA while (D) incubation with a blocking MoAb to the p80 TNF-αR (M1, 50 μg/mL) shows 32% of nuclei in the hypodiploid DNA fraction. (E) Cultures containing a nonreactive isotype control MoAb (50 μg/mL) show 64% of NK cell nuclei in the hypodiploid fraction of DNA and (F ) incubation with a blocking MoAb to the p60 TNF-αR (M50, 50 μg/mL) shows no measurable effect compared with control. Results were obtained with NK cells from a single donor and are representative of three experiments using different donors.
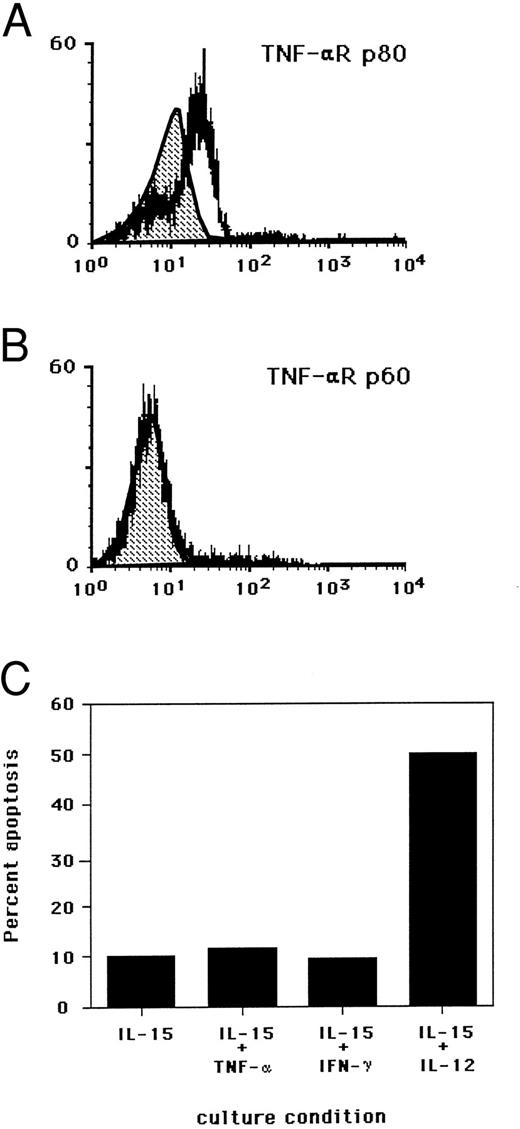
We investigated which TNF-αR might be responsible for the induction of NK cell apoptosis. Indirect staining of freshly isolated NK cells demonstrated the presence of the p80 TNF-αR (Fig 5A), with absent to low density expression of p60 TNF-αR (Fig 5B). The p80 TNF-αR protein has been shown to be markedly upregulated on the surface of NK cells following exposure to IL-2 or IL-12.19 Indeed, in the presence of an MoAb, which blocked TNF-α binding to the p80 TNF-αR, a marked reduction in NK cell apoptosis was observed in the presence of IL-15 and IL-12. An MoAb which blocked TNF-α binding to the p60 TNF-αR had no effect on cytokine-induced apoptosis (Fig 4C through F ). Further, neutralization of IFN-γ had no effect (data not shown).
Surface density expression of the (A) TNF-αR p80 and (B) TNF-αR p60 on fresh human NK cells by flow cytometry. Shaded area in each histogram represents isotype control antibody binding. Experiments were conducted with fresh NK cells from a single donor and are representative of two separate experiments. (C) Requirement for components of the IL-2R and IL-12R activation for the induction of NK cell apoptosis. Purified NK cells were cultured for 3 days in the presence of indicated cytokines at the following concentrations: IL-15 (15 ng/mL), TNF-α (5,000 U/mL), IFN-γ (10 ug/mL), and IL-12 (3 ng/mL). Results are expressed as the percent of cells undergoing apoptosis as determined by nuclear PI staining and flow cytometry. Standard deviation of the mean from duplicate wells was less than 2%. Results are representative of three individual experiments.
Surface density expression of the (A) TNF-αR p80 and (B) TNF-αR p60 on fresh human NK cells by flow cytometry. Shaded area in each histogram represents isotype control antibody binding. Experiments were conducted with fresh NK cells from a single donor and are representative of two separate experiments. (C) Requirement for components of the IL-2R and IL-12R activation for the induction of NK cell apoptosis. Purified NK cells were cultured for 3 days in the presence of indicated cytokines at the following concentrations: IL-15 (15 ng/mL), TNF-α (5,000 U/mL), IFN-γ (10 ug/mL), and IL-12 (3 ng/mL). Results are expressed as the percent of cells undergoing apoptosis as determined by nuclear PI staining and flow cytometry. Standard deviation of the mean from duplicate wells was less than 2%. Results are representative of three individual experiments.
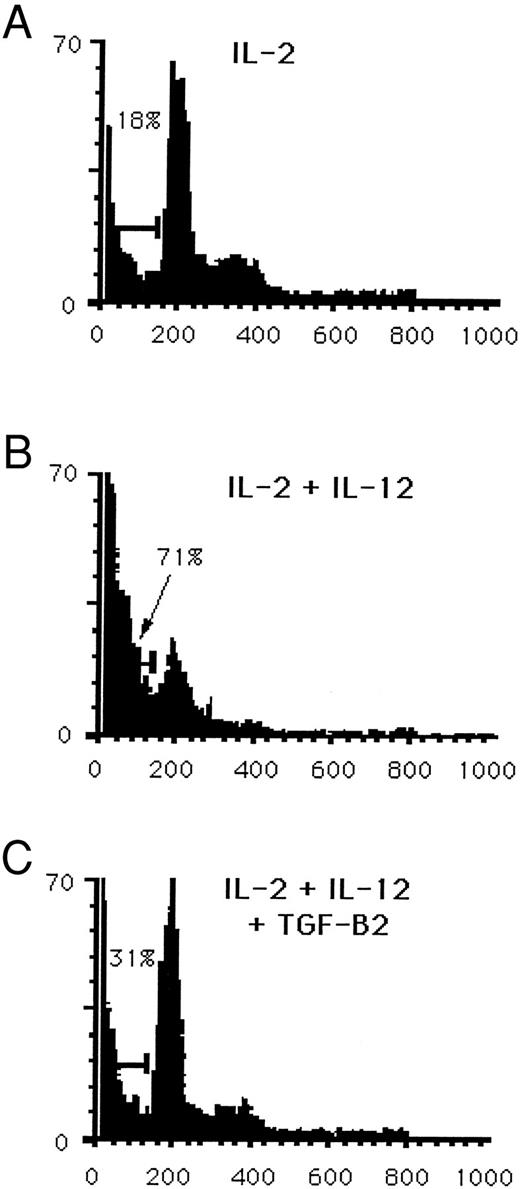
Costimulation of NK cells with IL-15 and IL-12 leads to the endogenous production of IFN-γ and TNF-α, followed by induction of NK cell apoptosis. Blocking TNF-α decreases the cytokine-induced apoptosis. However, culture of NK cells in the presence of IL-15 and IFN-γ or IL-15 and TNF-α did not lead to induction of apoptosis (Fig 5C). Thus, while TNF-α appears to participate in cytokine-induced apoptosis, simultaneous signaling via components of the IL-2R and the IL-12R appear to be critical for induction of programmed cell death in NK cells. Other studies from our laboratory have demonstrated that TGF-β2 suppresses TNF-α, IFN-γ, and chemokine production by NK cells cultured in IL-15 and IL-12 or IL-2 and IL-12.20 21 NK cells cultured for 3 days in IL-2, IL-12, and TGF-β2 showed a reduction in the percentage of cells undergoing apoptosis by 50% or greater as quantitated by PI. A representative experiment is shown in Fig 6A through C. However, addition of exogenous TNF-α to this culture did not reverse the partial inhibition of apoptosis (data not shown). This suggests that the inhibitory effect of TGF-β on cytokine-induced NK cell apoptosis is not mediated by an inhibition of TNF-α secretion.
Reduction in cytokine-induced apoptosis by TGF-β. Purified CD3−CD56+ human NK cells were cultured for 3 days in medium containing (A) 15 ng/mL IL-2, (B) IL-2 and 3 ng/mL IL-12, or (C) IL-2, IL-12 and 2 ng/mL TGF-β2 . Nuclei were stained with PI and then quantitated for apoptosis by flow cytometry. Results are representative of three independent experiments.
Reduction in cytokine-induced apoptosis by TGF-β. Purified CD3−CD56+ human NK cells were cultured for 3 days in medium containing (A) 15 ng/mL IL-2, (B) IL-2 and 3 ng/mL IL-12, or (C) IL-2, IL-12 and 2 ng/mL TGF-β2 . Nuclei were stained with PI and then quantitated for apoptosis by flow cytometry. Results are representative of three independent experiments.
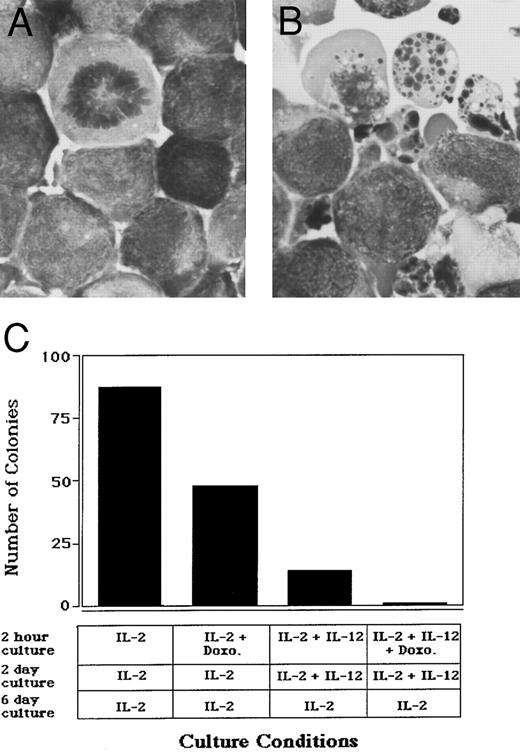
Cytokine-induced apoptosis of NK cell leukemia.The ability to induce apoptosis in normal human NK cells following costimulation with IL-15 and IL-12 or IL-2 and IL-12 suggested that this might have potential application in the treatment of NK cell malignancies with a CD3−CD56+ phenotype. The NK-92 cell line was developed from a patient with rapidly progressive non-Hodgkin's lymphoma who failed standard chemotherapy and died during the leukemic phase of his disease.11 NK-92 cells are CD3−CD56+, IL-2– or IL-15–dependent LGL leukemia cells that display cytotoxic activity against standard NK targets11 (and M. Caligiuri, unpublished observation). The incubation of NK-92 cells in the presence of IL-2 and IL-12 for 24 hours resulted in induction of apoptosis, compared with cells incubated in IL-2 alone (Fig 7A and B). In clonal assays, this effect was seen at the single cell level, implying that neither cell-to-cell contact nor cell-mediated cytotoxicity were required. Clones of cells surviving the first exposure to IL-2 and IL-12 showed susceptibility upon reexposure (data not shown). Further, synergy in cell kill was seen in the presence of IL-2, IL-12, and the antineoplastic agent doxorubicin (Fig 7C). Similar results were obtained when IL-15 was substituted for IL-2 (data not shown).
Cytokine-induced apoptosis of a human LGL leukemia cell line. NK-92 cells were plated at a concentration of 5 × 105/mL in culture medium containing either (A) 15 ng/mL IL-2 alone or (B) IL-2 and 3 ng/mL IL-12 for 24 hours, after which cells underwent cytocentrifuge preparation and Wright-Giemsa staining. Original magnification × 330. (C) NK-92 cells were first cultured at a concentration of 1 × 105 cells/mL for 2 hours in medium containing: 15 ng/mL IL-2; IL-2 and 600 ng/mL doxorubicin; IL-2 and 3 ng/mL IL-12; IL-2, IL-12, and doxorubicin. Cells were next washed three times with RPMI 1640 and plated in a 96-well plate at 10 cells/well in the presence of IL-2 alone or IL-2 and IL-12, as indicated. After 2 days, each well was washed three times with RPMI 1640, and cultures were then continued in medium containing IL-2 for 6 more days. Wells were then visually scored for the presence of a viable colony. Similar results were obtained when IL-15 was substituted for IL-2 in these experiments (data not shown).
Cytokine-induced apoptosis of a human LGL leukemia cell line. NK-92 cells were plated at a concentration of 5 × 105/mL in culture medium containing either (A) 15 ng/mL IL-2 alone or (B) IL-2 and 3 ng/mL IL-12 for 24 hours, after which cells underwent cytocentrifuge preparation and Wright-Giemsa staining. Original magnification × 330. (C) NK-92 cells were first cultured at a concentration of 1 × 105 cells/mL for 2 hours in medium containing: 15 ng/mL IL-2; IL-2 and 600 ng/mL doxorubicin; IL-2 and 3 ng/mL IL-12; IL-2, IL-12, and doxorubicin. Cells were next washed three times with RPMI 1640 and plated in a 96-well plate at 10 cells/well in the presence of IL-2 alone or IL-2 and IL-12, as indicated. After 2 days, each well was washed three times with RPMI 1640, and cultures were then continued in medium containing IL-2 for 6 more days. Wells were then visually scored for the presence of a viable colony. Similar results were obtained when IL-15 was substituted for IL-2 in these experiments (data not shown).
DISCUSSION
Animal studies demonstrating that excessive production of IFN-γ by NK cells is responsible for death following repeated exposure to endotoxin provide the most compelling in vivo data to support the importance of timely negative feedback during more limited infections.2 Monocytes activated by infectious organisms provide TNF-α, IL-1β, IL-15, and IL-12 for optimal IFN-γ production by NK cells.1-5,9 NK cells, in turn, provide monocytes with IFN-γ and TNF-α for an effective innate immune response against a number of obligate intracellular pathogens.1,2,4,5,22 Here we provide the first in vitro evidence that two of the same monocyte-derived cytokines, IL-15 and IL-12, that induce early IFN-γ and TNF-α production by NK cells, can also limit this response. The mechanism of downregulation involves an apoptotic pathway that uses endogenous TNF-α in a negative autocrine feedback loop. NK cells can undergo apoptosis following activation with IL-2 and engagement of the low affinity FcγIIIR23,24 or following cell-mediated lysis of a specific leukemia cell line.25 However, the current report provides evidence that monokines alone may have a role in limiting the innate immune response via apoptosis of NK cells, in the absence of antigen-specific humoral immunity or NK cytotoxic activity against a tumor cell. The data also offer an explanation for the observations that resting or IL-2–activated NK cells have decreased proliferation in the presence of IL-12 and IL-2, compared with IL-2 alone.26 27
Downregulation of NK cell IFN-γ production follows prolonged (18 hours) exposure to the same two cytokines that induce IFN-γ production and uses TNF-α and the p80 TNF-αR to induce apoptosis. The requisite costimulation (IL-15 and IL-12) of NK cells parallels that of the peripheral T-cell system where repeated antigen activation in combination with IL-2 induces apoptosis. Antigen-induced apoptosis of T cells has been shown to be mediated via Fas,28 and more recently, TNF-αR in vitro29 and in vivo.30 T cells and NK cells, therefore, appear to require costimulatory signals to initiate the cascade leading to apoptosis. T cells require T-cell receptor activation in combination with a single cytokine receptor signal, while NK cells can accomplish this via two distinct cytokine receptors in the absence of an antigen-specific receptor signal. This is consistent with the NK cell's role as an innate immune effector cell responding to monokines early and nonspecifically following microbial invasion.31 While the p60 TNF-αR has been more commonly noted to initiate the apoptotic signal, similar signaling has been seen with the p80 TNF-αR. Interestingly, apoptosis mediated via the p80 TNF-αR appears to be delayed for up to 48 hours in other systems,18 as was seen in the current study.
TGF-β can inhibit NK cell production of TNF-α and IFN-γ and can limit the induction of cytokine-induced NK cell apoptosis. This suggests that other factors may also serve to limit NK cell activation in vivo. There is good experimental evidence that monocyte-derived IL-10 can act as a negative regulator for NK cell IFN-γ production in the mouse.4 In humans, a similar mechanism involving IL-10 may be less likely, as IFN-γ inhibits monocyte production of IL-10,32 and IL-10 can enhance NK cell production of IFN-γ.33 Nonetheless, the fact that negative regulators such as TGF-β can be identified suggests that cytokine-induced apoptosis of NK cells may not be a primary mechanism of limiting IFN-γ production following microbial infection. Rather, the pathway may become operative in vivo during more sustained infectious insults when other more reversible mechanisms of negative feedback are inoperative or ineffective. One such example is that monocytes do not appear to produce TGF-β following lipopolysaccharide (LPS) activation.34 Lethal endotoxin-induced septic shock can occur as a consequence of overwhelming bacterial infection and is thought to be mediated in part by massive endogenous production of IFN-γ and TNF-α.2 This suggests that each of these postulated mechanisms of negative feedback can be overcome in vivo.
The data presented in this report would suggest that considerable caution should be exercised before simultaneously delivering combinations of IL-2 and IL-12 or IL-15 and IL-12 in vivo. Prolonged exposure to both agents could not only induce very high levels of proinflammatory cytokines such as TNF-α, IFN-γ, and macrophage inflammatory protein-1α (MIP-1α),21 but might also induce apoptosis in a majority of NK cells. This, in turn, could leave the host transiently more susceptible to obligate intracellular pathogens such as Toxoplasma gondii or Mycobacterium tuberculosis. Further, as IL-15 may be constitutively produced by a number of tissues in vivo,35 36 some of these effects could potentially be seen with the exogenous administration of IL-12 alone.
Apoptosis appears to be a final common pathway for a number of cancer therapies, including traditional chemotherapy, radiation therapy, and cell mediated cytotoxicity.37 The ability to costimulate a malignant counterpart of NK cells with IL-2 and IL-12 or IL-15 and IL-12 and the convergence of their signaling pathways to induce apoptosis may provide new opportunities to combine cytokine therapy with chemotherapy for the treatment of NK cell leukemia. Careful consideration would have to be given to the dose and schedule of such a regimen, given the potential toxicities of this cytokine combination discussed above.
ACKNOWLEDGMENT
We thank Drs Elizabeth Repasky, William E. Carson, Paul Anderson, and Douglas Williams for their insightful comments; Drs Stan Krajewski and John Reed for assistance with immunoblots. We thank Dr Eric Bluman, Yuko Kono, and other members of the laboratory for their technical assistance, and the Department of Flow Cytometry for cell sorting. We thank Dr H.E. Klingemann for the NK-92 cell line.
Supported by National Institutes of Health Grant No. CA-68458, Bethesda, MD and the Coleman Leukemia Research Fund, St Paul, MN.
Address reprint requests to Michael A. Caligiuri, MD, Roswell Park Cancer Institute, Buffalo, NY 14263.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal