Abstract
Patients with severe granulocytopenia are more susceptible to severe infections and sepsis. Proinflammatory cytokines such as tumor necrosis factor-α (TNF ), interleukin-1α (IL-1α), and IL-1β play an important role in the pathophysiology of sepsis. The profile of these proinflammatory cytokines after lipopolysccharide (LPS) challenge in cyclophosphamide-induced neutropenic mice was assessed, and possible mechanisms responsible for the modified cytokine production were studied. After LPS, both circulating concentrations of TNF and IL-1α in neutropenic mice were 50% to 200% higher than those of controls, whereas IL-1β concentrations were not modified. The kinetics of cytokine production were similar in neutropenic and control animals. The susceptibility of neutropenic mice to an LPS challenge was increased. The observed overproduction of TNF and IL-1α was not due to a direct effect of cyclophosphamide treatment. Because circulating concentrations of uric acid were increased in the neutropenic mice, the effect of hypouricemic treatment with allopurinol and sodium bicarbonate was investigated; such treatment in neutropenic mice challenged with LPS was followed by an improved survival and a reduced proinflammatory cytokine production towards the concentrations in control mice. Hyperuricemia induced by repeated administrations of uric acid in normal mice led to an increased TNF production after LPS. In conclusion, neutropenic mice respond with enhanced cytokine production and increased susceptibility to an LPS challenge, and hyperuricemia probably plays an important role in this phenomenon.
PATIENTS WITH granulocytopenia may develop severe and potentially lethal infections, with the relation between granulocytopenia and the incidence of infection being well documented.1 Interleukin-1α (IL-1α), IL-1β, and tumor necrosis factor-α (TNF ) are proinflammatory cytokines that play a central role in the pathophysiology of sepsis.2 Circulating concentrations of TNF and IL-1 can be found during sepsis, and clinical studies have shown a correlation between the concentration of these cytokines and the severity of disease and a poor outcome.3,4 Treatment with anti-TNF antibodies protects against septic shock in experimental gram-negative bacteremia.5,6 The administration of either of the naturally occuring antagonists of IL-1 and TNF, ie, IL-1 receptor antagonist and soluble TNF receptors, has been shown to be effective in lethal endotoxemia and in models of experimental infection.7-9 Despite their importance in sepsis and the higher incidence of severe infections in neutropenic hosts, little is known about the proinflammatory cytokine profile during neutropenia.
In a recent report, increased TNFα and IL-1β serum concentrations in neutropenic children with fever have been described.10 Patients with granulocytopenia reportedly had increased IL-6 serum levels preceding febrile episodes.11 Moreover, in animal models, cytokine concentrations during experimental Candida albicans infection were higher in neutropenic mice when compared with control animals,12,13 and TNF synthesis was increased in neutropenic animals after endotoxin challenge in vivo.14 These data indicate that neutropenic hosts may produce more proinflammatory cytokines when an infection occurs.
The aim of the present study was to investigate the increased cytokine production in mice rendered granulocytopenic by cyclophophamide and challenged with lipopolysaccharide (LPS) and to show the possible mechanisms responsible for this phenomenon. We investigated whether the cyclophosphamide treatment could have directly influenced the cytokine concentrations. Neutropenia induced by chemotherapy is often followed by hyperuricemia. Because it is known that urate crystals are able to stimulate the synthesis of proinflammatory cytokines,15 16 we tested the hypothesis that the increased uric acid concentrations are involved in the increased cytokine synthesis in neutropenic mice.
MATERIALS AND METHODS
Animals.Female Swiss mice who were 6 to 8 weeks old and weighed 20 to 25 g were obtained from a local colony. The animals were fed standard laboratory chow (Hope Farms, Woerden, The Netherlands) and housed under specific pathogen-free conditions. The experiments were approved by the ethical committee on animal experiments of the Catholic University Nijmegen (Nijmegen, The Netherlands).
Treatment of mice.Cyclophosphamide was obtained from Bristol-Myers Squibb B.V. (Weesp, The Netherlands), allopurinol from Multipharma B.V. (Weesp, The Netherlands), and catalase from Sigma Chemical Co (St Louis, MO). Uric acid and sodium bicarbonate were obtained from Boom B.V. (Meppel, The Netherlands). Neutropenia was induced in the animals by two subcutaneous injections of cyclophosphamide of 150 mg/kg on day −4 (before LPS challenge) and 100 mg/kg on day −1. As described before, in this model of neutropenia, the neutrophil counts are very low (105/mL).17 Also, the number of lymphocytes and monocytes are reduced, but not as strongly as the neutrophils. In some experiments, mice received allopurinol by gastric instillation (GI) at two daily doses of 40 mg/kg or sodium bicarbonate by GI at 100 mg/kg twice daily (BID), starting on day −4. Catalase (32,000 U/mouse) was administered intraperitoneally 1 hour before LPS challenge. Uric acid was administered by GI either as a single dose of 300 mg/kg 1 hour before LPS or as repeated administration of 300 mg/kg BID from day −4 to day −1. In all experiments, the control groups received 0.9% NaCl as placebo by the same route.
Endotoxemia model.LPS (Escherichia coli serotype O55:B5) was obtained from Sigma. Groups of normal and neutropenic mice were injected intraperitoneally with LPS (10 μg/mouse). Ninety minutes and 4 hours after challenge with LPS, 5 animals from each group were anesthetized with ether and bled from the retroorbital plexus for measurement of circulating cytokine concentrations. Uric acid concentrations were also assessed. In a separate experiment, survival was assessed daily for 7 days in groups of 10 normal and neutropenic mice injected with LPS at 1 mg/mouse. In additional experiments, normal and neutropenic mice were treated with sodium bicarbonate as described above and survival was assessed after LPS challenge.
Circulating cytokines.The kinetics of TNFα production during endotoxemia were studied in normal and neutropenic mice challenged with LPS (10 μg/mouse). Subgroups of 5 mice were killed, and plasma concentrations of TNF were determined before and 30 minutes, 90 minutes, 4 hours, and 8 hours after the LPS challenge.
In separate experiments, the direct influence of cyclophosphamide on the cytokine production was studied by injecting the animals with a single dose of cyclophosphamide (150 mg/kg) 1 hour before LPS (10 μg/mouse). Also, the influence of allopurinol, catalase, sodium bicarbonate, and uric acid on cytokine production in groups of 5 normal and neutropenic mice was studied by treating the mice with these drugs (as described in detail above) before the LPS challenge (10 μg/mouse).
Ex vivo cytokine production.Resident peritoneal macrophages of normal and neutropenic mice were harvested by rinsing the peritoneal cavity aseptically with cold phosphate-buffered saline containing 0.38% (wt/vol) sodium citrate. After centrifugation for 10 minutes at 1,800 cpm and 4°C, cells were resuspended in RPMI 1640 (Dutch modification; Flow Laboratories, Irvine, UK) containing 1 mmol/L pyruvate, 2 mmol/L L-glutamine, and 100 μg/mL of gentamicin. A total of 105 cells/well were cultured in 96-well microtiter plates (Costar Corp, Cambridge, MA) in culture medium (final volume, 200 μL) at 37°C. After 2 hours, the supernatant and the nonadherent cells were discarded and 200 μL medium with or without LPS at a final concentration of 1 μg/mL was added. The supernatants were collected after 24 hours of incubation at 37°C and stored at −70°C until assay.
Cytokine measurements.TNFα, IL-1α, and IL-1β concentrations were determined using specific radioimmunoassays developed in our laboratory, as described in detail earlier.18
Statistical analysis.Differences in concentrations of cytokines were analyzed using the Mann-Whitney test and by ANOVA where appropriate. Survival data were analyzed using the Kaplan-Meyer log rank test. Differences were considered significant at P < .05. All experiments were performed at least twice and used at least 5 mice/group/time point for the plasma cytokine concentrations and 10 mice/group for survival experiments. Data are presented as means ± standard deviation.
RESULTS
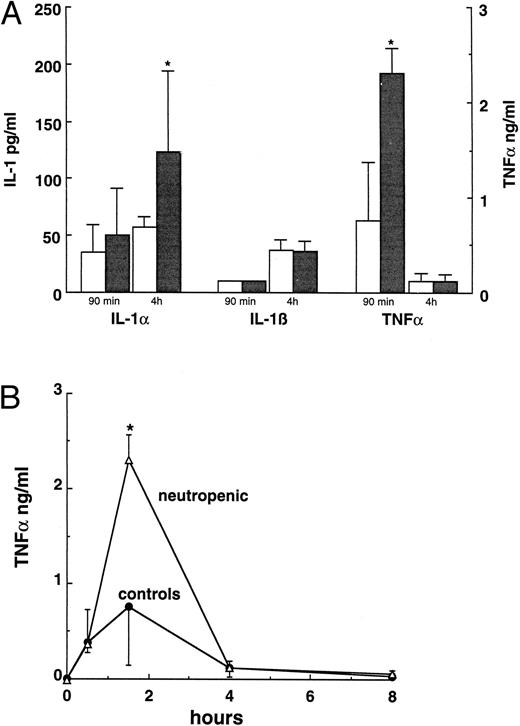
Cytokine production in neutropenic mice.Ninety minutes after challenge with 10 μg LPS, TNFα plasma concentrations in neutropenic mice were significantly higher than those of control mice (P < .001 by ANOVA in 5 separate experiments with similar results). A representative experiment is shown in Fig 1A. The IL-1α concentration was higher 4 hours after LPS (P < .05, Fig 1A). IL-1β concentrations were below detection limit at 90 minutes after the LPS challenge and did not differ between normal and neutropenic animals 4 hours after LPS (Fig 1A). The kinetics of the TNF in response upon LPS challenge was also studied. No differences were found between the kinetics of TNF production in normal and neutropenic mice (Fig 1B). In both groups, detectable levels of TNF appeared in the circulation 30 minutes after LPS, with a peak at 90 minutes and a decrease thereafter.
(A) Cytokine plasma concentrations 90 minutes and 4 hours after an LPS challenge (10 μg/mouse) in normal (□) and neutropenic (▪) mice. (B) Kinetics of TNF production in normal and neutropenic mice challenged with 10 μg LPS. Data represent the mean ± SD. * P < .05.
(A) Cytokine plasma concentrations 90 minutes and 4 hours after an LPS challenge (10 μg/mouse) in normal (□) and neutropenic (▪) mice. (B) Kinetics of TNF production in normal and neutropenic mice challenged with 10 μg LPS. Data represent the mean ± SD. * P < .05.
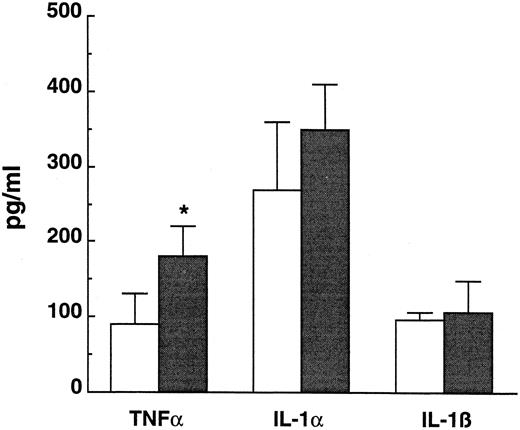
Ex vivo cytokine production.To investigate whether the tissue macrophages of neutropenic mice are able to produce more cytokines after an LPS challenge than normal macrophages, peritoneal macrophages were collected, adjusted to 105 cells/well, and stimulated with LPS (1 μg/mL) for 24 hours at 37°C. Macrophages from neutropenic animals secreted significantly more TNFα than those of control animals (Fig 2). IL-1α production by macrophages of neutropenic mice was slighltly, although not significantly, higher. No difference in IL-1β production was observed between the two groups (Fig 2).
Ex vivo cytokine production capacity of peritoneal macrophages collected from normal (□) and neutropenic (▪) mice stimulated with LPS (1 μg/mL) in vitro for 24 hours at 37°C. Data represent the mean ± SD. * P < .05.
Ex vivo cytokine production capacity of peritoneal macrophages collected from normal (□) and neutropenic (▪) mice stimulated with LPS (1 μg/mL) in vitro for 24 hours at 37°C. Data represent the mean ± SD. * P < .05.
Effect of cyclophosphamide on cytokine production.To exclude a direct effect of cyclophosphamide on cytokine production, mice were injected with cyclophosphamide (150 mg/kg) 1 hour before the LPS challenge. Ninety minutes after LPS challenge, the plasma concentration of TNF in the cyclophosphamide-treated mice was significantly lower than in placebo-treated mice (0.57 ± 0.09 v 1.78 ± 0.47 ng/mL, P < .05). No difference in IL-1α concentrations was detected (67 ± 44 v 82 ± 42 pg/mL, P > .05), whereas IL-1β concentrations were below the detection limit in both groups. These results indicate that the increased cytokine production in neutropenic mice is not due to a direct effect of cyclophosphamide.
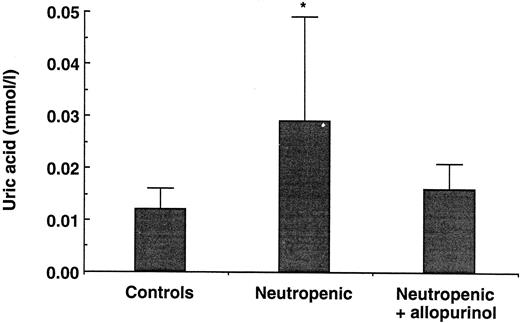
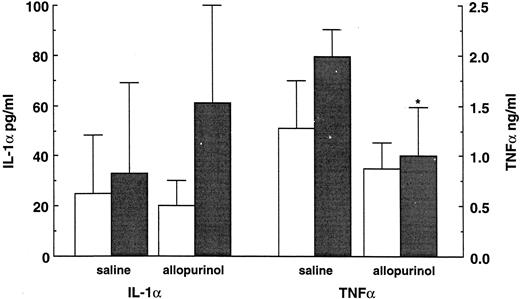
Modulation of uric acid concentrations.Normal mice and mice rendered neutropenic by cyclophosphamide were pretreated with allopurinol, an inhibitor of xanthine-oxidase known to decrease uric acid concentration. LPS challenge and cytokine measurements were performed as described above, and uric acid concentrations were determined in all mice. Uric acid concentrations were approximately threefold higher in neutropenic mice (0.029 ± 0.020 mmol/L, P < .02) than in controls (0.012 ± 0.004 mmol/L) and were restored towards normal values by treatment with allopurinol (0.016 ± 0.005 mmol/L; Fig 3). Allopurinol treatment was also able to significantly decrease the TNF concentrations in the neutropenic mice (P < .04; Fig 4). In normal mice treated with allopurinol, only a slight decrease in TNF concentration was observed. However, allopurinol did not reduce the circulating IL-1α concentration (Fig 4).
Uric acid concentrations in normal and neutropenic mice and in neutropenic mice treated with allopurinol (40 mg BID for 4 days). Data represent the mean ± SD. * P < .05.
Uric acid concentrations in normal and neutropenic mice and in neutropenic mice treated with allopurinol (40 mg BID for 4 days). Data represent the mean ± SD. * P < .05.
The influence of allopurinol treatment (40 mg/kg BID for 4 days) on cytokine production 90 minutes after an LPS administration (10 μg/mouse) in normal (□) and neutropenic (▪) mice. Data represent the mean ± SD. * P < .05.
The influence of allopurinol treatment (40 mg/kg BID for 4 days) on cytokine production 90 minutes after an LPS administration (10 μg/mouse) in normal (□) and neutropenic (▪) mice. Data represent the mean ± SD. * P < .05.
Because allopurinol is able not only to decrease the uric acid concentration, but also has antioxidant activities by decreasing the reactive oxigen intermediates (ROI), we investigated which of these two effects is involved in the allopurinol-induced modulation of cytokine synthesis. Therefore, subgroups of normal and neutropenic animals were pretreated with catalase or sodium bicarbonate. Catalase has antioxidant activities by scavenging ROI, with no effect on uric acid concentration. On the other hand, by alkalizing the urine and thus increasing the solubility of uric acid in urine, sodium bicarbonate was able to decrease the uric acid concentration in neutropenic animals. In this experiment, bicarbonate-treated animals had an uric acid concentration of 0.030 ± 0.008 mmol/L, versus 0.055 ± 0.011 mmol/L in the placebo group (P < .05). As shown in Table 1, catalase was able to decrease TNF concentration both in normal and neutropenic animals, whereas sodium bicarbonate decreased TNF significantly only in neutropenic animals. However, catalase was not able to influence the elevated IL-1α concentration in neutropenic mice and even increased IL-1α in normal animals. Sodium bicarbonate was able to restore the IL-1α concentration to normal in neutropenic mice, while having no effect in nonneutropenic animals (Table 1). These data suggest that the increased uric acid concentrations in neutropenic mice are responsible, at least in part, for the observed overproduction of proinflammatory cytokines after an LPS challenge.
Circulating TNF and IL-1α Concentrations 90 Minutes After an LPS Challenge (10 μg/Mouse) in Normal and Neutropenic Mice Treated With Saline (Control Group), Catalase (32,000 U/Mouse, 1 hour Before LPS), or Sodium Bicarbonate (100 mg/kg BID Starting on Day −4)
| . | TNFα (ng/mL) . | IL-1α (pg/mL) . |
|---|---|---|
| Nonneutropenic | ||
| Control | 2.1 ± 0.7 | 78 ± 13 |
| Catalase | 0.6 ± 0.6* | 242 ± 105* |
| Bicarbonate | 1.4 ± 0.9 | 58 ± 30 |
| Neutropenic | ||
| Control | 3.5 ± 0.8 | 114 ± 48 |
| Catalase | 0.15 ± 0.1† | 150 ± 59 |
| Bicarbonate | 0.7 ± 0.4* | 72 ± 19* |
| . | TNFα (ng/mL) . | IL-1α (pg/mL) . |
|---|---|---|
| Nonneutropenic | ||
| Control | 2.1 ± 0.7 | 78 ± 13 |
| Catalase | 0.6 ± 0.6* | 242 ± 105* |
| Bicarbonate | 1.4 ± 0.9 | 58 ± 30 |
| Neutropenic | ||
| Control | 3.5 ± 0.8 | 114 ± 48 |
| Catalase | 0.15 ± 0.1† | 150 ± 59 |
| Bicarbonate | 0.7 ± 0.4* | 72 ± 19* |
P < .05 v control.
P < .01 v control.
Hyperuricemia in normal animals.Hyperuricemia was induced in normal animals by the administration of uric acid (300 mg/kg BID) for 4 days. The uric acid concentration increased in treated animals from 0.010 ± 0.004 mmol/L to 0.018 ± 0.005 mmol/L (P < .03). A subgroup of mice received a single dose of uric acid to discern whether the potential effect of uric acid is rapid or long-lasting hyperuricemia is necessary for the activation of tissue macrophages. Whereas the single administration of uric acid did not influence the synthesis of TNF 90 minutes after LPS challenge (1.1 ± 0.4 ng/mL v 1.5 ± 0.5 ng/mL, P > .05), repeated administration of uric acid led to a significant increase in the TNF production (2.3 ± 0.9 ng/mL, P < .02), arguing for an influence of a sustained hyperuricemia on cytokine production when mice are challenged with LPS.
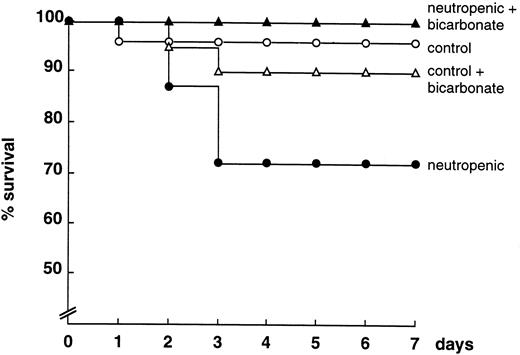
LPS-induced lethality.Because both TNF and IL-1 are central mediators of lethal endotoxemia, the effect of the increased cytokine response in neutropenic mice on lethality induced by LPS was studied. Normal and neutropenic mice were challenged intravenously with 1 mg of LPS, and mortality was assessed daily for 7 days. The neutropenic mice were more susceptible to endotoxemia when compared with normal mice (Fig 5). Treatment of mice with bicarbonate did not influence the outcome in control mice, whereas improving survival was seen in neutropenic animals, ie, 100% in bicarbonate-treated neutropenic mice versus 72% in nontreated animals (P < .05; Fig 5). The data represent two pooled experiments with 10 mice/group. The results were similar in both experiments.
Survival of normal and neutropenic mice treated with saline or bicarbonate (100 mg/kg BID starting on day −4) after LPS challenge (1 mg/mouse). The data represent the pooled results of two experiments with at least 10 animals/group.
Survival of normal and neutropenic mice treated with saline or bicarbonate (100 mg/kg BID starting on day −4) after LPS challenge (1 mg/mouse). The data represent the pooled results of two experiments with at least 10 animals/group.
DISCUSSION
In this study, we have shown that neutropenia leads to an increased cytokine production upon LPS challenge in mice. This enhanced production of cytokines is accompanied by an increased susceptibility of neutropenic mice to a lethal challenge with LPS. Neutropenic animals have higher plasma uric acid concentrations when compared with those of controls, and this hyperuricemia is responsible, at least in part, for the increased cytokine production.
Few studies have been performed to assess the production of cytokines during neutropenia. Two studies have reported increased cytokine production in neutropenic mice infected with C albicans.12 13 Another recent study reported greater cytokine production after an LPS challenge in neutropenic mice.14 This finding is in agreement with our observation that neutropenic mice produce greater amounts of proinflammatory cytokines after stimulation with LPS. Despite the increased peak of cytokine concentration, we observed similar kinetics of cytokine synthesis in normal and neutropenic mice, data that are also sustained by the study of Steinshamn et al.14
When macrophages from normal and neutropenic mice were stimulated ex vivo with LPS, the macrophages from neutropenic animals synthesized more cytokines in response to LPS. Xun et al19 observed circulating concentrations of cytokines in mice treated with daily injections of busulfan and cyclophosphamide, even without any stimulation of the animals. All of these data argue that the enhanced cytokine production in neutropenic animals is the result of an increased cytokine production by tissue macrophages that may already be in a state of activation at the time of the LPS challenge.
Little is known about the mechanisms responsible for the increased cytokine production during neutropenia. The observation that one single dose of cyclophosphamide shortly before the LPS challenge decreases the TNF concentration, while the IL-1 production is unmodified, indicates that cyclophosphamide is not directly responsible for the increased cytokine production observed during neutropenia. This finding is in agreement with the earlier observation that cyclophosphamide reduces the capacity of murine peritoneal macrophages to produce TNF and IL-1.20 In addition, similarly increased cytokine concentrations were found in mice rendered granulocytopenic after sublethal γ-irradiation (data not shown). This led us to the hypothesis that a product of leukocyte damage (eg, uric acid) may be responsible for the increased cytokine production during neutropenia.
Uric acid concentrations were significantly higher in neutropenic mice when compared with controls. It has been shown that uric acid crystals are able to stimulate cytokine production,15,16 although no data have been reported on the ability of soluble uric acid to influence cytokine production. In the present study, hypouricemic agents led to a decrease of both the levels of uric acid and the cytokine concentration in the circulation of neutropenic mice. Allopurinol is also able to influence the TNF production by inhibiting ROI,21 and this may explain the inhibition of TNFα production observed in normal mice treated with allopurinol. Therefore, the influence of ROI on TNF and IL-1α synthesis was assessed. Catalase, which decreases ROI21 and has no effects on uric acid concentrations, decreased TNF concentrations, indicating that indeed part of the effect of allopurinol may be due to ROI inhibition. In contrast, catalase was not able to inhibit the production of IL-1α. On the other hand, bicarbonate was able to inhibit both TNF and IL-1α production, by decreasing the concentration of uric acid without affecting ROI. The biologic significance of these observations is further stressed by the beneficial effect of bicarbonate on survival of neutropenic animals challenged with LPS. These data argue that hyperuricemia plays an important role in the increased cytokine production during neutropenia in mice.
This hypothesis was further tested by inducing hyperuricemia in nonneutropenic mice. An immediate increase in circulating uric acid concentration by a single administration of uric acid did not increase the cytokine production. However, when a sustained hyperuricemia was induced for 4 days, the TNF production after LPS was higher when compared with that of the placebo group. This finding suggests that the increased uric acid concentrations may induce an activation of tissue macrophages, leading to increased production of cytokines upon stimulation.
In contrast to humans, the final product of the purine metabolism in mice is not uric acid but allantoin, due to the presence of urate oxidase. For this reason, mice have much lower circulating concentrations of uric acid than do humans. It is to be expected that, in the present study, the increased concentrations of uric acid in the neutropenic mice and the mice that received oral uric acid were accompanied by a parallel increase in the concentration of allantoin. Thus, the possibility that the higher allantoin concentrations, rather than the hyperuricemia, are responsible for the increased cytokine production in these experiments cannot be excluded.
There are also other mechanisms that could have influenced the cytokine production. Steinshamn et al14 argued for a role of reduced release of soluble TNF receptor p75 in the enhanced TNF activity after LPS during neutropenia. However, although this mechanism may occur in vivo, it does not explain our observation that the cytokine production capacity of macrophages from neutropenic animals is increased when stimulated with LPS in vitro. It has also been shown that TNF and IL-1 can act as important hematopoietic regulatory cytokines,22 and it cannot be excluded that neutropenia leads to increased production of these cytokines through a direct feedback mechanism. Neutropenia may also activate other hematopoietic regulatory cytokines such as granulocyte-macrophage colony-stimulating factor,22,23 which may stimulate the production of proinflammatory cytokines.24
In conclusion, several mechanisms, including hyperuricemia, may lead to a higher production of proinflammatory cytokines during neutropenia. The results of our study and the other experimental studies, together with the observed increased production of proinflammatory cytokines in neutropenic patients,10 11 suggest that this overproduction of cytokines could contribute to the poor prognosis of infection in the neutropenic host.
ACKNOWLEDGMENT
The authors thank I. Verschueren for performing the cytokine determinations.
The results of this study were presented in part at the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 1995, San Francisco, CA.
Address reprint requests to Jos W.M. Van der Meer, MD, Department of Medicine (541), University Hospital Nijmegen, PO Box 9101, 6500 HB Nijmegen, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal