Abstract
We studied degranulation of single cord blood-derived mononuclear cells differentiating to eosinophils in cultures containing recombinant human interleukin-5 (rhIL-5) and rhIL-3 by whole-cell patch-clamp capacitance measurements. As in mature cells, degranulation can be stimulated by intracellular application of guanosine-5′-O-(3-thiotriphosphate) (GTP) γS after 10 days in culture, simultaneously with the first morphological appearance of granules. These results demonstrate that the fusion machineries for exocytotic fusion are present and functional as soon as the granules are formed, presumably at the myeloblast stage. In the third week, the total amount of granules exocytosed upon stimulation is similar to that in mature eosinophils from peripheral blood. The capacitance step size distributions in promyelocytes and myelocytes confirm that mature large specific granules are formed by homotypic fusion of unit granules with similar size. Homotypic fusion is facilitated during early stages of differentiation associated with granulogenesis. Between day 10 and day 35 in culture the plasma membrane area of resting cells decreases from ≈700 μm2 to ≈400 μm2, approaching the value of mature cells from peripheral blood. The most prominent decrease occurs between day 25 and day 35 and is accompanied by the appearance of an exocytotic component due to small vesicles. This suggests that a class of small secretory vesicles is formed by endocytosis during a late phase in maturation.
EOSINOPHILIC GRANULOCYTES (eosinophils) are packed with large specific secretory granules containing cytotoxic cationic proteins that are released by an exocytotic mechanism following stimulation.1-3 In addition, smaller granules and vesicles are also present in eosinophils.4-6 Hematopoiesis gives rise to distinct leukocyte lineages by committing stem cells to different paths of differentiation. Differentiation of eosinophils is indicated by a polylobed nucleus and the appearance of specific crystalloid containing eosinophil granules.7 A number of studies demonstrated that in the presence of recombinant human interleukin-3 (rhIL-3) and rhIL-5 human eosinophils differentiate in vitro from umbilical cord blood-derived mononuclear cells.8,9 The earliest recognized manifestation of differentiation was the appearance of large numbers of immature granules in the cytoplasm.10 The typical specific crystalloid-containing eosinophil granules may derive from these immature granules, but the relationship is not yet settled.11,12 In previous studies differentiation was thus defined morphologically, but functional competence, in particular the ability to perform exocytosis at different stages of maturation, has not been studied. We investigated the degranulation properties of cord blood-derived eosinophils at different states of maturation and those of mature human peripheral blood eosinophils using patch-clamp capacitance measurements.13 14
MATERIALS AND METHODS
Cell preparation and culture.Mature human eosinophils from peripheral blood were isolated by negative-immunomagnetic selection using an antibody against CD16, an FcRIII receptor found on neutrophils, but not on eosinophils.15 Briefly, cells were collected from heparinized fresh blood by dextran sedimentation. A granulocyte pellet was obtained by density centrifugation through a Ficoll-Paque (Biochrom, Berlin, Germany) layer at 1,000g for 20 minutes at 20°C. The residual contaminating erythrocytes were disrupted by hypotonic lysis. The granulocytes were pelleted (100g at 20°C for 5 minutes) and resuspended in Medium 199 (Biochrom) supplemented with 4 mmol/L glutamine (Sigma, St Louis, MO), 0.6 mg/mL penicillin and streptomycin (Biochrom), and 4.2 mmol/L NaHCO3 (pH 7.2). A volume of 250 μL containing 108 Dynabeads M450 coated with sheep antimouse IgG (Dynal, Oslo, Norway) was incubated with 80 μL mouse antihuman CD16 IgG (Dianova, Hamburg, Germany; 0.2 mg/mL in 0.02% sodium azide) in a rotary mixer overnight at 4°C. To remove unbound antibody, the beads were washed four times in Dulbecco's phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA; Sigma). A total of 108 coated beads were incubated with 107 cells in the rotary mixer for 30 minutes. During this time the beads bind neutrophils and are subsequently removed using a magnet. The resulting suspension of eosinophils was 90% pure with a yield of 40% to 60%. The cells were stored in medium 199 supplemented as described above at room temperature for up to 12 hours.
A cell fraction containing undifferentiated mononuclear precursor cells was isolated from cord blood under sterile conditions.8 A total of 5 to 15 mL cord blood was drawn into a heparin-containing syringe by the midwife with informed consent of the donor. A total of 30 mL of a 1:5 dilution of the blood with RPMI-1640 Medium (Biochrom; supplemented with 20 mmol/L HEPES) was loaded onto a 10-mL Ficoll-Paque cushion and centrifuged at 800g and 20°C for 25 minutes. The upper layer with the mononuclear cells was collected and washed with RPMI. Next, 106 cells/mL were cultivated in flasks with 7 mL RPMI supplemented with 10% fetal calf serum (FCS; GIBCO BRL, Eggenstein, Germany), 2 mmol/L glutamine, 50 μmol/L 2-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.05 mg/mL gentamycin (Boehringer, Mannheim, Germany) and incubated at 36°C in a 5% CO2 atmosphere. A total of 5 ng/mL rhIL-3 and 5 ng/mL rhIL-5 (Genzyme, Cambridge, UK) were added. On days 7, 14, 21, and 28, half of the medium (3.5 mL) was replaced by fresh medium containing rhIL-3 and 5 (10 ng/mL each). At different times of culture, the state of differentiation was assessed morphologically using Kimura stain. During the fourth week, basophils and their precursors were removed by immunomagnetic separation before the experiment: 2 × 107 beads (100 μL) were incubated overnight at 4°C with 60 μL anti-CD25 antibody and washed as described above for anti-CD16 IgG incubation. A total of 50 μL containing 2 × 107 coated beads and 450 μL of cell suspension (106 cells/mL) were incubated in a rotary shaker for 30 minutes at 4°C. The antibody recognized CD25 on basophils, which were subsequently removed from the cell suspension using a magnet.
Patch clamp experiments.About 50 to 100 μL of cell suspension were transferred into a petri dish having a coverslip as the bottom. After a few minutes to allow the cells to settle on the glass, the dish was perfused with standard external saline (see below). The whole-cell configuration, establishing contact between the cytosol and the solution in the micropipette, was used to dialyze the cells with the internal solution. The internal solution contained 125 mmol/L potassium-L–glutamate, 10 mmol/L NaCl, 7 mmol/L MgCl2 , 1 mmol/L Na2ATP, 10 mmol/L HEPES/ NaOH, 1 mmol/L EGTA, and 20 μmol/L GTPγS (pH 7.2 to 7.3); no CaCl2 was added. The external bath solution contained 140 mmol/L NaCl, 5 mmol/L KCl, 2 mmol/L CaCl2 , 1 mmol/L MgCl2 , 10 mmol/L HEPES/ NaOH, pH 7.2 to 7.3. The osmolality of the external solution was adjusted with D(+) glucose (Merck, Darmstadt, Germany) to surpass that of the internal solution by a few mOsm. All experiments were performed at room temperature.
Capacitance measurements.The plasma membrane area of single cells was measured as electrical capacitance (CM ), using the whole-cell mode of the patch-clamp technique with a patch clamp amplifier (EPC-9; List Electronics, Darmstadt, Germany) operating in the voltage clamp mode. Initial capacitance was determined using the automatic capacitance compensation of the EPC-9 amplifier. For high resolution measurements of plasma membrane area changes during degranulation, an 800 Hz, 20 mV (rms) sine wave was given as the command signal and the current output signal was analyzed by a two-phase lock-in amplifier (PAR 5210; EG&G, Princeton, NJ)13 and sampled by a computer every 20 to 25 milliseconds. In the whole-cell configuration, the bulk capacitance of the cell was compensated. The phase error of the lock-in was periodically determined using the phase tracking technique.16 The phase was adjusted such that one of the output signals directly indicated the membrane capacitance.13,14 With this method, the time course of the whole degranulation process could be reconstructed at high resolution. The capacitance recordings were done until the capacitance was stable for several minutes. The total capacitance increase was determined as the difference between initial and final value. Conversion of capacitance into membrane area was done assuming a specific capacitance of 9 fF/μm2 for human eosinophils.17
Data analysis.The size, ΔC, of a distinct stepwise capacitance increase due to fusion of a granule with the plasma membrane was determined by eye on the computer screen using horizontal cursor lines. To calculate the percentage of the capacitance increase generated by steps of a certain size, the number of steps in a specific size range (bin) was multiplied by the mid value of the bin and divided by the total stepwise capacitance increase generated by the degranulation of the cell (the sum of all detected steps, not including apparently continuous changes). To reduce the noise in the step size distribution, the moving bin technique was applied. The number of steps within a certain range (the bin size) was calculated for bin positions separated by the bin increment, which is smaller than or equal to the bin size.18
RESULTS
Cell morphology.The morphological appearance of cells in cultures of cord blood-derived mononuclear cells observed after different times in culture using Kimura staining and Nomarski-optics resembled the properties previously described.19 After 14 days in culture, cells were nongranular, had large round nuclei, and the cytoplasm stained blue-violet. During the third week (days 15 to 21), cells were found to contain large numbers of granules either clearly visible using Nomarski optics or as dark dots using Kimura stain. During this week, the fraction of granular cells increased progressively. In individual cells, granules were either absent or numerous. Cells with intermediate amounts of granules were rare. During the fourth week, granules became auto fluorescent and their color obtained with Kimura stain became greenish. Initially, granules in individual cells were partly green and partly dark violet. After 28 days, the cells remaining after immunomagnetic separation of basophils and their precursors were full of green granules when Kimura staining was applied and had two-lobed nuclei, similar to the mature eosinophils from peripheral blood.
Resting plasma membrane area.Plasma membrane area of single cells was determined by measuring its electrical capacitance, CM , which is directly proportional to the plasma membrane area.13 A glass micropipette made contact with the cytosol of a single cell, such that the contents of pipette and cytosol could mix and recordings of CM could be made. During the first 2 weeks in culture, cells frequently had large conductances that were not identified. The membrane capacitance, as measured with the automatic compensation of the amplifier, yielded variable values in the range of 5 to 14 pF. Assuming a specific capacitance of 0.9 μF/cm2 for human eosinophils,17 this range corresponds to plasma membrane areas between 550 and 1,600 μm2. However, due to the large conductances, in particular the higher capacitance, values may not be reliable. Between days 10 and 14, seven of 31 cells from four different cultures had a low conductance (<1 to 2 nS) and a similar initial capacitance of 6.3 ± 0.2 pF (standard deviation [SD]), corresponding to a membrane area of 700 ± 22 μm2. During the following weeks, the initial capacitance values decreased progressively (Fig 1). At the end of the third week, the initial capacitance was about 5.6 pF corresponding to 620 μm2 membrane area. After 5 weeks in culture, the cord blood cells had a resting capacitance of 3.6 ± 0.2 pF (SD, n = 13), ie, a plasma membrane area of ≈400 μm2. Mature eosinophils from healthy donors had only a slightly smaller plasma membrane area of ≈320 μm2, calculated from their resting capacitance of 2.9 ± 0.3 pF (SD, n = 30; dashed line in Fig 1). From capacitance measurements, a decrease in plasma membrane area is thus apparent during differentiation from myeloblasts to eosinophils, approaching closely the same area as that of mature peripheral blood eosinophils.
Decrease of plasma membrane area during differentiation. Resting plasma membrane area (left scale) was determined from initial capacitance (right scale) in 81 cells from three different cultures after different times in culture. Data points are mean ± SD. The continuous line is a third order polynomial fit. The mean value of mature cells is indicated by the dashed line (2.9 ± 0.3 pF, SD, n = 30).
Decrease of plasma membrane area during differentiation. Resting plasma membrane area (left scale) was determined from initial capacitance (right scale) in 81 cells from three different cultures after different times in culture. Data points are mean ± SD. The continuous line is a third order polynomial fit. The mean value of mature cells is indicated by the dashed line (2.9 ± 0.3 pF, SD, n = 30).
Stimulation of exocytosis.Exocytosis is associated with an increase in plasma membrane area due to addition of the granular membranes upon fusion. The increase in membrane area can accordingly be measured as a proportional increase in CM. In mature eosinophils, intracellular application of 20 μmol/L guanosine-5′-O-(3-thiotriphosphate) (GST) γS stimulates a capacitance increase extending over several minutes associated with complete degranulation (Fig 2A).1,17,20 Most of the increase occurs in well resolved steps of 3 to 30 fF.17 With this method GTPγS also stimulates degranulation in developing cells, as indicated by the capacitance traces shown in Fig 2B through E. Cells measured between days 10 and 14 (Fig 2B) showed either no or only small CM increases of 0.07 to 1.8 pF (0.9 ± 0.3 fF, standard error of mean [SEM], n = 7) (Fig 3A). In the third to fifth week, a much larger increase of 3 to 6 pF is typically observed (Fig 2C through E). During the third week in culture, dialysis with 20 μmol/L GTPγS stimulates a capacitance increase by 4.9 ± 0.3 pF (SEM, n = 23) corresponding to a 544 μm2 increase in membrane area. A similar increase by 5.7 ± 0.2 pF (SEM, n = 30) is observed in mature cells from peripheral blood (Fig 3A). The marked transition from cells showing only a very small capacitance change (if any) to cells showing a full degranulation response (Fig 2B and C and Fig 3A, second/third week) quantitatively confirms the morphological indication that a cell contains either very few granules or about as many as a mature cell. During the next 2 weeks in culture, the total capacitance increase is slightly decreasing by about 20% in parallel to the decrease in cell size, suggesting that smaller cells exocytose less granule membrane.
Representative traces of membrane capacitance increase measured during degranulation stimulated by 20 μmol/L intracellular GTPγS in a mature eosinophil from peripheral blood (A) and from cord blood cells after different times in culture as indicated (B through E).
Representative traces of membrane capacitance increase measured during degranulation stimulated by 20 μmol/L intracellular GTPγS in a mature eosinophil from peripheral blood (A) and from cord blood cells after different times in culture as indicated (B through E).
Size of capacitance increase after different times in culture. (A) Total capacitance increase, (B) apparently continuous part (steps <3 fF ). The cells measured during the second week showing no increase were not included.
Size of capacitance increase after different times in culture. (A) Total capacitance increase, (B) apparently continuous part (steps <3 fF ). The cells measured during the second week showing no increase were not included.
When a secretory granule fuses with the plasma membrane, the plasma membrane area, and hence its capacitance, increases by an amount directly corresponding to the membrane area of the fusing granule. During degranulation, the total capacitance increase occurs as a combination of well-resolved steps and of apparently continuous increases. The well-resolved steps correspond to individual exocytotic fusion events of large granules. The continuous changes presumably represent exocytosis of vesicles smaller than 300 nm, as the associated capacitance steps <3 fF were not resolved.21 In the second week, the degranulating cells showed only a change by 15 fF ± 12 fF (SEM, n = 6) that could not be resolved as steps. This value increased gradually during culture to 800 ± 130 fF (SEM, n = 10) in cells measured during the fifth week in culture (Fig 3B). In mature eosinophils from peripheral blood, the continuous increase was 480 ± 60 fF (SEM, n = 14).
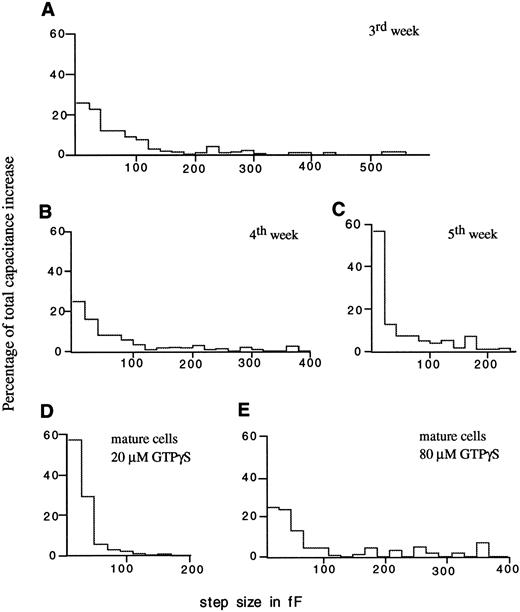
Granule-granule fusion.In Fig 2, the capacitance traces recorded in the second and third week of culture show much larger steps than the later recordings. The corresponding step size distributions shown in Fig 4A through C show a clear effect of differentiation on the step sizes. In the third week of culture, the capacitance increase contains large steps, some exceeding 500 fF, corresponding to exocytosis of vesicles up to 4 μm in diameter. In contrast, the granule diameters calculated from profile areas seen in myelocytes in cultures obtained at 3 weeks were 0.3 to 0.8 μm,19 corresponding to expected capacitance steps of only 2.5 to 18 fF. During degranulation stimulated with 20 μmol/L GTPγS, however, only 25% of the stepwise capacitance increase is due to steps <20 fF. This indicates that about 75% of the stepwise increase reflect exocytosis of multigranular compounds formed by granule-granule fusion inside the cell before exocytotic fusion with the plasma membrane. During the fifth week, all capacitance steps are <250 fF, and ≈60% of the stepwise increase occurs in steps <20 fF. In mature eosinophils stimulated with 20 μmol/L GTPγS, the step size distribution (Fig 4D) is very similar to that from the fifth week (Fig 4C). With the same concentration of GTPγS (20 μmol/L), the size of the steps thus decreases during differentiation approaching the step size distribution in mature eosinophils. With a higher concentration of 80 μmol/L GTPγS, even in mature eosinophils from peripheral blood, intergranular fusion is stimulated leading to exocytosis of large compounds (Fig 4E), as shown previously in horse eosinophils.20
Contribution of capacitance steps with different size to the total stepwise increase at different times in culture (A through C) and in mature cells from peripheral blood (D) stimulated with 20 μmol/L GTPγS. (E) Step size distribution from mature cells stimulated with 80 μmol/L GTPγS. The continuous increase was not included.
Contribution of capacitance steps with different size to the total stepwise increase at different times in culture (A through C) and in mature cells from peripheral blood (D) stimulated with 20 μmol/L GTPγS. (E) Step size distribution from mature cells stimulated with 80 μmol/L GTPγS. The continuous increase was not included.
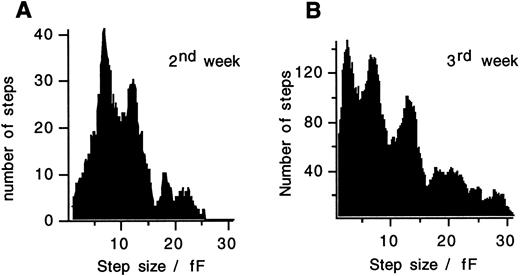
In both, developing cord blood cells (Fig 5A and B) and mature eosinophils from peripheral blood,17 a clear peak between 6 to 7 fF is found in the step size distributions and additional peaks occur at multiples of this value. In myelocytes of the third week, an additional peak occurs at <4 fF, presumably reflecting exocytosis of small granules or secretory vesicles. The left side of the distribution is incomplete since fusion of vesicles <3 fF cannot always be resolved as steps. Exocytosis in such small steps or in an apparently continuous manner as also observed in mature cells,17 does not occur early in differentiation during days 10 to 14 of culture.
Capacitance step size distributions measured in cells stimulated with 20 μmol/L GTPγS after 10 to 14 days in culture (A) and after 15 to 21 days in culture (B). A clear peak between 6 and 7 fF and additional peaks at multiples of this value are seen. The moving bin technique was used with a bin size of 1.8 fF and a bin increment of 0.1 fF in (A) and with a bin size of 2 fF and a bin increment of 0.2 fF in (B). The additional peak at <4 fF in (B) reflects exocytosis of a distinct class of small vesicles.
Capacitance step size distributions measured in cells stimulated with 20 μmol/L GTPγS after 10 to 14 days in culture (A) and after 15 to 21 days in culture (B). A clear peak between 6 and 7 fF and additional peaks at multiples of this value are seen. The moving bin technique was used with a bin size of 1.8 fF and a bin increment of 0.1 fF in (A) and with a bin size of 2 fF and a bin increment of 0.2 fF in (B). The additional peak at <4 fF in (B) reflects exocytosis of a distinct class of small vesicles.
DISCUSSION
Mononuclear cells isolated from cord blood differentiate into eosinophils in the presence of rhIL-3 and rhIL-5.8 The differentiation process manifests itself morphologically by the appearance of a polylobed nucleus and specific eosinophil granules.22 In the present report, we studied the degranulation properties during differentiation under the same conditions. We found that, as in mature eosinophils,1,17 23 degranulation can be stimulated in differentiating cells by intracellular application of GTPγS.
The first 3 weeks in culture.During the first 3 weeks in culture, the cells proliferate and mature. The slight decrease of resting plasma membrane capacitance between day 10 and day 21 indicates a corresponding decrease in plasma membrane area reflecting a small decrease in cell size. Exocytotic competence is present at the end of the second week. At this stage, the cells contain only very few granules and the total amount of granule membrane inserted into the plasma membrane upon stimulation is significantly less than in mature cells. This indicates that GTPγS stimulates exocytosis already at the myeloblast stage, as soon as granules are formed. Newly formed immature granules thus contain the G protein-stimulated fusion apparatus and other molecules required for exocytosis in an active form. In PC12 cells, immature secretory granules were also capable of undergoing regulated exocytosis24 and in an in vitro assay constitutive Golgi transport vesicles get the ability to function during their formation.25
In the third week, the degranulation amplitude was similar to that of mature cells from peripheral blood. Electron microscopy (EM) has shown that in the third week cells are predominantly at the myelocyte stage containing large amounts of immature or primary granules.19 The marked transition in degranulation amplitude indicates that formation of primary granules within an individual cell is confined to a short period of time, probably 1 day.
Granules of mature human eosinophils from peripheral blood have a multimodal size distribution with peaks at 6 to 7 fF and multiples of this value, suggesting that they are formed by homotypic fusion of unit granules with a mean diameter of 450 to 500 nm.17 We observed similar peaks at the promyelocyte and myelocyte stage, confirming the mechanism of unit granule fusion. Thus, maturation of eosinophil granules cannot be explained by a mechanism in which small vesicles bud from immature secretory granules simultaneously increasing their density and decreasing their size,26 but instead, it involves fusion among immature granules. This mechanism is in agreement with the long-standing observation that in eosinophilic myelocytes from rabbit bone marrow, immature granules with diameters between 0.3 and 0.5 μm merge into larger granules.11 Fusion of immature secretory granules with each other has also been discussed as a mechanism to explain the size distribution of secretory granules in mast cells,27-29 of pancreatic zymogen granules,30 and of dense core vesicles in PC12 cells.24
In contrast to mature eosinophils, the capacitance increase in promyelocytes and myelocytes stimulated with 20 μmol/L GTPγS included unusually large steps, corresponding to the exocytosis of granules with diameters of up to 4 μm. The diameters of granules in immature eosinophils from cord blood cultures as seen in electron micrographs, however, are only ≈ 0.3 to 0.8 μm.19 As the large granules were not seen in resting myelocytes, they must result from granule-granule fusion upon stimulation with GTPγS. In mature eosinophils large capacitance steps reflecting intracellular granule-granule fusion were previously observed only at very high GTPγS concentrations.20 Our results indicate that during early stages, when granules are formed, granule-granule fusion stimulated by GTPγS is facilitated in comparison with that observed at later stages or in mature cells. This suggests that formation of mature granules by constitutive fusion events associated with granule maturation are regulated by GTP-binding proteins and that the apparatus mediating fusion between granules functions with enhanced efficiency at the promyelocyte and myelocyte stage.
The molecular basis of the change in efficiency is not known. There may be a change in expression of particular GTP-binding proteins or some other regulatory mechanism exists restricting the extent of granule-granule fusion to prevent complete fusion of all granules in resting cells. This regulation leads to different granule sizes in different species.17 In patients or animals with the Chediak-Higashi syndrome, granules of eosinophils and other granulated cells are of giant size.31,32 These giant granules are the result of excessive initial fusion of immature granules during granulogenesis.33,34 Very recently, it was found that in beige mice, the gene Lyst is mutated and the homologous human gene LYST was found to contain a frame-shift mutation in a Chediak-Higashi syndrome patient.35 It was suggested that the role of the gene product might be in integration of cellular signal response coupling. The present results indicate that facilitated granule-granule fusion as part of their constitutive maturation may be restricted to an early period where granulogenesis is completed.
Maturation between the third and fifth week in culture.We measured an average decrease in resting capacitance or plasma membrane area by 35% from 620 to 400 μm2 (5.6 to 3.6 pF ) between the end of week 3 and the end of week 5 in culture. Under the same experimental conditions, a corresponding decrease in cell diameter from 10 μm to 8 μm was observed by EM19 between the third and the fifth week. Assuming a constant proportion of plasma membrane invaginations during development, this decrease in cell size should correspond to a 36% decrease in membrane capacitance in excellent agreement with the 35% decrease obtained here. The steepest decrease is observed between day 25 and day 35 in culture where the average membrane area decreases from 570 to 400 μm2 (5.1 to 3.6 pF, Fig 1). Because at this time the cells are at metamyelocyte and mature states and are thus postmitotic nondividing cells, the plasma membrane area decrease indicates strong endocytotic activity.
In the fourth and fifth week, an additional apparently continuous stimulated capacitance increase component appears (Fig 3B). This continuous increase component presumably reflects exocytosis of a pool of small secretory vesicles. Although some of these vesicles are present in week 4, they are much more abundant in week 5 (Fig 3B). The marked development of the continuous capacitance increase in the fifth week coincides with the steep decrease in plasma membrane area. The simultaneous decrease in plasma membrane area by endocytosis and formation of secretory vesicles suggests that these vesicles are formed by an endocytotic mechanism.
A corresponding pool of small vesicles, the so-called specific microgranules (due to their size of 20 to 200 nm), has been described in eosinophils from at least 20 species, including man.6 These vesicles are not present in early bone marrow precursors and appear only later, at the myelocyte or metamyelocyte stage. They are much more abundant as products of terminal differentiation in mature eosinophils, especially during eosinophilia.36 Previous EM studies showed that in the presence of rhIL-5 and rhIL-3, cord blood cells showed increasing numbers of small vesicles during culture,19 in accordance with the development of a pool of secretory vesicles generating the continuous capacitance increase observed in our experiments. In both neutrophilic and eosinophilic granulocytes, vesicles smaller than 200 nm contain albumin indicating that they are formed by an endocytotic mechanism during maturation.4 These easily exocytosable so-called secretory vesicles are believed to serve as a rapidly mobilizable pool of membrane proteins during activation.37 The decrease in plasma membrane area accompanied by the appearance of a stimulated capacitance increase due to small vesicles is in good agreement with the idea that formation of small secretory vesicles occurs by endocytosis during maturation.
The total degranulation amplitude decreases slightly from week 3 to week 5 (Fig 3A). Taking also into account the appearance of the continuous increase, the amount of the stepwise increase reflecting membrane from large granules, which is incorporated into the plasma membrane during degranulation, is significantly decreased between weeks 3 and 5. Since microscopic observations show that GTPγS-induced degranulation is always complete,20 this decrease reflects a decrease in granule number rather than partial fusion incompetence. The mean stepwise increase is 4.9 pF in the third week, 4.2 pF in the fourth week, and 3.1 pF in the fifth week. The myelocytes present in the third week may undergo cell division leading to cells with smaller numbers of granules and this could well explain the change from 4.9 to 4.2 pF between weeks 3 and 4. Interestingly, the decrease during this period is not very large suggesting that granules are formed de novo after cell division at this stage. After the fourth week, cell division should not occur or should at least be very rare. The loss of granules between weeks 4 and 5 must thus be explained by a different mechanism.
A loss of granules in eosinophils developing in cultures of cord blood mononuclear cells has also been observed by EM between the third and the fifth week.19 A comparable difference was found between eosinophilic myelocytes from the bone marrow and mature circulating eosinophils.3,10 These latter studies suggested that immature eosinophils degranulate during normal maturation in human bone marrow.10 Major basic protein (MBP) and Charcot-Leyden crystal (CLC) protein are secreted by eosinophil promyelocytes during maturation, as measured in culture supernatants of bone marrow cells.38 A decrease in granule content has also been observed in mature cells in culture stimulated by the presence of rhIL-5.39
If the reduction of the stepwise capacitance increase between weeks 4 and 5 reflects exocytosis of large granules during maturation in culture, then the plasma membrane area estimated from the initial capacitance should be increased by about 1 pF. In contrast, a decrease of about 1 pF is observed during this time (Fig 1). One possibility to explain this discrepancy would be that the exocytosed membrane is taken up again such that the total endocytotic activity between day 25 and day 35 is about twice as high as estimated from the data of Fig 1. Another possibility would be that the number and/or size of the large granules is decreased within the cell by a distinct mechanism. In mature eosinophils harvested from cord blood mononuclear cell cultures supplemented with rhIL-5 after 35 days, budding of small vesicles from eosinophil granules has been described.40 41 This mechanism has been called piecemeal degranulation. However, if this mechanism involves small vesicles budding from the granules and fusing with the plasma membrane, then the decrease in granule membrane should be accompanied by the same increase of plasma membrane area as in a conventional exocytotic process. Also in this case the exocytosed membrane has to be reinternalized by endocytosis. In any case, endocytotic activity is very high during the final stages of maturation and appears to be the mechanism forming small secretory vesicles.
ACKNOWLEDGMENT
We thank Dr A.B. Kay and his staff for introducing to us the cord blood cell culture, and we are grateful to Drs J. Coorsen and H.-H. Gerdes for critically reading the manuscript. We thank the Universitäts-Frauenklinik, Heidelberg for providing the cord blood.
Supported by the Deutsche Forschungsgemeinschaft (SFB 352TP C5, Li443/9-1).
Address reprint requests to Manfred Lindau, PhD, Abt. Molekulare Zellforschung, Max-Planck-Institut f. medizinische Forschung, Jahnstrasse 29, D-69120 Heidelberg, Germany.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal