Abstract
Thrombomodulin (TM) is a multidomain protein that serves as a cofactor in a major natural anticoagulant system. To further characterize the structure-function of TM, we have transfected COS cells with different truncated forms of TM. In the first form, COS cells expressing TM that lacks the putative signal peptide (17 residues); the lectin-like, hydrophobic N-terminal domain (226 residues); and 12 residues of the first epidermal growth factor (EGF )-like repeat (COSdel.238 cells) were found to function normally with respect to TM transport to the cell surface and thrombin-dependent protein C activation. However, in contrast to wild-type TM, as visually studied by immunofluorescence and immunogold electron microscopy, the COSdel.238 cells did not constitutively internalize anti-TM–TM or thrombin-TM complexes. To identify the region responsible for mediating the endocytic process, deletant forms of TM lacking either the lectin-like region (residues 2-155) or the hydrophobic region of the N-terminal domain (residues 161-202) were expressed in COS cells (COSdel.2-155 and COSdel.161-202, respectively). Protein C cofactor activity was maintained in both cells. Although the COSdel.161-202 cells behaved similarly to wild-type TM-transfected cells, visual studies showed a lack of constitutive internalization of thrombin-TM or anti-TM–TM complexes in the COSdel.2-155 cells. We conclude that the lectin-like domain of human TM serves to regulate cell surface expression of TM via the endocytic route and therefore may also play a major physiologic role in controlling intracellular and extracellular accumulation of thrombin in a variety of biologic systems.
THROMBOMODULIN (TM) is a vascular endothelial cell receptor that is a critical cofactor in a major physiologically relevant natural anticoagulant system.1,2 By enhancing the activation of protein C by thrombin, TM leads to the cleavage and inactivation of factors V and VIII by activated protein C, thereby downregulating further production of thrombin. Functional and quantitative abnormalities of protein C have commonly been reported to be associated with a hypercoagulable state3,4 and recently there has been a family described with a mutation in the gene encoding TM that was associated with a thrombotic diathesis.5
Studies by several investigators have elucidated the putative structural organization of TM and the regions responsible for its anticoagulant function.6-11 The mature single-chain glycoprotein in the human is 557 amino acids long and is structurally divided into five domains. The N-terminal region (residues 1-226)10 has a module (residues 1-154) with homology to the lectin domains of the hepatic asialoglycoprotein receptor12 and IgE13 as well as to members of the selectin family14 and has two potential sites for N-linked glycosylation. From residues 155 to 226, there is a hydrophobic region that may be associated with the plasma membrane15 and contains two potential sites for O-linked glycosylation. The next domain is composed of six epidermal growth factor (EGF )-like repeats, the last two of which form the binding site for thrombin and the last three of which are necessary for association and activation of protein C. The function of the other EGF-like repeats is unknown. The third domain between the EGF-like repeats and the membrane-spanning region is rich in serines and threonines and contains four potential sites for O-linked glycosylation, to one of which is attached a chondroitin sulfate, which is important for full anticoagulant activity of TM.2 Fourthly, there is a highly conserved transmembrane domain and, finally, a short cytoplasmic tail that contains potential sites of phosphorylation and a single cysteine that may mediate multimerization of the molecule.16
Considerable attention has been paid to identifying the structural determinants critical for normal anticoagulant function of TM. However, few studies have been directed towards elucidating the role of the other structural domains. We have recently determined that TM undergoes constitutive endocytosis and that this process is not significantly affected by deletion of the cytoplasmic tail.16 17 The results suggested that alternative signals were necessary for the endocytic process to occur. In view of the fact that the N-terminal domain of TM has a hydrophobic region as well as a lectin-like module, we hypothesized that some or part of this region may interact with the plasma membrane and play a role in regulating intracellular trafficking of TM. To explore this possibility, we used COS cells to express human TM lacking the following: (1) the entire N-terminal domain of TM, (2) the lectin-like module, and (3) the hydrophobic module. The results, which are visually demonstrated by immunofluorescent and immunogold electron microscopy studies, confirm that the lectin-like region of TM (residues 2-155) is required for its constitutive internalization.
MATERIALS AND METHODS
Materials
Purified bovine protein C was obtained from Enzyme Research Laboratories (South Bend, IN), and the chromogenic substrate, HD-Phe-Pip-Arg-pNA (S2238), was provided by Helena Laboratories (Beaumont, TX). Bovine thrombin with a specific activity of 1,900 U/mg was purified as previously detailed.18 Murine monoclonal antibodies 24FM and 3E2 directed against human TM were a gift of Stago (Paris, France) and have previously been shown not to induce internalization and/or aggregation of TM.17 CD71 is a murine monoclonal antihuman transferrin receptor antibody obtained from Sigma (St Louis, MO). HL12-21 is a murine monoclonal antihuman factor IX antibody kindly provided by Dr E. Yeo (Toronto, Ontario, Canada). Each of the antibodies was isotypically the same (IgG1κ). Restriction enzymes were from Boehringer Mannheim Canada (Dorval, Quebec, Canada), and radiolabeled products were from ICN Biomedicals, Mississauga, Ontario, Canada).
Vector Construction
Deletion of residues −17 to 238 from N-terminus of TM.Using the convenient restriction enzyme sites Xho I and Mlu I, a 950-bp fragment of human TM cDNA that encodes the amino-terminal 256 residues of nascent TM was removed from a 3,071-bp Xho I-BamHI fragment of the TM DNA; the latter includes the entire coding region and was previously subcloned into the plasmid Bluescript (pBS; Stratagene, La Jolla, CA).17 The Xho I and Mlu I sites of the remaining linearized vector were filled in with dNTPs using Klenow and dephosphorylated with calf intestinal alkaline phosphatase (Pharmacia, d'Urfe, Quebec, Canada). The Cla I linker [New England Biolabs, Beverly, MA; #1077 c(pCCCATCGATGGG)] containing an initiation codon was ligated to each end, resulting in circularized DNA with the ATG codon in-frame for translation of the remainder of TM. After transformation of this recombinant plasmid, the 2.1-kb Cla I-Xba I fragment was subcloned into Cla I-Xba I cut pBS and transformed into bacteria. Several colonies were picked, checked for the expected insert, and confirmed by DNA sequencing. An appropriate clone was selected and grown to large scale, and the entire Xho I-Xba I insert coding for TM without the N-terminal 255 amino acids (5′ of the initiation methionine) of the molecule (a putative 17-residue signal peptide + 238 residues of mature TM)10 was subsequently subcloned into the expression plasmid vector pSR1neo17 and introduced by electroporation into COS-7 cells to yield “COSdel.238” cells.
Deletion of lectin-like and hydrophobic regions of N-terminal domain.The lectin-like module within the N-terminal domain of TM was removed by recombination polymerase chain reaction (PCR) in the following way. Oligonucleotide primers P1 (5′CAACTCGAGCCCTGGCCGATCCGCAT) and P2(5′ATTGCACGCGTGCTCGCAGC) that flank the region from nucleotide 1 to 95919 and encoding the N-terminal domain of human TM were synthesized for the purposes of PCR amplification. Two primers (a 36-mer [5′GGCCTGGGGTTCCCCGCAACCTGCAGGCCACTGGCT] and its reverse complement) were constructed such that they overlapped and skipped the lectin-like module from residue 2 to 155, inclusive. Each of these primers were matched with primers P2 and P1, respectively, for PCR amplification, with the target DNA being pBS containing the entire human TM cDNA (see above). The two PCR products of expected sizes 249 bp and 247 bp were purified, denatured, annealed, and amplified by PCR using P1 and P2 as the primers. The recombination product of 496 bp was digested with Xho I-Mlu I and subcloned to replace the wild-type N-terminal domain of human TM in the expression vector pSR1neo. The hydrophobic module within the N-terminal domain of TM (residues 161-202, inclusive) was removed by similar techniques; however, the primer -( 5 ;pr CTTCCCAGCCACCTGCAGGCCCTTACAGCTAATGTGCAC ) and its reverse complement were designed to delete the DNA encoding the region from residues 161 to 202, inclusive. These oligonucleotide primers were paired, respectively, with primers P2 and P1 in PCR reactions to yield amplicons of 112 and 709 bp. The recombination product of 821 bp was then subcloned as described above into Xho I-Mlu I=ndigested wild-type TM cDNA in pSR1neo. Both of the deletant constructs were confirmed by DNA sequencing, and the expression vectors were introduced into COS-7 cells by electroporation, resulting in COSdel.2-155 and COSdel.161-202 cells.
RNA Isolation and Northern Analysis
Total RNA was isolated from confluent cell monolayers by the method of Chomczynski and Sacchi.20 Northern analyses and detections were performed as previously described.21 The cDNA probe used to detect TM mRNA was a Xho I-Xba I 3.1-kb fragment from the TM gene that spans the entire coding region for the protein. It was purified on a low melting point agarose gel followed by labeling with ;ga-32P-dCTP using the random primer synthesis method.22 The specific activity of the probe was 1 to 2 ;ts 106 cpm/ng of DNA.
Cell Culture
COS-7 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 mg/mL penicillin, and 100 mg/mL streptomycin. Transfected COS cells expressing the neomycin-resistant gene were selected and continually grown in the presence of 1 mg/mL Geneticin (GIBCO/BRL, Grand Island, NY). Cell cultures were incubated at 37;dgC in a humidified atmosphere of 5% CO2 and 95% air, and media was changed 24 hours before all experiments.
Protein C Activation
The levels of functional cell surface TM were evaluated as previously reported17 by incubating purified protein C and thrombin over the cells and quantitating the generation of activated protein C using the synthetic amidolytic substrate, S2238. Controls containing the same concentrations of thrombin and protein C in the absence of cells resulted in no activation of protein C.
Immunofluorescent Studies
Cells were grown on glass coverslip slides and washed three times in phosphate-buffered saline (PBS). Cells were fixed with 3.0% paraformaldehyde in 0.1 mol/L sodium phosphate pH 7.2, for 20 minutes and permeabilized with 0.2% Triton X-100 in PBS for 2 minutes. After further washes in PBS, the cells were treated for 30 minutes with blocking solution (PBS with 1% bovine serum albumin) and washed with PBS, and the first murine monoclonal antibody was added at 10 mg/mL for 30 minutes at 37;dgC in blocking solution. After further washes, detection was accomplished with rabbit-antimouse Fab;pr bound to fluorescein isothiocyanate (FITC). Parallel experiments using an irrelevant first antibody (HL12-21) were performed to exclude nonspecific immunofluorescence.
Conjugation of Proteins to Colloidal Gold
Colloidal gold with a mean diameter of 15 nm (SD ;eq 1.8), made using the procedure of Frens,23 was stably conjugated with thrombin or murine monoclonal antibodies by methods previously reported.16,17,24 The minimum amount of protein needed to ensure complete labeling of a given volume of colloidal gold solution was established using the dilution-electrolyte flocculation test.25 The gold conjugates were examined under electron microscopy (EM) before use to ensure the absence of aggregates.
Sample Preparation for Electron Microscopic Studies
Before protein-gold labeling, cell layers were washed with ice cold serum-free media and incubated at 0;dgC for 40 minutes. Gold-labeled anti-TM antibody or thrombin was then added to a final concentration of 5 mg/mL and incubated for 60 minutes on ice. The cell monolayers were washed with media and incubated for various times at 37;dgC, after which the cells were placed on ice, washed with cold PBS, quickly scraped into a microfuge tube, and fixed with 2.5% glutaraldehyde in PBS. After postfixation in 1% osmium tetroxide and ethanol dehydration, the pellets were treated with propylene oxide, followed by propylene oxide/Epon 1:1 mix, dessicated, and polymerized at 70;dgC in Epon. Thin sections were stained with uranyl acetate, lightly counter-stained with lead citrate, and examined at 60 kV using a JEOL (Peabody, MA) transmission electron microscope (JEM 1200EXII). Representative fields were photographed and presented as Figs 6-11.
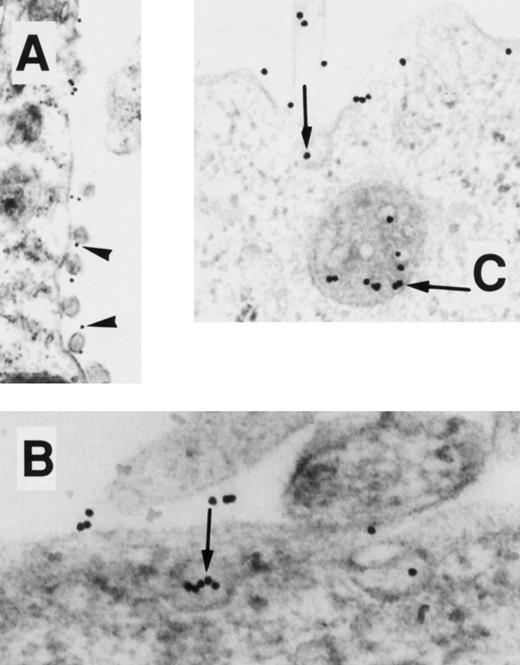
EM localization of gold-conjugated 24FM in COS.TM-CR cells. After preincubation of cells with gold-complexed antibody, the cells were exposed to 37°C for 0, 10, 20, and 45 minutes (A, B, C, and D, respectively). (A) Gold particles are diffusely distributed over the cell membrane surface (arrowheads). (B and C) Clusters of particles are seen on the cell surface and in predominantly noncoated pits and vesicles. (D) At 45 minutes, particles appear in deeper vesicles and multivesicular bodies (arrow), coincidentally with particles at the cell surface.
EM localization of gold-conjugated 24FM in COS.TM-CR cells. After preincubation of cells with gold-complexed antibody, the cells were exposed to 37°C for 0, 10, 20, and 45 minutes (A, B, C, and D, respectively). (A) Gold particles are diffusely distributed over the cell membrane surface (arrowheads). (B and C) Clusters of particles are seen on the cell surface and in predominantly noncoated pits and vesicles. (D) At 45 minutes, particles appear in deeper vesicles and multivesicular bodies (arrow), coincidentally with particles at the cell surface.
Analysis of Data
Statistical analyses of data were conducted by standard techniques26 with the aid of StatView computer program for the Macintosh (Abacus Concepts Inc, Berkeley, CA). The means are provided with associated standard errors (SE).
RESULTS
Expression of Truncated TM by COS Cells
COS cells have previously been stably transfected with wild-type TM (COS.TM-CR cells) that express high cell-surface levels of a functional protein that is identical by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and endocytic routing to that from human umbilical vein endothelial cells (HUVEC).17 For this report, we have transfected COS cells to express three truncated forms of TM. The first lacks the 17 residue signal peptide after the initiation methionine, the entire N-terminal domain (residues 1-226), and 12 amino acids of the first EGF-like repeat (Fig 1). The second lacks the lectin-like module (residues 2-155) and the third lacks the hydrophobic region of the N-terminal domain (residues 161-202). In each case, several clones selected by neomycin resistance were isolated by limited dilution and the ones expressing the highest amounts of cell surface TM as evaluated by thrombin-dependent activation of protein C (Table 1) were maintained for the following experiments and are referred to as COSdel.238 cells, COSdel.2-155 cells, and COSdel.161-202 cells. Functional cell surface TM per cell was similar in those cells expressing wild-type and the truncated forms of TM (Table 1). In addition to verification of the cDNA construct by direct DNA sequence analysis and Southern blotting (data not shown), Northern analysis ensured transcription of the expected size mRNAs from the transfected cell lines (Fig 2).
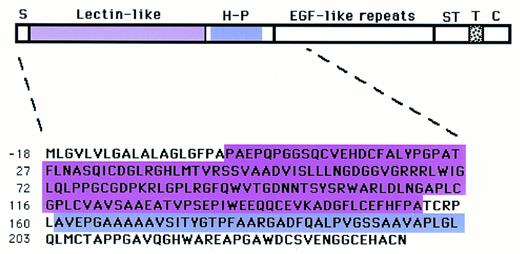
Model of human TM and amino acid sequence of deleted amino terminal end for expression in COS.del.Nterm cells. Nascent human TM has 575 amino acid residues starting with a putative 18-residue signal peptide (S) at the N-terminal end. This is followed by 5 structural domains: (1) the N-terminal domain composed of a lectin-like module and a hydrophobic region (H-P), (2) six EGF-like repeats, (3) a serine-threonine (ST)-rich region, (4) the transmembrane domain (T), and (5) the cytoplasmic domain at the C-terminal. Numbering of amino acids is according to Suzuki et al.10 TM cDNA constructs lacking the encoding regions for the entire N-terminal domain (residues −17 to 238), the lectin-like module (residues 2-155 in pink), or the hydrophobic module (residues 161-202 in purple) were transfected for expression as COSdel.238 cells, COSdel.2-155, or COSdel.161-202 cells, respectively, as detailed in the Materials and Methods.
Model of human TM and amino acid sequence of deleted amino terminal end for expression in COS.del.Nterm cells. Nascent human TM has 575 amino acid residues starting with a putative 18-residue signal peptide (S) at the N-terminal end. This is followed by 5 structural domains: (1) the N-terminal domain composed of a lectin-like module and a hydrophobic region (H-P), (2) six EGF-like repeats, (3) a serine-threonine (ST)-rich region, (4) the transmembrane domain (T), and (5) the cytoplasmic domain at the C-terminal. Numbering of amino acids is according to Suzuki et al.10 TM cDNA constructs lacking the encoding regions for the entire N-terminal domain (residues −17 to 238), the lectin-like module (residues 2-155 in pink), or the hydrophobic module (residues 161-202 in purple) were transfected for expression as COSdel.238 cells, COSdel.2-155, or COSdel.161-202 cells, respectively, as detailed in the Materials and Methods.
Cell Surface Thrombomodulin Function
| Cell Type . | ;gDOD405/min/108 Cells . |
|---|---|
| COS | 0 |
| COS.pSR1neo | 0 |
| HUVEC | 2.7 ;pm 0.9 |
| COS.TM-CR | 196 ;pm 28 |
| COSdel.238 | 206 ;pm 36 |
| COSdel.2-155 | 184 ;pm 24 |
| COSdel.161-202 | 201 ;pm 34 |
| Cell Type . | ;gDOD405/min/108 Cells . |
|---|---|
| COS | 0 |
| COS.pSR1neo | 0 |
| HUVEC | 2.7 ;pm 0.9 |
| COS.TM-CR | 196 ;pm 28 |
| COSdel.238 | 206 ;pm 36 |
| COSdel.2-155 | 184 ;pm 24 |
| COSdel.161-202 | 201 ;pm 34 |
Cells were grown in 2-cm2 tissue culture wells until 48 hours postconfluent. TM-dependent protein C activation was determined using S2238 amidolytic substrate as detailed in the Materials and Methods. Results reflect the means of three individual experiments performed in duplicate with associated standard errors (SE).
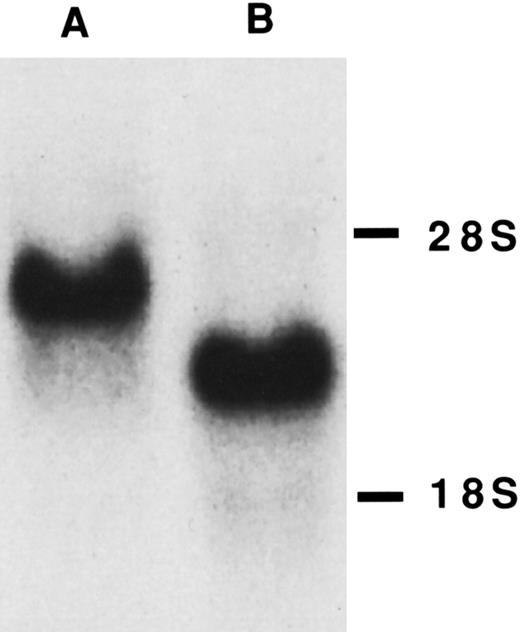
Northern blot of TM-transfected COS cells. Ten micrograms of total RNA from COS.TM-CR (A) and COSdel.238 (B) cells was separated on a denaturing formaldehyde-agarose gel as detailed in the Materials and Methods. mRNA for TM was detected with a specific radiolabeled cDNA probe. 28S and 18S ribosomal bands are shown on the right.
Northern blot of TM-transfected COS cells. Ten micrograms of total RNA from COS.TM-CR (A) and COSdel.238 (B) cells was separated on a denaturing formaldehyde-agarose gel as detailed in the Materials and Methods. mRNA for TM was detected with a specific radiolabeled cDNA probe. 28S and 18S ribosomal bands are shown on the right.
Immunofluorescent Studies
Direct visualization of expression of TM by COS-TM.CR and COSdel.238 cells was accomplished by using immunofluorescent microscopy with monoclonal antibodies 24FM or 3E2 specifically directed against human TM, followed by detection with FITC-secondarily labeled antibody (Figs 3 and 4). This confirmed that the deletant and wild-type forms of TM could be synthesized, processed, and inserted into the plasma membrane and that it could associate with thrombin, protein C, and the specific monoclonal antibodies. No cross-reactivity with the irrelevant monoclonal antibody HL12-21 (antihuman factor IX antibody) was detected.
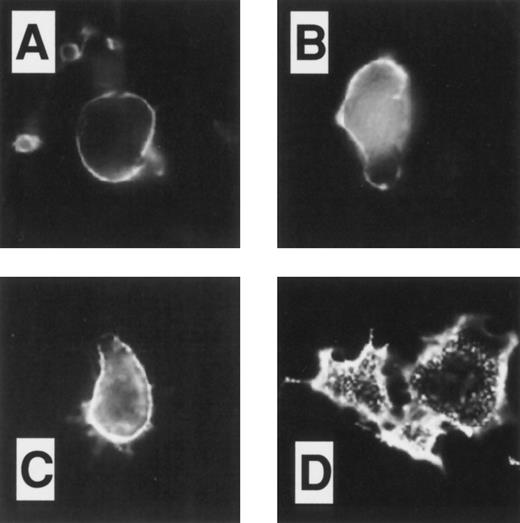
Immunofluorescent studies on COS.TM-CR cells. Cells were preincubated on ice for 45 minutes with 24FM, washed, and placed at 37°C for 0, 10, and 30 minutes (A, B, and C, respectively). After permeabilization and fixation, antibody localization was detected using FITC as described in the Materials and Methods. In (B) and (C), antibody-TM complex was present intracellularly in clusters, with less on the plasma membrane as compared with (A). (D) After incubation at 37°C for 30 minutes as in (C), the cells were fixed without permeabilization. The intensity of labeled TM at the cell surface was reduced, indicating that constitutive internalization of the receptor had occurred.
Immunofluorescent studies on COS.TM-CR cells. Cells were preincubated on ice for 45 minutes with 24FM, washed, and placed at 37°C for 0, 10, and 30 minutes (A, B, and C, respectively). After permeabilization and fixation, antibody localization was detected using FITC as described in the Materials and Methods. In (B) and (C), antibody-TM complex was present intracellularly in clusters, with less on the plasma membrane as compared with (A). (D) After incubation at 37°C for 30 minutes as in (C), the cells were fixed without permeabilization. The intensity of labeled TM at the cell surface was reduced, indicating that constitutive internalization of the receptor had occurred.
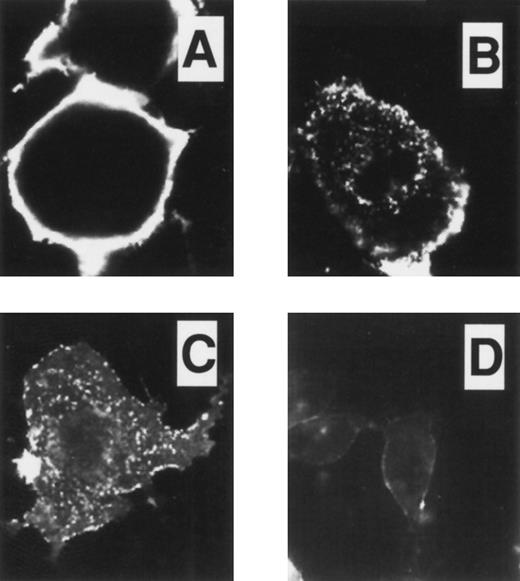
Immunofluorescent studies on COSdel.238 cells. Cells were preincubated on ice for 45 minutes with 24FM, washed, and exposed to 37°C for 0, 10, and 30 minutes (A, B, and C, respectively). After permeabilization and fixation, antibody localization was detected using FITC conjugated to a secondary antibody. In representative fields as shown in (A), (B), and (C), antibody-TM complex was seen predominantly on the cell surface, with little evidence of internalization or significant shedding of the complex from the cell surface over 30 minutes. In (D), cells were prepared as in (C), but with murine monoclonal anti-transferrin receptor antibodies CD71. In this case, intracellular clusters of antibody-receptor complexes were observed, indicating that the cells were capable of supporting constitutive endocytosis.
Immunofluorescent studies on COSdel.238 cells. Cells were preincubated on ice for 45 minutes with 24FM, washed, and exposed to 37°C for 0, 10, and 30 minutes (A, B, and C, respectively). After permeabilization and fixation, antibody localization was detected using FITC conjugated to a secondary antibody. In representative fields as shown in (A), (B), and (C), antibody-TM complex was seen predominantly on the cell surface, with little evidence of internalization or significant shedding of the complex from the cell surface over 30 minutes. In (D), cells were prepared as in (C), but with murine monoclonal anti-transferrin receptor antibodies CD71. In this case, intracellular clusters of antibody-receptor complexes were observed, indicating that the cells were capable of supporting constitutive endocytosis.
After preincubation of the COS-TM.CR and COSdel.238 cells on ice, 24FM antibodies were added for a further 30 minutes on ice. At this temperature (4;dgC), where endocytosis is absent, the anti-TM antibody=nTM complexes were detected in both cell lines (Figs 3A and 4A) by immunofluorescence and observed to be evenly distributed predominantly on the plasma membrane surface, with little evidence of intracellular localization. Cells were exposed to 37;dgC for 10 and 30 minutes to attempt to initiate the process of endocytosis. Under these conditions, internalization of the complexes was induced in the COS-TM.CR cells, as seen in Fig 3B and C. An intracellular punctate pattern was observed with a coincident decrease in intensity of the cell surface membrane fluorescence. The intracellular location was confirmed by parallel experiments in which permeabilization was not performed before fixation (Fig 3D). In this case, fluorescence was markedly diminished and restricted to the cell surface.
In contrast to the intracellular routing of TM in the COS-TM.CR cells, internalization of 24FM-TM complexes was not seen with the COSdel.238 cells (Fig 4). After exposure of the cells to 37;dgC for 10 and 30 minutes, the expression of TM was largely restricted to the cell surface and was barely detectable intracellularly. These studies excluded the possibility that the loss of intracellular TM in the COSdel.238 cells was due to augmentation in ligand or ligand-receptor shedding from the cell surface, because the surface labeling remained fairly constant during exposure to 37;dgC. As a further control, we showed that the COSdel.238 cells could support the process of constitutive endocytosis by observing internalization of the transferrin receptor as detected by anti-transferrin receptor antibodies CD71 and immunofluorescence under the same conditions over a 30-minute period (Fig 4D).
Because of the possibility that either the signal peptide or the first EGF-like repeat of TM might be responsible for the observed effect on the endocytosis of the molecule, we repeated the above studies with COS cells expressing deletant forms of TM that either lacked only the lectin-like module (residues 2-155; COSdel.2-155 cells) or the hydrophobic region from residues 161 to 202 (COSdel.161-202 cells; Fig 1). Transcription of the expected size mRNAs in each cell line was confirmed by Northern blot analyses, and expression of functional cell-surface TM was verified by determination of thrombin-dependent protein C activity (Table 1).
After preincubation of the COSdel.2-155 cells on ice with 24FM antibodies, we examined the endocytic role of the lectin-like module of TM by increasing the temperature to 37;dgC for varying periods of time. As with the COSdel.238 cells, there was almost no evidence of internalization of the antibody-TM complexes over a 30-minute period (Fig 5). Furthermore, the intensity of surface labeling on the cells remained fairly constant, suggesting that there was little shedding of the ligand from the surface during the period studied. The observation of internalization of transferrin receptor in the COSdel.2-155 cells again confirmed that these cells were still capable of supporting the process of endocytosis (not shown).
Immunofluorescent studies on COSdel.2-155 cells. Cells were preincubated on ice for 45 minutes with 24FM, washed, and exposed to 37°C for 0, 10, and 30 minutes (A, B, and C, respectively). After permeabilization and fixation, 24FM localization was identified as in Figs 3 and 4. Antibody-TM complex remained exclusively on the cell surface in these representative pictures (A, B, and C) over a 30-minute period. As with the COSdel.238 cells (Fig 4), there was no evidence of internalization or shedding of the complex.
Immunofluorescent studies on COSdel.2-155 cells. Cells were preincubated on ice for 45 minutes with 24FM, washed, and exposed to 37°C for 0, 10, and 30 minutes (A, B, and C, respectively). After permeabilization and fixation, 24FM localization was identified as in Figs 3 and 4. Antibody-TM complex remained exclusively on the cell surface in these representative pictures (A, B, and C) over a 30-minute period. As with the COSdel.238 cells (Fig 4), there was no evidence of internalization or shedding of the complex.
The hydrophobic region of the N-terminal domain of TM between residues 161 and 202 was similarly examined by immunofluorescence studies using the COSdel.161-202 cells. In contrast to the effect of deleting residues 2 to 155, there was no apparent effect on internalization of TM when residues 161-202 were deleted. Using the 24FM antibodies, exposure of the COSdel.161-202 cells to 37;dgC for 10 and 30 minutes resulted in rapid internalization of the antibody-TM complexes in a pattern apparently unchanged from that seen with the wild-type COS.TM-CR cells (data not shown).
Electron Microscopy Studies
When the COS-TM.CR cells were incubated at 4;dgC with the 24FM-gold complexes, gold particles became randomly distributed on the cell membrane surface as individual particles or in clusters of 2 or 3 (Fig 6A). Controls using 24FM-gold complexes incubated with either COS cells or pSR1neo-transfected cells or an irrelevant gold-complexed antibody (HL12-21) incubated with the COS.TM-CR or COSdel.238 cells resulted in an absence of binding to the cell surface. As shown previously,17 internalization of specific anti-TM gold particles in the COS-TM.CR cells was evident as the cells were exposed to 37;dgC for varying periods of time (Fig 6B through D). This was characterized by redistribution of many of the particles into clusters and progressive accumulation into mostly noncoated plasma membrane invaginations, pits, and vesicles. By 10 to 20 minutes, gold particles were observed in deeper, larger-sized vesicles. By 45 minutes, they were seen in multivesicular bodies and lysosomes, with the internalized fraction representing a range of 40% to 80% of the total number of gold particles. Nonspecific binding of the gold-complexed antibody was excluded as previously reported17 using the same immunogold complexes on non-TM=nexpressing COS cells and gold-complexed HL12-21 antibody on the COS-TM.CR cells.
Routing of gold-labeled thrombin was also re-examined in the COS-TM.CR and the results, depicted in Fig 7, are consistent with those previously reported by us.16 17 Without induction of internalization, the thrombin-gold complexes were seen at the cell surface singly and in clusters of 1 or 2 (Fig 7A). As endocytosis was induced for 10 to 45 minutes, internalization of the gold was evident predominantly via noncoated pits and vesicles, with ultimate localization in multivesicular bodies and lysosomes (Fig 7B and C).
EM localization of gold-conjugated thrombin in COS.TM-CR cells. After preincubation of cells with gold-complexed thrombin, the cells were exposed to 37°C for 0, 10, and 45 minutes (A, B, and C, respectively). (A) Gold particles are diffusely distributed over the cell membrane surface (arrowheads). (B and C) Particles, singly and in clusters (arrow), appear in pits, vesicles, and multivesicular bodies.
EM localization of gold-conjugated thrombin in COS.TM-CR cells. After preincubation of cells with gold-complexed thrombin, the cells were exposed to 37°C for 0, 10, and 45 minutes (A, B, and C, respectively). (A) Gold particles are diffusely distributed over the cell membrane surface (arrowheads). (B and C) Particles, singly and in clusters (arrow), appear in pits, vesicles, and multivesicular bodies.
The effects of exposing either the COSdel.238 cells or the COSdel.2-155 cells to 37;dgC on the routing of 24FM-gold and thrombin-gold complexes were similarly studied (Figs 8-11). Before induction of internalization, the 24FM-gold (Figs 8A and 10A) and thrombin-gold (Figs 9A and 11A) complexes distributed singly or in small clusters exclusively on the cell surface in a pattern similar to that seen with the COS-TM.CR cells. However, in marked contrast to the intracellular pathway characterized by the COS.TM-CR cells, internalization of the gold complexes was notably absent in both the COSdel.238 (Figs 8 and 9) and COSdel.2-155 cells (Figs 10 and 11). With incubation of these cells at 37;dgC for 10 to 45 minutes, complexes of 24FM-gold (Figs 8B through D and 10B through D) and thrombin-gold (Figs 9B and C and 11B and C) were almost exclusively seen on the cell surface, whereas intracellular particles represented less than 2% of all gold particles. Empty clathrin-coated and noncoated vesicles were easily identified. Using a gold-complexed monoclonal anti-TM antibody (3E2) known to identify a different epitope than 24FM, the above experiments were repeated with identical results, in that constitutive endocytosis was not seen using the COSdel.2-155 cells, whereas it was readily apparent in the COS.TM-CR cells. There was no discernible difference in the endocytic routing of gold-complexes of 24FM or thrombin in COSdel.161-202 and COS.TM-CR cells, further illustrating the specificity of the region (residues 2-155) that is critical for internalization of TM.
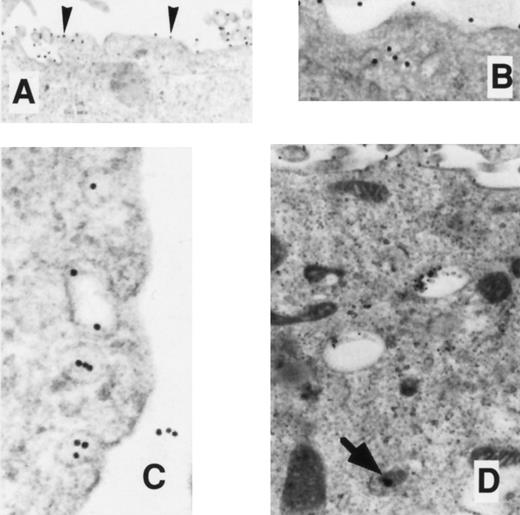
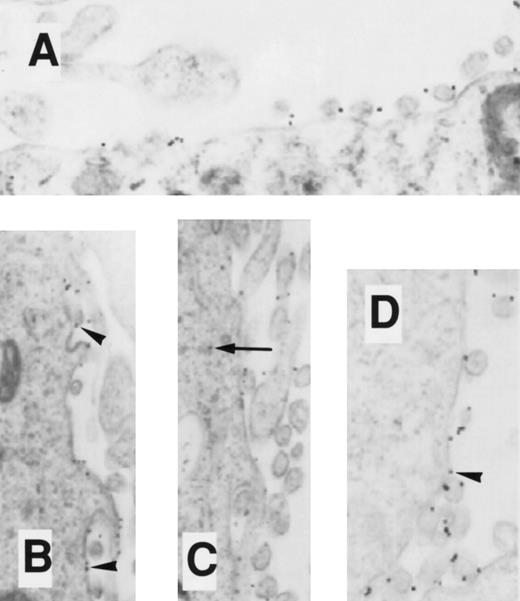
EM localization of gold-conjugated 24FM in COSdel.238 cells. Cells were preincubated with gold-complexed 24FM as in Fig 5 and exposed to 37°C for 0, 10, 30, and 45 minutes (A, B, C, and D, respectively). In contrast to what is seen in Fig 5, gold particles remained on the cell surface (arrowheads), although clusters formed within 10 minutes of exposure to 37°C. Rare intracellular particles are seen (arrow).
EM localization of gold-conjugated 24FM in COSdel.238 cells. Cells were preincubated with gold-complexed 24FM as in Fig 5 and exposed to 37°C for 0, 10, 30, and 45 minutes (A, B, C, and D, respectively). In contrast to what is seen in Fig 5, gold particles remained on the cell surface (arrowheads), although clusters formed within 10 minutes of exposure to 37°C. Rare intracellular particles are seen (arrow).
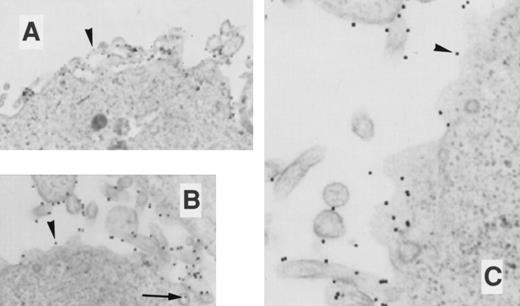
EM localization of gold-conjugated 24FM in COSdel.2-155 cells. Cells were preincubated with gold-complexed 24FM as in Fig 5 and exposed to 37°C for 0, 10, 30, and 45 minutes (A, B, C, and D, respectively). In contrast to that seen in Fig 5, gold particles remained on the cell surface (arrowheads), although clusters formed within 10 minutes of exposure to 37°C. Rare intracellular particles are seen (arrow).
EM localization of gold-conjugated 24FM in COSdel.2-155 cells. Cells were preincubated with gold-complexed 24FM as in Fig 5 and exposed to 37°C for 0, 10, 30, and 45 minutes (A, B, C, and D, respectively). In contrast to that seen in Fig 5, gold particles remained on the cell surface (arrowheads), although clusters formed within 10 minutes of exposure to 37°C. Rare intracellular particles are seen (arrow).
EM localization of gold-conjugated thrombin in COSdel.238 cells. Cells were preincubated with gold-complexed thrombin as in Fig 6 and exposed to 37°C for 0, 10, and 45 minutes (A, B, and C, respectively). Gold particles remained distributed on the cell surface during this period (arrowheads), with only a rare intracellular particle found (arrow).
EM localization of gold-conjugated thrombin in COSdel.238 cells. Cells were preincubated with gold-complexed thrombin as in Fig 6 and exposed to 37°C for 0, 10, and 45 minutes (A, B, and C, respectively). Gold particles remained distributed on the cell surface during this period (arrowheads), with only a rare intracellular particle found (arrow).
EM localization of gold-conjugated thrombin in COSdel.2-155 cells. Cells were preincubated with gold-complexed thrombin as in Fig 6 and exposed to 37°C for 0, 10, and 45 minutes (A, B, and C, respectively). Gold particles remained distributed on the cell surface during this period (arrowheads), with only a rare intracellular particle found.
EM localization of gold-conjugated thrombin in COSdel.2-155 cells. Cells were preincubated with gold-complexed thrombin as in Fig 6 and exposed to 37°C for 0, 10, and 45 minutes (A, B, and C, respectively). Gold particles remained distributed on the cell surface during this period (arrowheads), with only a rare intracellular particle found.
DISCUSSION
In ongoing studies to characterize the structure-function correlations of thrombomodulin, we have recently shown that the cytoplasmic tail of the molecule is not required for efficient constitutive endocytosis via non=nclathrin-coated pits, yet it may be important both for clathrin-coated pit-mediated endocytosis and for multimerization of TM.16,17 The mechanism by which TM might be internalized without a cytoplasmic tail remained a mystery and prompted us to investigate other regions of TM. Previous studies have suggested that the nonpolar amino terminus end of TM that has lectin-like homology and a hydrophobic region may be associated with the plasma membrane surface.15 We hypothesized that this domain may, under appropriate conditions, confer information to initiate the internalization of TM.16 Consequently, we deleted parts of this region of TM to evaluate effects on the endocytic process. The visual evidence of a lack of constitutive internalization of complexes of thrombin-TM or anti-TM antibody=nTM in the COSdel.2-155 cells, both by immunofluorescence and immunoelectron microscopy, was strong evidence that the lectin-like module of TM, which contains two potential sites for N-linked glycosylation, was critical in mediating its constitutive endocytic routing from the cell surface membrane. Future similar studies with more subtle mutations will determine whether the observed findings are dependent on the lectin-like properties of this domain, its glycosylation, or, alternatively, due to tertiary structural changes in other regions of the molecule that may have been induced by the deletion of this module.
The deletant form of TM expressed by the COSdel.238 cells was initially constructed using convenient restriction enzyme sites. Not only was the entire N-terminal domain removed, but the recombinant deletant TM was also lacking the putative signal peptide and 12 residues of the first EGF-like domain. It was interesting to note that, even without a signal peptide, TM could be transported intact to the cell surface. Because N-terminal amino acid sequencing of nascent TM has not been reported, it is possible that there is no functional or cleavable signal peptide. Indeed, it has been noted that the putative signal peptide lacks those basic amino acid residues often marking each end of such sequences.6 Transmembrane proteins that lack typical N-terminal signal peptides are not uncommon and include, for example, the asialoglycoprotein receptor,27 the transferrin receptor,28 hepsin,29 and the effector protease receptor-1,30 all of which are able to insert correctly across the membrane, presumably due to their possessing a hydrophobic domain. Thus, it is not surprising that recombinant TM synthesized by COSdel.238 cells was transported to the cell surface apparently normally.
The in vitro expression of TM is regulated by both transcriptional and posttranscriptional mechanisms (for review, see Hirokawa and Aoki31 ), with considerable variability noted between cell and tissue types. For example, there is evidence that internalization of thrombin-TM does not occur in human saphenous vein endothelial cells,31-33 whereas in HUVEC, bovine aortic endothelial cells, A549 cells, and wild-type TM-transfected COS cells, endocytosis of thrombin-TM is well documented.17,31-36 There are several mechanisms by which the lectin-like module of TM may contribute to the variability in its expression between cells and/or tissues. For example, changes in N-linked glycosylation within the lectin-like region may be cell/tissue specific and/or may be altered during in vivo stresses, preventing the molecule from being internalized, and thereby serving to maintain thromboresistance. Alternatively, the release of soluble forms of TM (containing all of the extracellular domains) that occurs during inflammation and a variety of vascular disorders37 may accumulate in regions of the vasculature and saturate the binding sites of an as yet unidentified carbohydrate ligand that may otherwise interact with the intact cell surface TM lectin module and induce internalization of the molecule. This competitive effect would then result in the prevention of constitutive endocytosis of thrombin-TM complexes and complement the heat shock-induced transcriptional upregulatory effects on TM hypothesized to occur in vivo during inflammation and infection,38 possibly as a vaso-protective measure. Finally, cell/tissue-specific expression of carbohydrates or lectins that bind to TM's lectin module or glycosaminoglycans within that module, respectively, may result in differential properties of TM with respect to its ability to undergo constitutive endocytosis. This may be important in the low-flow circulation of saphenous veins in which continuous expression of cell-surface TM is important; whereas, in high-flow vascular beds, the response to injury may be critical, and suppression of cell-surface TM may be important to allow appropriate local hemostasis and eventual healing.
In addition to that of TM, the lectin domains of receptors on, eg, activated macrophages, hepatocytes, and fibroblasts have also been implicated in regulating receptor-mediated endocytosis of glycoconjugates.39-41 The mechanism(s) of initiation of these processes is also largely speculative and the focus of considerable investigation. Although our studies support a hypothesis that the lectin-like properties of TM may mediate its internalization, it is equally possible that signaling via this region in response to a stimulus (such as following the association of TM with thrombin and/or protein C) and unrelated to its lectin-homology leads to transmission of information required for initiation of the steps necessary for endocytosis to occur.
Beyond thrombin's action to cleave fibrinogen to generate a fibrin clot and to activate protein C when associated with one of its major receptors, the serine protease has several profound biologic effects on a variety of cells and organ systems. Thrombin activates platelets, inducing phosphoinositide hydrolysis, eicosanoid formation, and protein phosphorylation, leading to adhesion, aggregation, and secretion. Vascular endothelial cell expression and/or release of nitric oxide,42 platelet-activating factor,43 tissue-type plasminogen activator, plasminogen activator inhibitor-1, and urokinase-type plasminogen activator have been reported to be augmented by thrombin,44 whereas urokinase receptor (u-PAR) may be decreased in endothelial cells45 or increased in smooth muscle cells.46 Furthermore, adhesion molecules may be upregulated by thrombin, promoting neutrophil adhesion, and thus contribute to a proinflammatory response. Thrombin also has mitogenic activity on a variety of cells, including lymphocytes, fibroblasts, macrophages, mesangial cells, neuroblastoma cells, and osteoblasts.44,47-50 Finally, in the inflamed joint, thrombin has recently been implicated in the pathology of arthritis by inducing proteoglycan release in the degradation of cartilage.51
Many of the above-mentioned biologic actions of thrombin are mediated by the thrombin receptor, a G-protein-linked 7-transmembrane protein.52,53 Current evidence suggests that thrombin binds to and cleaves the N-terminus of the thrombin receptor, exposing a so-called tethered ligand that activates the receptor, after which it may be internalized and recycled or degraded. The fate of thrombin is unknown, but it appears that each thrombin molecule is restricted to activating a limited number of thrombin receptors. This led to the conclusion that, for thrombin to become inaccessible to other thrombin receptors, it must (1) be similarly internalized via the activated thrombin receptor, (2) become neutralized by thrombin inhibitors, or (3) associate with other thrombin receptors, such as TM.54 The recent in vivo thrombin receptor gene-inactivation study55 supports the existence of at least one other thrombin receptor. There is currently no evidence to indicate that thrombin is internalized via the thrombin receptor and, although serine protease inhibitors such as anti-thrombin III and protease nexin I may neutralize thrombin, it is likely that TM plays a critical role in mopping up the excess thrombin, rendering it inaccessible to further activation of the thrombin receptor.
The extent of the potential importance of TM in regulating thrombin's diverse actions is illustrated by the fact that the expression of TM is not restricted to the vascular endothelium. Rather, it is also synthesized by vascular smooth muscle cells, synovial lining cells, osteoblasts, syncytiotrophoblasts, keratinocytes, cells of the central nervous system, and several hematopoietic cells.21,56-59 The developmental expression of TM in both vascular tissues and at sites of cell-cell contact in epithelial cells,60,61 as well as the embryonic lethality of TM knockout mice without evidence of thrombosis,62 further supports the possibility of alternative, nonanticoagulant functions for the protein. In this report, we have shown that the lectin-like module within the N-terminal domain of TM is critical for directing the molecule intracellularly from the membrane surface. Although the in vivo significance of this process is currently unknown, it is worth noting that lectins and their corresponding carbohydrate ligands may contribute to a variety of physiologically important processes including tumor cell recognition, cell adhesion, cell activation, signal transduction, differentiation, and apoptosis.63 In vivo studies using targeted deletion strategies in mice will eventually provide us with further insights into the structure-function correlates of TM not only in adult pathophysiology, but also in normal development.
Supported by The Heart and Stroke Foundation of Ontario, Canada, and Miles/Canadian Red Cross Research Fund.
Address reprint requests to Edward M. Conway, MD, The Toronto Hospital, 585 University Ave, Mulock Larkin Building 2-031a, Toronto, Ontario, Canada M5G 2C4.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal