Abstract
The nonerythroid expression of the Duffy blood group protein (gp-Fy) was confined to certain cell types. Immunocytochemistry studies of the kidney showed gp-Fy in the endothelium of glomeruli, peritubular capillaries, vasa recta, and the principal cells (epithelial) of collecting ducts. Gp-Fy was also produced in the endothelial cells of large venules and epithelial cells (type-I) of pulmonary alveoli. In the thyroid, only the endothelial cells of capillaries produced gp-Fy. In the spleen, the endothelial cells of capillaries, high endothelial venule, and sinusoids produced abundant gp-Fy. Ultrastructural studies showed that apical and basolateral plasma membrane domains, including caveolae, had gp-Fy. Immunoblot analysis showed substantially less gp-Fy in nonerythroid cells than in erythrocytes. Moreover, the analyzed nonerythroid organs of Duffy-negative individuals did not produce more gp-Fy to compensate for the lack of this protein in their erythrocytes. The nucleotide sequence and the size of kidney mRNA from a Duffy-positive individual were the same as that of bone marrow. It is assumed, therefore, that nonerythroid Duffy protein is the product of the same gene as that of bone marrow. This notion is reinforced by the fact that nonerythroid and erythroid gp-Fy have the same antigenic domains.
THE DUFFY BLOOD GROUP system consists of two principal antigens, Fya and Fyb, produced by FY*A and FY*B codominant alleles. Antisera, anti-Fy a and anti-Fy b, define four phenotypes: Fy(a+b−), Fy(a−b+), Fy(a+b+), and Fy(a−b−).1 2 Neither antiserum agglutinates Fy(a−b−) cells, the predominant phenotype in African and American Blacks. Outside these populations, the Fy(a−b−) phenotype is very rare.
The product of the Duffy blood group gene (FY), a single copy gene localized in the long arm of chromosome 1,3,4 is a glycoprotein (gp-Fy) of 337 amino acid residues with a Mr of 35,733. Hydropathy analysis of the protein predicts an exocellular N-terminal domain of 62 residues, seven transmembrane α-helices, and an endocellular C-terminal domain of 28 residues.5-7 Nine α-helices were originally predicted, based on the hydrophobicity scale of Engelman et al,5,7 to be the transmembrane domain of gp-Fy. However, using another hydrophobicity scale, Neote et al6 identified seven α-helices as the transmembrane domain of gp-Fy. These seven transmembrane spanning regions are compatible with the hydropathy plots of all chemokine receptors and therefore may reflect the real basic structure of the transmembrane domain of gp-Fy. However, neither the seven nor the nine α-helices has been validated experimentally. Thus far, only the exocellular N-terminal domain has been experimentally proved.5 The major Duffy blood group determinants are in gp-Fy, though, purification of the Duffy antigen by immunochromatography yields several proteins of which gp-Fy is the major subunit.8 The molecular difference of FY*A and FY*B polymorphism is a single G→A nucleotide substitution at position 306 of the gene. This produces a glycine→ aspartic acid substitution at residue 43 of gp-Fy.9-12
The significance of gp-Fy stems from its role as the receptor for the human malarial parasite Plasmodium vivax and simian malarial parasite P knowlesi13 14 and as a new class of chemokine receptor for several proinflammatory cytokines.6,15-18 Binding studies of chemokines to human erythroleukemia K562 and human embryonic kidney 293 cell lines, transfected with cDNA encoding for the cloned gp-Fy, have shown that gp-Fy and the human erythrocyte chemokine receptor are the same protein.6 16 Moreover, this study has demonstrated that gp-Fy is sufficient for chemokines to bind.
Most African and American Blacks have red blood cells that lack Fya and Fyb antigens. This class of erythrocytes resists invasion by Pvivax and P knowlesi parasites.13,14 Duffy-negative African and American Blacks carry a FY*B allele that is bone marrow silent, but active in nonerythroid tissues.9,19 Present studies have determined that a single T→C substitution is present in the promoter region of Duffy-negative individuals.20 This mutation affects the promoter activity in the erythroid progeny by disrupting a binding site for GATA I erythroid transcription factor.20 However, the mutation does not affect the expression of Duffy gene in other organs.9 19
Because gp-Fy mRNA is produced in the nonerythroid organs of Duffy-positive and Duffy-negative individuals, it is important to determine which cells and subcellular structures contain gp-Fy. Recently, it was reported that gp-Fy was confined to endothelial cells of postcapillary venules in the kidney, breast, and splenic sinusoid.18,19 The investigators extrapolated their findings to the endothelial cells of small venules of all organs except liver.19 However, by using a potent Duffy-specific antibody, we report here that gp-Fy is present in the endothelium of some vessels of the kidney, lung, thyroid, and spleen. It is present also in the epithelial cells of the kidney collecting ducts and lung alveoli. Furthermore, ultrastructural studies showed gp-Fy in plasma membranes including caveolae (noncoated plasmalemmal vesicles).
MATERIALS AND METHODS
Material.Human tissues were acquired from the National Cancer Institute Cooperative Human Tissue Network (Birmingham, AL). The blood of the donors was typed in our institution according to standard procedures. In addition, for immunocytochemistry and immunoelectron microscopy studies, human organs of Duffy-positive individuals were acquired from the Department of Pathology, Aarhus University Hospital and Randers Centralsygehus, Aarhus, Denmark.
Nucleotide sequence of gp-Fy mRNA from nonerythroid organs of Duffy-positive and Duffy-negative individuals.RNA extraction and sequence analysis by reverse transcription polymerase chain reaction (RT-PCR) technology was performed as explained elsewhere.5 9 The amplified product was subcloned in TA vector (Invitrogen Corp, San Diego, CA) and several clones were sequenced.
Preparation of erythrocyte membrane and protein determination.Erythrocyte isolation and production of erythrocyte ghosts were performed as reported elsewhere.8 Membrane proteins were solubilized in 1% dodecyl β-D-maltoside (CalBiochem-NovaBiochem Corp, San Diego, CA) and were determined by the Pierce BCA protein assay reagent (Pierce, Rockford, IL). Bovine serum albumin was used as a standard.
Preparation of membranes from nonerythroid organs.Fresh or frozen organs were suspended in 10 volumes of a buffer solution containing 0.25 mol/L sucrose, 25 mmol/L Tris-HCl (pH 7.2), and 1 mmol/L EDTA to which the following freshly prepared protease inhibitors were added: 100 KIU aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride, 100 μmol/L leupeptin, and 1.4 μmol/L pepstatin A. The organs were disrupted in a Polytron homogenizer (Brinkmann, Westbury, NY) for 1 minute at setting 5. Nuclei and cellular debris were removed by centrifugation for 15 minutes at 500g. The supernatant was then centrifuged in the Spinco (Beckman, Columbia, MD) centrifuge rotor SW 50-1 for 30 minutes at 100,000g. The pellet, consisting of a crude membrane preparation, was dissolved in 1% dodecyl β-D-maltoside. Protein concentration was determined as explained above.
Preparation of a Duffy-specific rabbit polyclonal antibody.The rabbit polyclonal antibody, 6615, was prepared as follows: Gp-Fy was purified by immunoaffinity chromatography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as explained elsewhere.8 The electroeluted polymeric form of gp-Fy was acetone precipitated, oxidized with performic acid, and lyophilized as explained.21 The lyophilized sample was dissolved in 70% formic acid and digested overnight at room temperature with CNBr. CNBr was removed by lyophilization and the sample was dissolved in a sterile 150 mmol/L NaCl solution containing 0.1% SDS, and emulsified in the MPL+TDM+CWS adjuvant (Ribi ImmunoChem Research, Hamilton, MO). Three young, New Zealand white rabbits were prebled and injected subcutaneously and intramuscularly with 25 μg of the formic acid-treated and CNBr-digested gp-Fy. After 3 weeks, the animals were boosted with the same emulsion. After 2 additional weeks, the rabbits were bled and IgG purified by column chromatography with Bakerbond AB resin (J.T. Baker Chemical, Phillipsburg, NJ) following the manufacturer's protocol.
Immunopurification of rabbit polyclonal antibody 6615.Affinity purified gp-Fy was separated by preparative 10% SDS-PAGE and the polymeric form of the protein (90 to 200 kD) was extracted from the gel with 0.1 mol/L NH4HCO3 (pH 8.3) and 0.2% SDS by gently shaking overnight at room temperature. The extract was concentrated to ≈30 mL with AMICON (W.R. Grace & Co, Beverly, MA) filters and dialyzed overnight against 0.1 mol/L sodium phosphate buffer (pH 7.0). Approximately 8 mg of gp-Fy in 30 mL of 0.1 mol/L sodium phosphate buffer (pH 7.0) was cross-linked to 2 mL of sepharose beads using the Amino Link Immobilization Kit (Pierce) following the manufacturer's protocol. Approximately 20 mg of purified IgG solubilized in 0.01 mol/L sodium phosphate buffer (pH 7.3)/0.15 mol/L sodium chloride (PBS) was incubated with the gp-Fy cross-linked beads overnight at 4°C. Unbound IgG was removed by washing extensively with PBS and the bound IgG was eluted with 0.1 mol/L glycine-HCl buffer (pH 3.0). The eluted fractions were immediately neutralized with 1 mol/L Tris-HCl buffer (pH 9.0) and peak fractions were pooled, dialyzed against PBS, and concentrated using Centricon microconcentrators (AMICON). The activity of the affinity purified antibody was determined by protein immunoblots against purified gp-Fy.
Immunoblot analysis.Proteins were separated by SDS-PAGE in Bio-Rad minigels as explained elsewhere.8 After the run, the gels were soaked in 10 mmol/L 3-cyclohexylamino-1-propanesulfonic acid, pH 11 containing 10% methanol. The proteins were electrophoretically transferred onto Immobilon-P membranes (Millipore Corp, Bedford, MA) at 4°C in the same buffer for 1.5 hours at 400 mA. After transfer, the membranes were blocked overnight at 4°C in 10 mmol/L Tris-HCl (pH 8.0), containing 150 mmol/L NaCl, 5% fat free milk, and 0.05% Tween 20 (blocking buffer). The membranes were incubated for 1.5 hours at room temperature with the corresponding primary antibody. Subsequently, the membranes were washed three times at room temperature (15 minutes each) in the same buffer and incubated for 30 minutes at room temperature in the blocking buffer containing 3% donkey serum. Finally, the membranes were incubated with horseradish peroxidase-conjugated donkey antimouse or antirabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) at a 1:5,000 dilution in blocking buffer for 1.5 hours at room temperature.
Deglycosylation of purified gp-Fy. Gp-Fy was purified by affinity chromatography, separated by SDS-PAGE, and the 36 to 46 kD band was eluted. The protein was digested with N-glycosidase (0.4 U) in 50 mmol/L potassium phosphate buffer pH 6.8 containing 1% n-octyl-β-D-glucopyranoside (Sigma, St Louis, MO), 0.1 mmol/L phenylmethylsulfonylfluoride, 10 KIU aprotinin, 10 mmol/L 1,10-phenanthroline monohydrate in a final volume of 50 μL and incubated at 37°C for 18 hours. After digestion, the samples were run on SDS-PAGE minigel and immunoblotted as explained above.
Preparation of organs for immunolocalization.Human kidneys (from three white male donors), thyroid, lung, and spleen (from different white donors) were fixed with 8% paraformaldehyde in 0.1 sodium cacodylate buffer, pH 7.2. Blocks of organs were trimmed and further fixed by immersion in the fixative for 2 hours, infiltrated for 30 minutes with 2.3 mol/L sucrose containing 2% paraformaldehyde. Afterwards, the blocks were mounted on holders and rapidly frozen in liquid nitrogen. Cryosections for the light and electron microscope were obtained with a Reichert-Jung Ultracut S cryoultramicrotome (Leica, Inc, Wetzlar, Germany).
Freeze-substitution of kidney.The frozen samples were freeze-substituted22 in a Reichert AFS (Reichert Vienna, Austria) or in a Balzers freeze-substitution unit (FSU 010, Balzers AG, Liechtenstein) as published elsewhere.23 The samples were sequentially equilibrated over 3 days with 0.5% uranyl acetate in methanol from −8°C decreasing gradually to −70°C. They were rinsed in pure methanol from −70°C to −45°C. At −45°C, the samples were infiltrated with Lowicryl HM20 and methanol 1:1, 2:1 (1 day in each solution). Before UV polymerization, they were infiltrated with pure Lowicryl HM20 for 2 days at −45°C and 2 days at 0°C.
Immunohistochemistry and immunoelectron microscopy.The procedures used for immunocytochemistry have been reported elsewhere.23-25 For examination under the light microscope, cryosections of 0.85 μm were placed on gelatin-coated glass slides, preincubated with PBS containing 1% bovine serum albumin (BSA) and 0.05 mol/L glycine and thereafter incubated with antibody 6615 at 1/2,000 dilution, immunopurified 6615 at 1/100 dilution, or murine monoclonal antibody anti-Fy6* at 1/50 to 1/100 dilution. The reactions were visualized with horseradish peroxidase-conjugated second antibody (P448 DAKO, Copenhagen, Denmark) at a dilution of 1/100. The sections were counterstained with Meier stain. The spleen samples were subjected to blockage of endogenous peroxidase before labeling as explained elsewhere.23 Sections of the samples were analyzed in a Leica Laborlux S microscope (Leica, Inc). For immunoelectron microscopy, the labeling was performed on ultrathin Lowicryl sections (40 to 60 nm), which were incubated with antibody 6615 (1/2,000 dilution) and visualized with goat antirabbit-gold antibody, 10 nm gold particles (BioCell Research Laboratories, Cardiff, UK). Afterwards, the sections were stained with uranyl acetate for 10 minutes and examined with either a Philips CM100 or EM208 electron microscope (Philips Electronic Instruments Co, Eindhoven, The Netherlands). The following controls were performed for light and electron microscopic preparations: (1) incubation with preimmune serum 6615; (2) incubation with a nonspecific mouse IgG1; (3) incubation without primary antibody; and (4) incubation without primary and secondary antibodies. All controls showed no labeling.
RESULTS
Characterization of a Duffy-specific antibody for the identification of cell types that express gp-Fy.The murine monoclonal antibody, anti-Fy6,26 27 yielded inadequate labeling for immunoelectron microscopic studies (not shown). Of the several rabbit polyclonal antibodies that we developed, antibody 6615 was preferred because it yielded the proper resolution for ultrastructural localization of the Duffy antigen (see below). Although the other rabbit polyclonal antibodies were robust in immunoblots, they yielded a very weak signal in fixed tissues, possibly due to the hindrance of the antigenic sites (not shown).
Antibody 6615 was generated with performic acid and CNBr-treated gp-Fy and did not react with deglycosylated gp-Fy (Fig 1). It appears that the performic acid treatment, which is the most effective procedure to disassemble the polymeric form of gp-Fy21 and CNBr cleavage, had converted the carbohydrate component of gp-Fy into a potent immunogen. The lack of immunoreactivity with erythrocyte ghosts of Duffy-negative individuals and deglycosylated gp-Fy, proved the immunological and chemical specificities of antibody 6615, respectively (Figs 1 and 2).
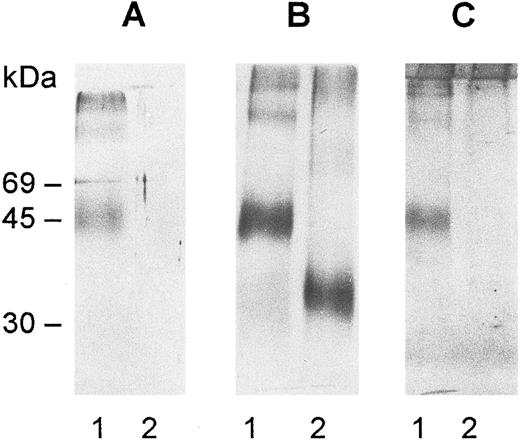
Immunoblots of deglycosylated gp-Fy. The protein was purified by affinity chromatography and SDS-PAGE and digested with N-glycosidase F as explained in Materials and Methods. After digestion, the samples were run on SDS-PAGE minigels, electrotransferred onto nitrocellulose membranes, and immunoblotted with antibody 6615 (A) and antibody anti-Fy6 (B). The antibodies were detected by the horseradish peroxidase-conjugate substrate kit (Bio-Rad Laboratories, Inc, Richmond, CA). The carbohydrate determination was performed with the DIG glycan detection kit (Mannheim Boehringer Biochemicals Co, Indianapolis, IN) (C). A 0.5 μg aliquot of gp-Fy was analyzed in each lane. Lane 1, control and lane 2, treated samples.
Immunoblots of deglycosylated gp-Fy. The protein was purified by affinity chromatography and SDS-PAGE and digested with N-glycosidase F as explained in Materials and Methods. After digestion, the samples were run on SDS-PAGE minigels, electrotransferred onto nitrocellulose membranes, and immunoblotted with antibody 6615 (A) and antibody anti-Fy6 (B). The antibodies were detected by the horseradish peroxidase-conjugate substrate kit (Bio-Rad Laboratories, Inc, Richmond, CA). The carbohydrate determination was performed with the DIG glycan detection kit (Mannheim Boehringer Biochemicals Co, Indianapolis, IN) (C). A 0.5 μg aliquot of gp-Fy was analyzed in each lane. Lane 1, control and lane 2, treated samples.
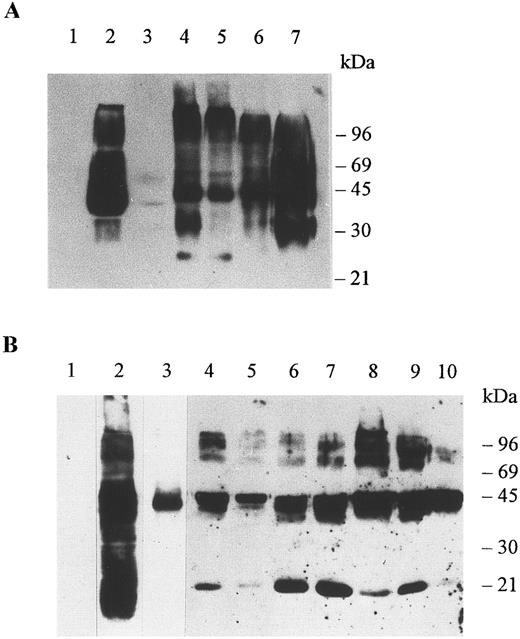
Immunoblots of membrane proteins from erythrocytes and organs. Membranes were prepared as explained in Materials and Methods and the detergent soluble proteins were run in SDS-PAGE minigels, electrotransferred onto nitrocellulose membranes, and immunoblotted with antibody Fy6 at a dilution of 1/500 (A) and antibody 6615 at a dilution of 1/5000 (B). The antibodies were detected by chemiluminescence using the ECL Western blotting procedure as explained by the manufacturer (Amersham Life Science, Arlington Hts, IL). (A) Erythrocytes of a Duffy-negative individual (lane 1), erythrocytes of a Duffy-positive individual (lane 2), adrenal tumor of a Duffy-negative individual (lane 3), colon of a Duffy-negative individual (lane 4), colon of a Duffy-positive individual (lane 5), kidney of a Duffy-positive individual (lane 6), and spleen of a Duffy-positive individual (lane 7). Aliquots of 10 μg of erythrocyte membrane proteins and 60 μg of organ membrane proteins were analyzed per lane. (B) Erythrocytes of a Duffy-negative individual (lane 1), erythrocytes of a Duffy-positive individual (lanes 2 and 3), adrenal gland (lane 4), adrenal tumor of a Duffy-negative individual (lane 5), colon of a Duffy-negative individual (lane 6), colon of a Duffy-positive individual (lane 7), kidney of a Duffy-positive individual (lane 8), thyroid of a Duffy-positive individual (lane 9), spleen of a Duffy-positive individual (lane 10). For the negative control the spleen of a Duffy-positive individual was immunoblotted with preimmune serum and with a second antibody (not shown). A 25-μg aliquot of erythrocyte membrane proteins was analyzed in lanes 1 and 2 and a 2-μg aliquot of erythrocyte membrane proteins was analyzed in lane 3. A 60-μg aliquot was analyzed in each lane of membrane protein of tissues.
Immunoblots of membrane proteins from erythrocytes and organs. Membranes were prepared as explained in Materials and Methods and the detergent soluble proteins were run in SDS-PAGE minigels, electrotransferred onto nitrocellulose membranes, and immunoblotted with antibody Fy6 at a dilution of 1/500 (A) and antibody 6615 at a dilution of 1/5000 (B). The antibodies were detected by chemiluminescence using the ECL Western blotting procedure as explained by the manufacturer (Amersham Life Science, Arlington Hts, IL). (A) Erythrocytes of a Duffy-negative individual (lane 1), erythrocytes of a Duffy-positive individual (lane 2), adrenal tumor of a Duffy-negative individual (lane 3), colon of a Duffy-negative individual (lane 4), colon of a Duffy-positive individual (lane 5), kidney of a Duffy-positive individual (lane 6), and spleen of a Duffy-positive individual (lane 7). Aliquots of 10 μg of erythrocyte membrane proteins and 60 μg of organ membrane proteins were analyzed per lane. (B) Erythrocytes of a Duffy-negative individual (lane 1), erythrocytes of a Duffy-positive individual (lanes 2 and 3), adrenal gland (lane 4), adrenal tumor of a Duffy-negative individual (lane 5), colon of a Duffy-negative individual (lane 6), colon of a Duffy-positive individual (lane 7), kidney of a Duffy-positive individual (lane 8), thyroid of a Duffy-positive individual (lane 9), spleen of a Duffy-positive individual (lane 10). For the negative control the spleen of a Duffy-positive individual was immunoblotted with preimmune serum and with a second antibody (not shown). A 25-μg aliquot of erythrocyte membrane proteins was analyzed in lanes 1 and 2 and a 2-μg aliquot of erythrocyte membrane proteins was analyzed in lane 3. A 60-μg aliquot was analyzed in each lane of membrane protein of tissues.
A striking feature of gp-Fy is its display of numerous bands in SDS-PAGE.8,28 This phenomenon is primarily due to protein-protein interactions of a highly hydrophobic protein5,8 and is compounded by the large amount of gp-Fy in the sample (Fig 2A and B). This unusual feature was noticed since the protein was first identified28 and later purified.8 Furthermore, the degree of glycosylation increases the number of reactive bands.
Multiple immunoreactive bands were observed in organs and erythrocytes immunoblotted with either monoclonal antibody Fy6 or rabbit polyclonal antibody 6615 (Fig 2A and B). Both antibodies yielded a similar arrangement of bands, as they reacted with the same protein. An aliquot of 2 μg of erythrocyte membrane proteins yielded a single immunoreactive band of about 45 kD, which is the monomeric form of gp-Fy (Fig 2B).8 The same single band was seen in an aliquot of 60 μg of membrane proteins of an adrenal tumor indicating that gp-Fy was less abundant in this tumor (Fig 2B).
Several reactive bands were observed in membranes of the adrenal gland of a Duffy-negative individual, the colon of Duffy-negative and Duffy-positive individuals, and the kidney, spleen, and thyroid of Duffy-positive individuals (Fig 2). It appeared that the nonerythroid organs of Duffy-negative individuals did not produce more gp-Fy than those of Duffy-positive individuals.
It is interesting that antibody 6615 did not react with proteins having electrophoretic mobilities of approximately 36 kD, however, these proteins reacted with antibody Fy6 (Fig 2A and B). The 36-kD was observed in colon, kidney, and spleen and was a nonglycosylated form of gp-Fy.21 High mobility components of approximately 25 kD observed in the adrenal glands, colon, kidney, and thyroid were probably glycopolypeptides generated by partial proteolysis of gp-Fy.
Since antibody 6615 is against carbohydrates, it could be argued that it reacted with a different membrane protein that contained the same sugar as gp-Fy. However, immunocytochemical studies excluded this possibility. As indicated in the immunocytochemistry of the kidney, the same cell types that immunoreacted with antibody 6615 also immunoreacted with antibody anti-Fy6 (Fig 3f ). The specificity of 6615 was further confirmed by using an immunopurified preparation of this antibody for the histochemical characterization of kidney cells producing gp-Fy (Fig 3b, inset D). The same cell types reacted with antibody 6615 and the immunopurified antibody 6615. Finally, neither antibody reacted with stroma and parenchymal cells of adult liver, which is an organ that does not produce gp-Fy mRNA (not shown).
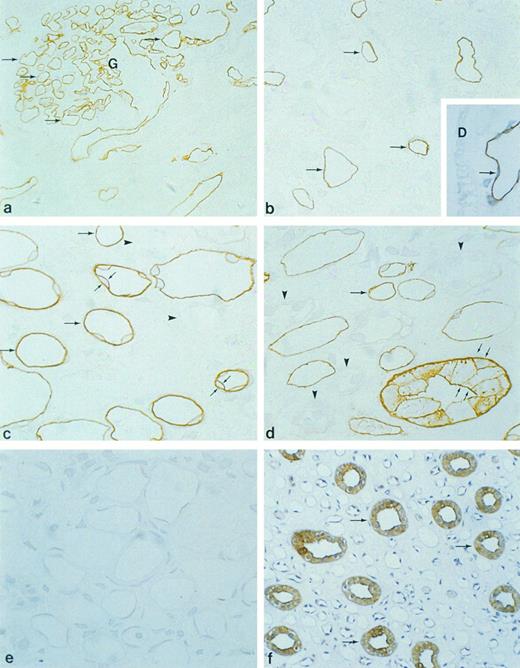
Immunocytochemical localization of gp-Fy in semithin cryosections of human kidney cortex (a and b), outer medulla (c), and inner medulla (d and e). Antibody 6615 was used for labeling except in (f ). (a) Capillary endothelial cells within the glomerulus (arrows) and peritubular capillaries exhibited strong labeling. (Original magnification × 500.) (b) Cortical peritubular capillaries were labeled, while proximal and distal tubules were unlabeled. (Original magnification × 1,100.) (c) In outer medulla, strong labeling of vascular structures was seen (arrows), whereas multiple thin walled structures (descending thin limbs) were unlabeled (arrowheads). (d) In the inner medulla, strong labeling of vascular structures was seen (arrows), whereas multiple thin walled structures (descending and ascending thin limbs) were unlabeled (arrowheads). Collecting ducts exhibited strong labeling in the apical and basolateral plasma membrane domains (small arrows). (Original magnification × 1,100.) (e) When preimmune serum was used, no labeling was observed. (f ) Paraffin section of human kidney inner medulla labeled with anti-Fy6. There was faint labeling seen in some thin structures, presumably capillaries, whereas collecting ducts (arrows) exhibited strong labeling. (Original magnification × 240.) (Inset D) Paraffin section of human kidney labeled with immunopurified antibody 6615. The abundant labeling of capillary endothelium (arrow) confirmed the specificity of antibody 6615.
Immunocytochemical localization of gp-Fy in semithin cryosections of human kidney cortex (a and b), outer medulla (c), and inner medulla (d and e). Antibody 6615 was used for labeling except in (f ). (a) Capillary endothelial cells within the glomerulus (arrows) and peritubular capillaries exhibited strong labeling. (Original magnification × 500.) (b) Cortical peritubular capillaries were labeled, while proximal and distal tubules were unlabeled. (Original magnification × 1,100.) (c) In outer medulla, strong labeling of vascular structures was seen (arrows), whereas multiple thin walled structures (descending thin limbs) were unlabeled (arrowheads). (d) In the inner medulla, strong labeling of vascular structures was seen (arrows), whereas multiple thin walled structures (descending and ascending thin limbs) were unlabeled (arrowheads). Collecting ducts exhibited strong labeling in the apical and basolateral plasma membrane domains (small arrows). (Original magnification × 1,100.) (e) When preimmune serum was used, no labeling was observed. (f ) Paraffin section of human kidney inner medulla labeled with anti-Fy6. There was faint labeling seen in some thin structures, presumably capillaries, whereas collecting ducts (arrows) exhibited strong labeling. (Original magnification × 240.) (Inset D) Paraffin section of human kidney labeled with immunopurified antibody 6615. The abundant labeling of capillary endothelium (arrow) confirmed the specificity of antibody 6615.
Immunolocalization of gp-Fy.Immunocytochemistry of cryosections of three human kidneys showed abundant gp-Fy in glomerulus, with labeling confined to capillary endothelium and no labeling of podocytes (Figs 3a and 4c). In addition, fenestrated peritubular capillaries exhibited abundant labeling in the cortex and outer and inner medulla of the kidney (Fig 3a through d). The labeling of capillary endothelial cells was localized to apical and basal plasma membrane domains (Fig 3b through d). The endothelial cells of vasa recta also exhibited abundant labeling (Fig 3c and d). Within the tubular system, the proximal tubule, thin limbs and distal tubule, did not show labeling (Fig 3a through d). In contrast, the principal cells of collecting ducts were extensively labeled in the cortex, outer medulla (not shown), and inner medulla (Fig 3d). Both apical and basolateral plasma membrane domains were labeled. Controls challenged with preimmune serum did not show labeling (Fig 3e). Furthermore, specific labeling of gp-Fy was confirmed with anti-Fy6 (Fig 3f ). Thus, the same cell type reacted with anti-Fy6 and rabbit polyclonal antibody 6615.
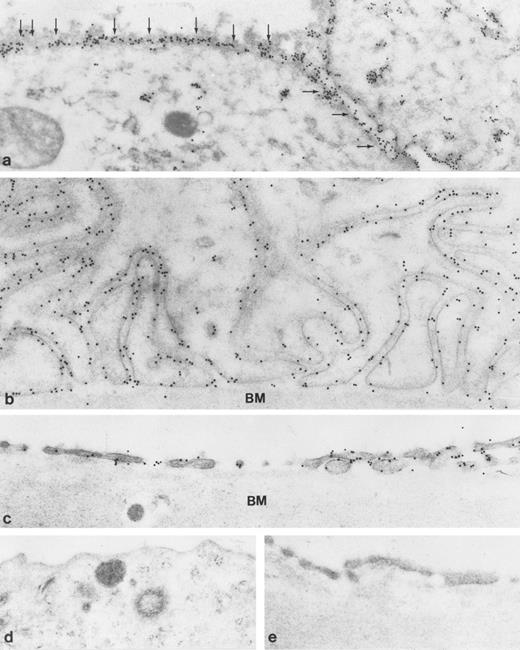
Immunoelectron microscopic localization of gp-Fy in kidney glomerulus. Antibody 6615 was used for labeling. (a) Low magnification of the glomerular capillaries (original magnification × 6,000) and (b through e) higher magnification showed strong labeling (original magnification × 60,000). Endothelial cells (E) showed labeling of the apical and basal plasma membranes, including fenestration and caveolae. In contrast epithelial cells (podocytes, P) were unlabeled. (e) When preimmune serum was used, no labeling was observed. BM, basement membrane.
Immunoelectron microscopic localization of gp-Fy in kidney glomerulus. Antibody 6615 was used for labeling. (a) Low magnification of the glomerular capillaries (original magnification × 6,000) and (b through e) higher magnification showed strong labeling (original magnification × 60,000). Endothelial cells (E) showed labeling of the apical and basal plasma membranes, including fenestration and caveolae. In contrast epithelial cells (podocytes, P) were unlabeled. (e) When preimmune serum was used, no labeling was observed. BM, basement membrane.
To define the subcellular localization of gp-Fy, ultrathin sections were analyzed under the electron microscope. Capillary endothelial cells exhibited abundant labeling of apical and basal plasma membrane domains with labeling extending into fenestrations (Fig 4). This labeling pattern was evident in glomerular capillaries (Fig 4b) and peritubular capillaries in the cortex (Fig 5c). The principal epithelial cells of collecting ducts exhibited extensive labeling (Fig 5b) and strong labeling of caveoli was also observed (Fig 6a and b). Podocytes were not labeled (Fig 4c). Immunolabeling controls were negative (Figs 4e and and 5d and e).
Immunoelectron microscopic localization of gp-Fy in principal cells of collecting duct and peritubular capillaries in kidney medulla. Antibody 6615 was used for labeling. (a) Strong labeling of the apical plasma membrane and small vesicles of the principal cells of the collecting duct is seen. (Original magnification × 39,000.) (b) The basolateral membrane domain, including membrane infolding, of the principal cells of the collecting duct was also heavily labeled. (Original magnification × 60,000.) (c) Peritubular fenestrated endothelial cells of capillaries of the inner medulla exhibited strong labeling. (Original magnification × 48,000.) (d and e). When preimmune serum was used, no immunolabeling of the principal cells of the collecting duct (d) and capillary endothelial cells (e) was observed. (Original magnification × 39,000.) BM, basement membrane.
Immunoelectron microscopic localization of gp-Fy in principal cells of collecting duct and peritubular capillaries in kidney medulla. Antibody 6615 was used for labeling. (a) Strong labeling of the apical plasma membrane and small vesicles of the principal cells of the collecting duct is seen. (Original magnification × 39,000.) (b) The basolateral membrane domain, including membrane infolding, of the principal cells of the collecting duct was also heavily labeled. (Original magnification × 60,000.) (c) Peritubular fenestrated endothelial cells of capillaries of the inner medulla exhibited strong labeling. (Original magnification × 48,000.) (d and e). When preimmune serum was used, no immunolabeling of the principal cells of the collecting duct (d) and capillary endothelial cells (e) was observed. (Original magnification × 39,000.) BM, basement membrane.
Immunoelectron microscopic localization of gp-Fy in the inner medulla of kidney vascular structures. Extensive labeling is seen of plasma membranes and multiple caveoli (arrows). BM, basement membrane; N, nucleus. (Original magnification × 35,000 [a] and × 24,000 [b].)
Immunoelectron microscopic localization of gp-Fy in the inner medulla of kidney vascular structures. Extensive labeling is seen of plasma membranes and multiple caveoli (arrows). BM, basement membrane; N, nucleus. (Original magnification × 35,000 [a] and × 24,000 [b].)
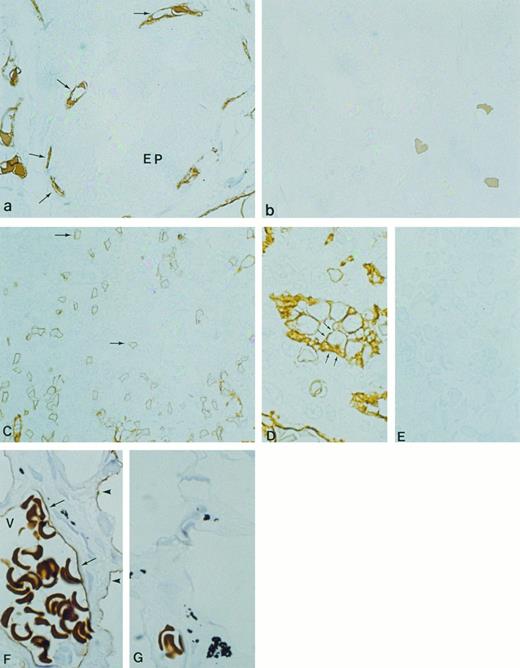
We studied thyroid, lung, and spleen to further analyze other nonerythroid cells that express gp-Fy. In the thyroid, abundant labeling was observed in fenestrated capillaries with no labeling of epithelial cells (Fig 7a). Controls did not show immunolabeling (Fig 7b).
Immunocytochemical localization of gp-Fy in semithin cryosections of human thyroid (a and b), spleen (C through E), and lung (F and G). Antibody 6615 was used for labeling. (a) Fenestrated capillary endothelial cells (arrows) exhibited strong labeling, whereas epithelial cells (EP) were unlabeled. (b) When preimmune serum was used, no labeling was observed. (C) The capillaries and postcapillary venules of spleen were labeled. (D) The cuboidal cells of HEV showed strong labeling of both apical and basal plasma membrane domains. (E) When preimmune serum was used, no labeling was observed. (F ) The endothelial cells of the large venule (arrows) with multiple erythrocytes (V) exhibited strong labeling. Arrowheads show labeling of the squamous alveolar cell (pneumocyte type 1). (G) When preimmune serum was used, no labeling was observed. (Original magnification × 1,100.)
Immunocytochemical localization of gp-Fy in semithin cryosections of human thyroid (a and b), spleen (C through E), and lung (F and G). Antibody 6615 was used for labeling. (a) Fenestrated capillary endothelial cells (arrows) exhibited strong labeling, whereas epithelial cells (EP) were unlabeled. (b) When preimmune serum was used, no labeling was observed. (C) The capillaries and postcapillary venules of spleen were labeled. (D) The cuboidal cells of HEV showed strong labeling of both apical and basal plasma membrane domains. (E) When preimmune serum was used, no labeling was observed. (F ) The endothelial cells of the large venule (arrows) with multiple erythrocytes (V) exhibited strong labeling. Arrowheads show labeling of the squamous alveolar cell (pneumocyte type 1). (G) When preimmune serum was used, no labeling was observed. (Original magnification × 1,100.)
Because the lung and spleen showed significant expression of gp-Fy, we identified which cells produced the Duffy antigen. Cryosections of human lung showed predominant labeling of peri-bronchiolar capillaries, postcapillary venules, and epithelial squamous cells (type-I alveolar cells; Fig 7F ). Controls did not show labeling (Fig 7G). Cryosections from the human spleen showed abundant gp-Fy in capillaries (Fig 7C). Notably, the cuboidal cells of the endothelium of postcapillary venules in the white pulp of the spleen showed a substantial amount of gp-Fy (Fig 7D). This domain of venules, where lymphocyte emigration from the blood occurs, is referred to as the high endothelial venule (HEV). Incubation with preimmune serum did not show labeling (Fig 7E).
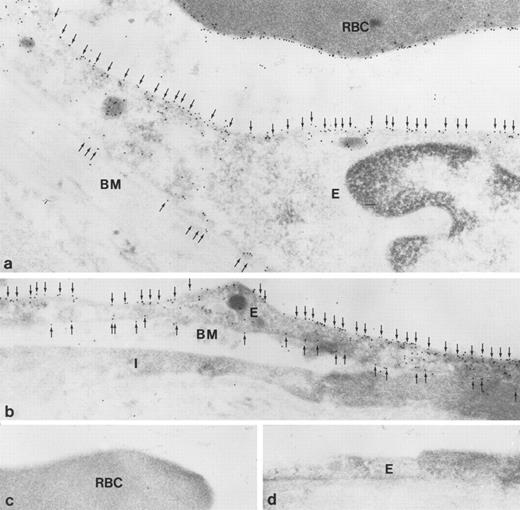
Immunoelectron microscopy of ultrathin cryosections confirmed strong labeling of apical and basolateral plasma membrane domains (Fig 8a and b). Labeling of the red blood cell membrane was also observed (Fig 8a). Controls using preimmune serum did not show labeling of red blood cells or endothelial cells (Fig 8c and d).
Immunoelectron microscopic localization of gp-Fy in ultrathin cryosections of human lung. Antibody 6615 was used. (a) Extensive labeling of both apical and basal membrane domains of vascular endothelial cells (E) was seen. Membranes of red blood cells (RBC) were extensively labeled. (b) The endothelial cells (E) of a peribronchiolar venule exhibited strong labeling in both apical and basal membrane domains (arrows). An interstitial cell was unlabeled (I). BM, basement membrane. (c and d) When using preimmune serum, no labeling was seen in red blood cells (c) and endothelial cells (d). (Original magnification × 60,000.)
Immunoelectron microscopic localization of gp-Fy in ultrathin cryosections of human lung. Antibody 6615 was used. (a) Extensive labeling of both apical and basal membrane domains of vascular endothelial cells (E) was seen. Membranes of red blood cells (RBC) were extensively labeled. (b) The endothelial cells (E) of a peribronchiolar venule exhibited strong labeling in both apical and basal membrane domains (arrows). An interstitial cell was unlabeled (I). BM, basement membrane. (c and d) When using preimmune serum, no labeling was seen in red blood cells (c) and endothelial cells (d). (Original magnification × 60,000.)
Primary structure of gp-Fy in nonerythroid tissues.Oligonucleotide primers, designed from 3′ and 5′ untranslated regions of kidney mRNA from a Duffy-positive individual, were used in RT-PCR and cloned. The size (≈1.3 kb) of the polymerase chain reaction (PCR) product was similar to that of bone marrow (not shown). All clones yielded the same nucleotide sequence as that of bone marrow mRNA, indicating that the kidney had the same gp-Fy as erythrocytes (not shown). The observation is in agreement to what has been recently reported.18,19 Moreover, adult kidney, like bone marrow, produced the spliced and nonspliced variants of Duffy mRNA (not shown).29 Although we did not sequence mRNA from the other tissues, we assume they have the same sequence as bone marrow and kidney, since antibodies Fy6 and 6615 immunoreacted with membrane proteins of these tissues.
DISCUSSION
There are major structural proteins of erythrocyte membrane present in other cell types, which are products of genes different from the erythroid homologue, with a substantial difference in antigenic sites, although the major functional features have been conserved.30 There are other structural proteins of erythrocyte membrane in nonerythroid cells, which are the products of the same gene arising by alternative splicing of coding exons or by alternative use of tissue specific promoters.31-33 However, gp-Fy appears to be the product of the same gene and is produced by the same molecular mechanisms in bone marrow, kidney, spleen, colon, adrenal gland, lung, and thyroid. In the brain, in addition to the same gp-Fy, there are proteins having partial similarity to erythroid gp-Fy (unpublished results).
In kidney, the protein is present in two types of tissue: endothelium and epithelium. Only the endothelial cells in glomeruli, peritubular capillaries, and vasa recta synthesize gp-Fy. Within the tubular system, only the principal cells of collecting ducts produce gp-Fy. The antibodies, 6615, immunopurified 6615, and anti-Fy6 label gp-Fy throughout the entire collecting duct, but the labeling appears stronger in the inner medulla and very weak in the cortex with anti-Fy6 only. This may be the reason why Hadley et al18 did not detect gp-Fy in collecting ducts with anti-Fy6. They not only used a weak antibody, but had limited their studies to the cortex of the kidney.
It is intriguing that cell types such as the fenestrated endothelium of the glomerular capillaries, which is a component of the renal filter, the fenestrated and nonfenestrated endothelium of the vasa recta, which functions as a countercurrent exchanger of fluid, the collecting duct, which reabsorbs water and electrolytes under the control of vasopressin, all produce the Duffy protein. Furthermore, dissimilar cells, such as the endothelium of large venules, capillaries, and the type-I alveolar squamous cell, produce Duffy protein in the lung.
As opposed to the kidney and lung, only capillary endothelial cells of the thyroid produced gp-Fy. Although we did not study the adrenal glands, we assume that as an endocrine gland, it will be similar to the thyroid. The only common feature of endothelial cells of capillaries in endocrine glands is that like glomeruli, they are fenestrated. It is interesting that spleen HEV produces gp-Fy. These cuboidal endothelial cells are morphologically distinct from the flat endothelium of the microvasculature and are the sites for lymphocyte emigration.34 It is possible that gp-Fy is involved with local lymphocytic traffic.
Recent studies have indicated that gp-Fy is expressed along postcapillary venules throughout the body except in the liver.18 19 The use of monoclonal antibody anti-Fy6, which gives a weaker and unreliable signal in immunocytochemistry, is the main shortcoming of these studies. The expression of Duffy protein is not limited to the endothelium of postcapillary venules when a potent antibody is used as shown here. Moreover, not all endothelial cells of capillary and postcapillary venules produce this protein. The nonerythroid expression of gp-Fy is confined to certain organ and cell types.
Endothelial cells are known for having caveolae or noncoated plasmalemmal vesicles, glycolipid microdomains rich in cholesterol, glycosyl phosphatidylinositol (GPI)-anchored protein and caveolin.35 Duffy protein, as a transmembrane protein, is present also in caveolae (Fig 6) and may participate in receptor-mediated endocytosis.36 Recently, the endocytosis of radiolabeled chemokines in K562, transfected with gp-Fy cDNA was shown.19 Possibly, gp-Fy, as a receptor for small molecules (polypetide or amino acids), uses this membrane domain for endocytosis.
Chemokines play a major role in the mobilization and activation of leukocytes.6,15,17 The observation that gp-Fy is the human erythrocyte chemokine receptor, albeit promiscuous, inspired the idea that it may function in the red blood cell as a “sink” or scavenger for chemokines.17 Therefore, Duffy-negative individuals may have a greater vulnerability to septic shock than Duffy-positive individuals.37 It can be argued that the expression of gp-Fy in nonerythroid organs may compensate for the erythroid deficiency in Duffy-negative individuals. Since all the blood must flow throughout the kidney and lung, gp-Fy in such organs has the potential to remove harmful chemokines. However, it is unlikely that the endothelial cells of renal glomeruli and lung capillaries, which have less gp-Fy and do not compensate for the absence of this protein in the erythrocytes, can function as effective scavengers in Duffy-negative individuals. It is conceivable that Duffy-negative individuals have other clearance mechanisms. It will be important to determine whether Duffy-negative individuals have greater vulnerability to septic shock than Duffy-positive individuals.
The study of Duffy protein in nonerythroid cells has indicated that cells with markedly different roles produce this protein. Perhaps it is a receptor of an unknown ligand (polypeptide), which remains to be discovered. The ligand may be necessary for the normal function of the kidney, lung, thyroid, adrenal glands or organs, such as the brain, where the Duffy gene is expressed. Now the challenge is to discover whether gp-Fy is a protein with multiple functions or whether there is a common function that gp-Fy performs in these cell types.
ACKNOWLEDGMENT
The authors thank I. Kristoffersen for excellent technical assistance, and gratefully acknowledge Drs S. Olsen and P. Ottosen for the surgical human specimens, Drs M. Nichols, P. Rubinstein, and D. Blanchard for samples of anti-Fy6, T. Huima for photographic/printing assistance, and V.M. Sarnicole for secretarial assistance.
A.C. and S.N. contributed equally to this report.
Supported by Novo Nordisk Foundation, the Danish Medical Research Council, the Biomembrane Research Center at University of Aarhus, University of Aarhus Research Foundation, Danish Foundation for the Advancement of Medical Science, and Grant No. HL 53297 of the National Heart, Lung, and Blood Institute of the National Institutes of Health, Bethesda, MD.
Address reprint requests to A. Oscar Pogo, MD, DMSc, Laboratory of Cell Biology, Lindsley F. Kimball Research Institute, New York Blood Center, 310 E 67th St, New York, NY 10021.


![Fig. 6. Immunoelectron microscopic localization of gp-Fy in the inner medulla of kidney vascular structures. Extensive labeling is seen of plasma membranes and multiple caveoli (arrows). BM, basement membrane; N, nucleus. (Original magnification × 35,000 [a] and × 24,000 [b].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/2/10.1182_blood.v89.2.701/4/m_bl_0005f6.jpeg?Expires=1769096284&Signature=Uf3MhfUOBv--FWw6-gJwVosLjGzVQE705pMmNIqOVkv5rW0P0pNAYVst8WfnIDYZJdF5G2Kvn6ujOjkP-C91h2i7p4WrwoRKCIfmE~3a4VE1ZmYL3vtncDw2njbGhJEquudmGshLLVXTfCmsG4xhU2kqmVQZuV-74GYtYCW4f2m4Ayq6-O9rqVC5H1boAhgh4BRAn-PIWClsIMKV74clsY4kzDLe2eug9pQh5~aEZyed~kmel92kc7jBujAmVmOjBDZAGOarjBFllGgnzj2yUCv-mPLgKM6CM9fL-y-W1iKCaE9Ggr7uX-ad6WOflpxhfXMibccXxnIVREUmEs3AUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal