Abstract
The role of interleukin-12 (IL-12) in Th1 cell differentiation is well established. The heterodimer p70, composed of a p40 and a p35 chain, is the biologically active form. IL-12 production by human monocytes is enhanced by interferon-γ (IFN-γ) and inhibited by IL-10 and prostaglandin E2 (PGE2 ). Peripheral blood mononuclear cells from human immunodeficiency virus (HIV)-infected individuals reportedly have impaired IL-12 p40 and p70 production on stimulation with Staphylococcus aureus Cowan I (SAC) in vitro. Both PGE2 and IL-10 previously were proposed to be instrumental in this defect in IL-12 production. Here, we studied IL-12 p40 and p70 production in relation to IL-10 and PGE2 production in whole blood cultures from HIV-infected individuals. On stimulation with lipopolysaccharide, IL-12 production was normal. However, on stimulation with SAC, IL-12 p40 and p70 production was decreased in HIV-infected individuals and correlated significantly with decreased peripheral blood CD4+ T-cell number and T-cell reactivity to CD3 monoclonal antibody in vitro. However, IL-10 and PGE2 production in cultures from HIV-infected individuals was normal and did not relate to IL-12 production. In conclusion, IL-12 production by cells from HIV-infected individuals is impaired under certain conditions in vitro and this decrease is independent of IL-10 or PGE2 production.
HUMAN IMMUNODEFICIENCY virus-1 (HIV-1) infection is characterized by functional defects of T cells in vitro, which are observed in early infection, even before the loss of peripheral blood CD4+ T cells. The fact that both CD4+ and CD8+ T cells of HIV-infected individuals are disturbed in their function1,2 in a stage of infection in which the number of infected T cells is low3 asks for a systemic explanation for the observed T-cell dysfunction. One powerful mechanism of immune dysregulation is a disturbance of cytokine networks induced by persistent HIV infection. When T-cell dysfunction in HIV-infected individuals is studied in more detail, it is clear that functional properties ascribed to Th1 cells are specifically disturbed. Proliferation of T cells in response to ligation of the T-cell receptor CD3 complex is impaired1,4-6 and interleukin-2 (IL-2) production is decreased.2,7 Furthermore, antigen-induced interferon-γ (IFN-γ) production8 and delayed-type hypersensitivity (DTH) reactions are decreased.9,10 Shearer et al11 have been the first to propose a shift to Th2 cytokine patterns to explain T-cell dysfunction and failure of immune control of HIV-1 replication. Indeed, on generation of T-cell clones an increase in Th0 clones can be observed in HIV-infected individuals.12 13 The general conclusion from these data might be that there is a decrease in Th1-type cytokine production causing a disturbance of the balance between Th1 and Th2 responses, leading to a Th0-like cytokine profile.
In addition to the Th cytokines themselves, cytokines that are responsible for polarization of Th-type responses, are of interest. Disturbance of the production of these cytokines might provide a systemic explanation for the changes in the cytokine balance on HIV infection. IL-12 plays a critical role in Th1 cell differentiation.14 It is a cytokine consisting of two chains, p35 and p40, that is only biologically active in the heterodimeric p70 form.15 The single p40 chain is secreted in excess over p70, but no biological function is known for this protein. Chehimi et al16 have reported that peripheral blood mononuclear cells (PBMCs) from HIV-infected individuals are impaired in IL-12 p40 and p70 production on stimulation with Staphylococcus aureus antigen. This would be suggestive for an underlying failure to produce the required amount of IL-12 to mount proper Th1 responses. So far, there have been few other reports supporting the data of Chehimi et al, and we here set out to study these findings in more detail.
In recent years, several possible regulators of IL-12 production have been identified. IL-12 production is initiated by pathogens in antigen-presenting cells and can then be further enhanced by IFN-γ, the production of which is induced by IL-12 itself in T cells and natural killer cells.17 Inhibition of IL-12 production can be achieved by IL-10,18 which is also induced by IL-12 itself in T cells, possibly as a negative feedback mechanism.19 Although Chehimi et al16 suggested that increased IL-10 production does not play a role in the decreased IL-12 production in HIV infection, others have argued that increased IL-10 production does play a key role in the Th1 deficiency in HIV infection.20 Next to IL-10, prostaglandin E2 (PGE2 ) was shown to be a potent inhibitor of IL-12 production in an IL-10–independent fashion and was proposed to play a role in decreased IL-12 production in HIV infection.21
Here, we studied IL-12 p40 and p70 production in vitro from HIV-infected individuals. To avoid preactivation of monocytes, a whole blood culture system was used, which allows for rapid analysis of many samples.21 Furthermore, we quantified IL-10 and PGE2 in the same samples, to study whether they might be responsible for altered IL-12 production in HIV infection.
MATERIALS AND METHODS
Study population.PB from HIV-infected individuals enrolled in the Amsterdam cohort study on HIV infection in homosexual men was used. From all participants, absolute lymphocyte counts and T-cell subset analyses were routinely determined by fluorescence-activated cell sorting analysis using standard procedures. As controls, PBMC from non-HIV–infected healthy cohort participants was used. These controls belonged to the same risk-group as the HIV-infected cohort participants.
Cell cultures.Blood obtained by venipuncture in the morning in sodium heparin-containing sterile blood collecting tubes (VT-100 H tubes; Venoject, Terumo Europe N.V., Leuven, Belgium) was 1:10 diluted in Iscove's modified Dulbecco's medium containing 0.1% fetal calf serum, 15 IU/mL heparin (Leo Pharmaceutical Products, Weesp, The Netherlands), and antibiotics. All media were ultrafiltrated by means of a hollow fiber dialyzer (Hemoflow F5; Fresenius A.G., Bad Homburg, Germany). The same day, diluted whole blood was cultured in triplicate cultures in 96-well culture plates containing 200 μL/well.
Cultures were either stimulated or not stimulated with Neisseria meningitides-derived lipopolysaccharide (LPS; kind gift from Dr J. Poolman, RIVM, The Netherlands) or S aureus Cowan strain (SAC; Pansorbin; Calbiochem-Behring Corp, La Jolla, CA) at various concentrations as indicated in the figures. Supernatants were harvested after 20 hours of culture and stored at −70°C until further analysis.
Cytokine assays.IL-12 p40 production was determined in an enzyme-linked immunosorbent assay (ELISA) as previously described.21 IL-12 p70 production was determined in an ELISA as described,22 identical to the IL-12 p40 ELISA using p70-specific monoclonal antibody (MoAb) 20C2 as a coating antibody (kindly provided by Dr M. Gately, Hoffmann La Roche, Nutley, NJ). The IL-6 ELISA was performed as described21 using a modification of the procedure described by Helle et al.23 IL-10 production was determined in an ELISA following the protocol described by Abrams24 using MoAb 9D7 and 12G8, which were purchased from Pharmigen (San Diego, CA). For assessment of PGE2 production, a commercial ELISA was used following the instructions of the manufacturer (Boehringer Mannheim, Germany).
Statistical analysis.Data were statistically analyzed using SPSS software. For comparison of groups, the Mann Whitney U-test was used; correlations were tested for significance using the Spearman's rank correlation test. Differences and correlations were considered significant at P < .05.
RESULTS
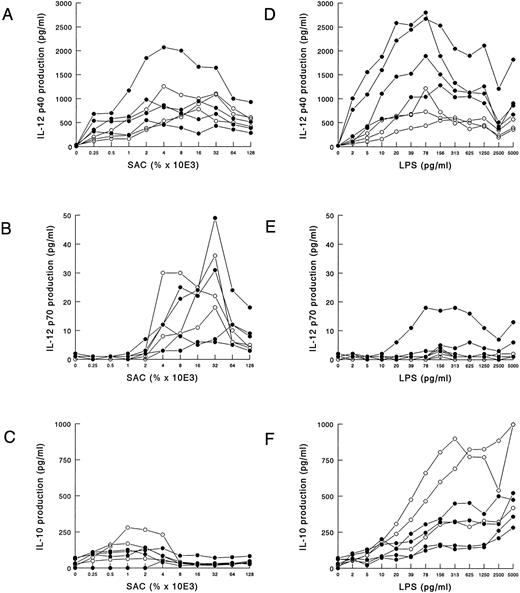
Analysis of cytokine production in whole blood cultures.A whole blood culture system was used to assess monokine production by blood cells essentially following procedures as previously described.21 The cells were not processed by density gradient centrifugation; thus, the chances that the peripheral blood monocytes were preactivated are as small as possible. The IL-12 production in PBMCs is approximately 10-fold less than in that of whole blood cultures.21 Moreover, the latter is not as laborious and requires only very little patient material. In all experiments cells were cultured for 20 hours. IL-12 production was optimal after 16 hours and stayed at the same level for up to 48 hours (data not shown). We determined the optimal concentration of SAC and LPS to induce IL-12 and IL-10 production in these cultures. Whole blood from four HIV-infected individuals and three controls was stimulated with increasing concentrations of SAC and LPS, and IL-12 p40 and p70 as well as IL-10 production in the supernatant was measured. SAC was a poor inducer of IL-10 as compared with LPS (Fig 1C), whereas IL-12 p40 and p70 were induced well (Figs 1A and B). The optimal concentration for IL-12 p70 production was 0.032%, whereas p40 production had a less restricted range for optimal induction, ranging from 0.002% to 0.016%. LPS was a potent inducer of IL-12 p40 (Fig 1D) and IL-10 (Fig 1F ), whereas it was a poor inducer of IL-12 p70 (Fig 1E). For IL-12 p40 production, the highest production was achieved with LPS concentrations ranging from 10 to 150 pg/mL, whereas IL-10 increased with higher concentrations of LPS.
SAC and LPS induced IL-12 p40 and p70 production in whole blood cultures. Whole blood (diluted 1:10) from four HIV-infected individuals (closed symbols) and three noninfected controls (open symbols) was cultured in the presence of various concentrations of SAC (A through C) or LPS (D through F ). After 20 hours of culture, supernatants were harvested, and IL-12 p40 (A and D), IL-12 p70 (B and E), and IL-10 (C and F ) concentrations were measured in ELISA. The results of one representative experiment of three are shown.
SAC and LPS induced IL-12 p40 and p70 production in whole blood cultures. Whole blood (diluted 1:10) from four HIV-infected individuals (closed symbols) and three noninfected controls (open symbols) was cultured in the presence of various concentrations of SAC (A through C) or LPS (D through F ). After 20 hours of culture, supernatants were harvested, and IL-12 p40 (A and D), IL-12 p70 (B and E), and IL-10 (C and F ) concentrations were measured in ELISA. The results of one representative experiment of three are shown.
It is important to note that the time period between the moment of blood collection and initiation of the culture negatively influenced IL-12 production. IL-12 p40 production typically decreased with about 50% over the first 8-hour time period, whereas p70 production started to decrease almost immediately and continued to decrease over time until, at 8 hours, only 10% to 30% of the production after 1 hour was left (data not shown).
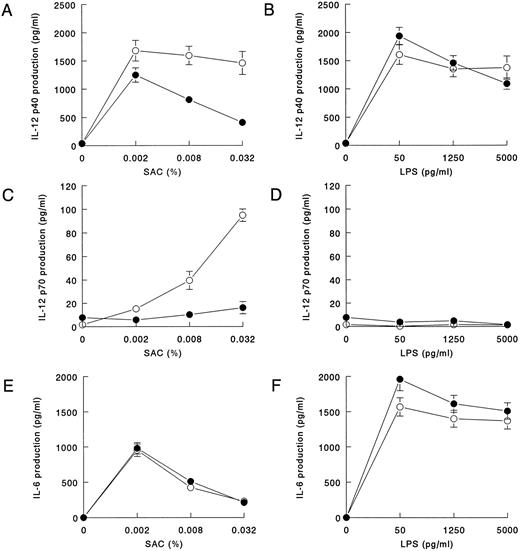
SAC-induced IL-12 production is decreased in HIV-infected individuals.For experiments with groups of HIV-infected individuals and controls, the blood was processed as soon as possible, and the average time between collection of blood and the start of the experiments was the same in HIV-infected individuals and control groups. To be able to study both suboptimal and optimal stimulation conditions for all cytokines, we used 0.002%, 0.008%, and 0.032% SAC and 50, 1,250, and 5,000 pg/mL LPS for stimulation of the cultures. IL-12 p40 production was induced to about the same extent by SAC and LPS in controls. In contrast, after stimulation with SAC we observed a significantly lower IL-12 p40 production in the HIV-infected individuals (Figs 2A and B). Under the same conditions, IL-12 p70 production, which in the control group was strongly induced at high concentrations of SAC, was significantly decreased in HIV-infected individuals (Fig 2C). LPS is a very poor inducer of IL-12 p70, and there was no difference between the two groups (Fig 2D). IL-6 was induced more potently by LPS than by SAC, but, under both conditions, IL-6 production was similar in the HIV-infected individuals and in the control group.
IL-12 p40 and p70 production in whole blood cultures of HIV-infected individuals in response to LPS is normal but, in response to SAC, is decreased. Whole blood (diluted 1:10) from HIV-infected individuals (closed symbols; n = 58) and noninfected controls (open symbols, n = 36) was cultured in the presence of various concentrations SAC (A,C, and E) or LPS (B,D, and F ). After 20 hours of culture, supernatants were harvested, and IL-12 p40 (A and B), IL-12 p70 (C and D), and IL-6 (E and F ) concentrations were measured in ELISA. Mean cytokine production is shown; bars indicate the standard error of the mean. IL-12 p40 and IL-12 p70 production in HIV-infected individuals differed significantly from that in noninfected controls at 0.002% SAC (P = .03), 0.008% SAC (P < .005), and 0.032% SAC (P < .005) and at 0.002% SAC (P < .005), 0.008% SAC (P < .005), and 0.032% SAC (P < .005), respectively, as determined in the Mann-Whitney U-test.
IL-12 p40 and p70 production in whole blood cultures of HIV-infected individuals in response to LPS is normal but, in response to SAC, is decreased. Whole blood (diluted 1:10) from HIV-infected individuals (closed symbols; n = 58) and noninfected controls (open symbols, n = 36) was cultured in the presence of various concentrations SAC (A,C, and E) or LPS (B,D, and F ). After 20 hours of culture, supernatants were harvested, and IL-12 p40 (A and B), IL-12 p70 (C and D), and IL-6 (E and F ) concentrations were measured in ELISA. Mean cytokine production is shown; bars indicate the standard error of the mean. IL-12 p40 and IL-12 p70 production in HIV-infected individuals differed significantly from that in noninfected controls at 0.002% SAC (P = .03), 0.008% SAC (P < .005), and 0.032% SAC (P < .005) and at 0.002% SAC (P < .005), 0.008% SAC (P < .005), and 0.032% SAC (P < .005), respectively, as determined in the Mann-Whitney U-test.
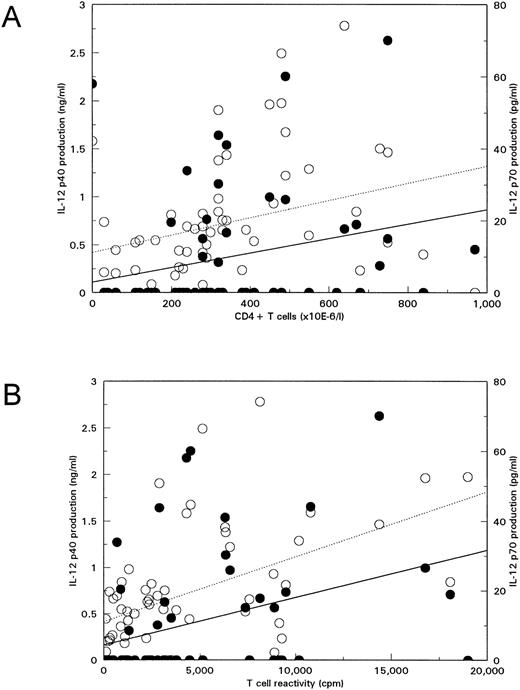
SAC-induced IL-12 production decreases with progression to disease.Thus, IL-12 p40 and p70 production on SAC stimulation at certain concentrations was decreased in HIV-infected individuals. We investigated whether this was related to the stage of infection of the patients as reflected by the number of peripheral blood CD4+ T cells, a predictive marker for progression to disease. IL-12 p40 and p70 production on SAC stimulation was positively correlated with CD4+ T-cell number, implying that IL-12 production decreases with progression to disease (Fig 3A). In addition, in the Amsterdam Cohort, T-cell reactivity in vitro to CD3 MoAb is measured routinely, which is a progression marker independent of CD4+ T-cell counts.25 There was a significant correlation of SAC-induced IL-12 production with T-cell reactivity (Fig 3B). In Fig 3, this is shown for stimulation with 0.008% SAC, but IL-12 p40 and p70 production also correlated significantly with CD4+ T-cell number and reactivity to CD3 MoAb on stimulation with 0.032% SAC (data not shown). One should note that, although correlations of CD4+ T-cell counts, T-cell reactivity, and IL-12 production are significant, the correlation coefficients are not very high.
IL-12 production in response to SAC decreases with progression to disease. IL-12 p40 (open symbols, dotted lines) and IL-12 p70 (closed symbols, solid lines) production in whole blood cultures from 55 HIV-infected individuals on 20 hours culture in the presence of 0.008% SAC was plotted against CD4+ T-cell number as PB (A) or T-cell reactivity to CD3 MoAb in vitro25 (B). Correlations were analyzed with the Spearman's rank correlation test. Correlations of CD4+ T-cell counts with IL-12 p40 and with IL-12 p70 were R = .50, P < .005, and R = .44, P < .005, respectively. Correlations of T-cell reactivity with IL-12 p40 and with IL-12 p70 were R = .50, P < .005, and R = .45, P < .005, respectively.
IL-12 production in response to SAC decreases with progression to disease. IL-12 p40 (open symbols, dotted lines) and IL-12 p70 (closed symbols, solid lines) production in whole blood cultures from 55 HIV-infected individuals on 20 hours culture in the presence of 0.008% SAC was plotted against CD4+ T-cell number as PB (A) or T-cell reactivity to CD3 MoAb in vitro25 (B). Correlations were analyzed with the Spearman's rank correlation test. Correlations of CD4+ T-cell counts with IL-12 p40 and with IL-12 p70 were R = .50, P < .005, and R = .44, P < .005, respectively. Correlations of T-cell reactivity with IL-12 p40 and with IL-12 p70 were R = .50, P < .005, and R = .45, P < .005, respectively.
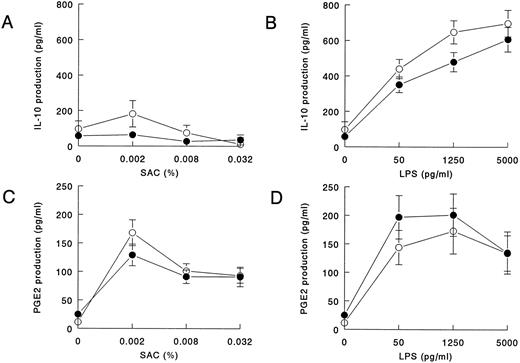
Decreased SAC-induced IL-12 production is not associated with increased IL-10 or increased PGE2 production in HIV-infected individuals.Because IL-10 and PGE2 are potential inhibitors of IL-12, we investigated the levels in the same supernatants in which we measured IL-12. IL-10 was induced by SAC to the same extent in HIV-infected individuals and controls (Fig 4A). High-level production of IL-10 was induced by LPS, which was not increased in HIV-infected individuals. At the two lowest concentrations of LPS, IL-10 release was even significantly decreased in HIV-infected individuals (Fig 4B). PGE2 induced by SAC and LPS was produced to the same extent in HIV-infected individuals and the control group (Figs 4C and D).
IL-10 and PGE2 production in whole blood cultures of HIV-infected individuals in response to LPS and SAC is normal. Whole blood (diluted 1:10) from HIV-infected individuals (closed symbols) and noninfected controls (open symbols) was cultured in the presence of various concentrations SAC (A and C) or LPS (B and D). After 20 hours of culture, supernatants were harvested, and IL-10 (A and B; HIV−, n = 35; HIV+, n = 55) and PGE2 (C and D; HIV−, n = 20; HIV+, n = 22) concentrations were measured in ELISA. Mean cytokine production is shown; bars indicate the standard error of the mean. IL-10 production in the HIV-infected group was significantly decreased from that in noninfected controls at 50 pg/mL LPS (P = .049) and 1,250 pg/mL (P = .01) as determined in the Mann-Whitney U-test.
IL-10 and PGE2 production in whole blood cultures of HIV-infected individuals in response to LPS and SAC is normal. Whole blood (diluted 1:10) from HIV-infected individuals (closed symbols) and noninfected controls (open symbols) was cultured in the presence of various concentrations SAC (A and C) or LPS (B and D). After 20 hours of culture, supernatants were harvested, and IL-10 (A and B; HIV−, n = 35; HIV+, n = 55) and PGE2 (C and D; HIV−, n = 20; HIV+, n = 22) concentrations were measured in ELISA. Mean cytokine production is shown; bars indicate the standard error of the mean. IL-10 production in the HIV-infected group was significantly decreased from that in noninfected controls at 50 pg/mL LPS (P = .049) and 1,250 pg/mL (P = .01) as determined in the Mann-Whitney U-test.
Thus, whereas SAC-induced IL-12 production is decreased in HIV-infected individuals, PGE2 and IL-10 production are normal. This suggests that there is no inverse relation between PGE2 or IL-10 production with IL-12 p40 or p70 production in HIV-infected individuals. We calculated the correlation coefficients of IL-12 p40 or p70 production with PGE2 or IL-10 production, and, indeed, no significant correlation was found.
DISCUSSION
We showed that, on stimulation with LPS in vitro, IL-12 production in whole blood cultures from HIV-infected individuals equals that of controls. However, on stimulation with SAC, IL-12 p40 and p70 production is decreased in HIV-infected individuals, and this decrease in IL-12 production significantly correlates with decreased peripheral blood CD4+ T-cell number and T-cell reactivity to CD3 MoAb in vitro. However, IL-12 production was already lower in the patients with CD4+ T-cell counts greater than 500/μL, which suggests, as has been observed before,16 that the IL-12 deficiency may be a relatively early phenomenon in HIV infection.
In addition, we found that IL-10 and PGE2 production in cultures from HIV-infected individuals is normal and does not relate to IL-12 production. The decreased IL-12 production in HIV-infected individuals was not caused by decreased numbers of monocytes present in the cultures or by a general refractoriness in monocytes, because IL-6 could be induced to normal levels in cells from HIV-infected individuals by SAC.
The decreased IL-12 p40 and p70 on SAC stimulation in whole blood cultures is in agreement with the data obtained in PBMCs with SAC stimulation by Chehimi et al.16 A similar finding was obtained after stimulation with T gondii antigen for IL-12 p40.26 However, alveolar macrophages from asymptomatically infected individuals produce increased amounts of IL-12 p70 when stimulated with SAC, and decreased IL-12 secretion was only found in patients with acquired immunodeficiency syndrome (AIDS).27 Denis et al,27 as well as Chougnet et al20 who studied IL-12 and IL-10 mRNA, proposed an important role for IL-10 in the downregulation of IL-12 production in HIV-infected individuals. However, we found that IL-10 production does not relate to IL-12 production in HIV-infected individuals, which is in agreement with Chehimi et al,16 who reported that the decrease in IL-12 production in PBMCs from HIV-infected individuals, in their hands, is not accompanied by high IL-10 production. Although IL-10 is capable of downregulating IL-12 production and anti–IL-10 will upregulate IL-12 production,18 20 this occurs to the same extent in cultures from HIV-infected individuals and controls16; therefore, there is no proof for a causal role of IL-10 in decreased IL-12 production in HIV infection in vitro or in vivo.
The potent downregulation in vitro of IL-12 production by PGE221 was also proposed as a mechanism by which IL-12 production by monocytes from HIV-infected individuals is suppressed,21,28 although, so far, no experimental data on this have been published. Our data argue against a direct role for PGE2 in downregulating IL-12 production. This might be because of the fact that optimal PGE2 levels are reached around the moment of optimal IL-12 production and are too late to inhibit IL-12 production.29 However, because IL-12 and PGE2 have opposing roles in the development of Th1 cells,29 preserved PGE2 production in the presence of decreased IL-12 production may be instrumental in shifting the cytokine balance away from Th1 cell development in HIV-infected individuals. Furthermore, increased PGE2 concentrations have been reported in serum from AIDS patients,30 which might imply a role for PGE2 in downregulating IL-12 production in vivo.
Although SAC and LPS are capable of inducing IL-12 p40 production to the same extent, SAC is more potent in inducing IL-12 p70 production in this system. This is probably related to the finding that, in whole blood cultures stimulated with LPS, IL-10 is induced up to seven times as efficiently as in cultures stimulated with SAC. Endogenous IL-10 might be responsible for lower IL-12 p70 production in these cultures. Indeed, the addition of neutralizing IL-10 MoAb to LPS-stimulated cultures enhances IL-12 p70 production, whereas no effect of neutralizing IL-10 on p70 production was observed in SAC-stimulated cultures (van der Pouw Kraan et al, unpublished data).
Although SAC and LPS are equally efficient in inducing IL-12 p40 production, p40 production was only decreased in whole blood cultures from HIV-infected individuals stimulated with SAC. This suggests that SAC and LPS induce IL-12 production via a different mechanism, and only the pathway used by SAC is affected in HIV-infected individuals. LPS exclusively stimulates monocytes via the CD14 molecule. The mechanism by which SAC stimulates monocytes is not exactly known; however, because it is a crude bacterium preparation, it may well be possible that it also contains antigens that are able to stimulate T cells.31 If so, IL-12 production in SAC-stimulated cultures might be more dependent on T-cell–derived factors such as IFN-γ17 or CD40L expression32,33 than that in LPS-stimulated cultures would be. T cells of HIV-infected individuals are impaired in IFN-γ production8 and CD40L expression (Brugnoni et al34 and Wolthers et al, manuscript submitted). Thus, the possibility that decreased IL-12 production in HIV infection, in fact, is secondary, reflecting a defect in CD4+ T cells, cannot be ruled out. However, this defect in Th1 function of T cells could still reflect an IL-12 defect in vivo. HIV infection in vitro of monocyte-derived macrophages results in decreased IL-12 production (Chehimi et al16 and our own unpublished observations). Monocytes in PB are not infected with HIV,3 but the macrophages in the tissues, important for antigen presentation, are and might be incapable of inducing proper Th1 responses because of impaired IL-12 production.35
Because of its potent immunoregulatory function in vitro, IL-12 has been proposed as an immunotherapeutic agent in HIV infection. Indeed, IL-12 is capable of enhancing proliferation of T cells from HIV-1–infected individuals in response to influenza, HIV-1 peptides,36,37Mycobacterium avium,38,39 and polyclonal T-cell stimulators.39 Furthermore, IFN-γ production in response to several T-cell stimulators in vitro is enhanced on IL-12 addition,36,39,40 and cloning T cells from HIV-1–infected individuals in the presence of IL-12 results in an increased outgrowth of IFN-γ–producing cells.41 It should be noted that, in most publications, it was reported that proliferation and IFN-γ release by T cells from non-HIV–infected controls is also enhanced by IL-12.38,39 The enhanced outgrowth of IFN-γ–producing T cells in the presence of IL-12 is also observed when T cells from noninfected controls are used.41-44 This suggests that lack of IL-12 is not the only factor responsible for decreased Th1 function in HIV infection but, at least in vitro, enhances Th1-mediated responses, which is also observable in normal individuals. However, it can be anticipated that IL-12 treatment will support Th1 cellular immune responses in HIV-infected individuals to a certain extent.
ACKNOWLEDGMENT
We are indebted to all participants in the Amsterdam Cohort Studies for their continuous cooperation. We thank N. Albrecht-van Lent, M. Knapen, and R. Keet at the Municipal Health Service for their help in collecting the patient samples and Dr R. van Lier for critical review of the manuscript.
Supported by a grant from the Netherlands Organization for Scientific Research (900-506-208). Performed as part of the Amsterdam Cohort Studies on AIDS, a collaboration between the Municipal Health Service, the Academic Medical Centre, and the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands.
Address reprint requests to Frank Miedema, Department of Clinical Viro-Immunology, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal