Abstract
The induction by hypoxia of genes such as erythropoietin, vascular endothelial growth factor (VEGF ), and glucose transporter-1 (Glut-1) is mediated in part by a transcriptional complex termed hypoxia-inducible factor-1 (HIF-1). Several lines of evidence have implicated protein phosphorylation in the mechanism of activation of HIF-1 by hypoxia. Recent reports have described the activation of the tyrosine kinase src by severe hypoxia, and a role in the induction of VEGF by severe hypoxia has been proposed. This led us to examine whether src and related kinases operated more widely in the hypoxic induction of HIF-1 and HIF-1–dependent genes regulated by hypoxia. Measurements of src kinase activity in cells exposed to varying severities of hypoxia showed activation by severe hypoxia (0.1% oxygen or catalyst induced anoxia), but not 1% oxygen. This contrasted with the marked induction of HIF-1 by exposure to 1% oxygen. Manipulations of src activity were produced by transient and stable transfection of Hep3B cells. Despite substantial changes in src activity, no alteration was seen in the normoxic or hypoxic expression of erythropoietin, VEGF, or Glut-1, or in the regulation of HIF-1–dependent reporter genes inducible by hypoxia. Similarly, we found that the expression of these genes in src- or c-src kinase-deficient cells did not differ from wild-type cells at either 1% oxygen or more severe hypoxia. These results indicate that src is not critical for the hypoxic induction of HIF-1, erythropoietin, VEGF, or Glut-1.
THE REGULATION OF gene expression is an important adaptive response to the availability of oxygen.1 For a number of groups of mammalian genes that are induced in a characteristic way by hypoxia, regulatory studies have implicated an inducible transcriptional complex termed hypoxia inducible factor-1 (HIF-1).2-8 These genes include the hematopoietic growth factor erythropoietin,2 angiogenic growth factors such as vascular endothelial growth factor (VEGF ),4,5,9,10 and genes involved in glucose transport and metabolism such as the glucose transporter-1 (Glut-1).3,6,11 For several such genes, transfection assays have defined oxygen-regulated cis-acting control sequences that bind HIF-1,2-7 and induction of these genes by hypoxia is reduced or absent in cells that are defective for one component of the HIF-1 heterodimer (HIF-1β) and are unable to form a HIF-1 complex.12
The HIF-1 DNA-binding complex, which consists of a heterodimer of two basic-helix-loop-helix proteins, HIF-1α and HIF-1β,13 is strongly induced by moderate hypoxia, such as exposure of cells to an atmosphere of 1% oxygen.2 Although the mechanisms that underlie oxygen sensing and HIF-1 activation remain unclear, several lines of evidence have implicated protein phosphorylation in the signal transduction mechanism. Extraction of HIF-1 in its DNA-binding form requires the addition of the phosphatase inhibitor sodium vanadate to the preparative buffers,2 and exposure of HIF-1 in vitro to phosphatases abolishes binding.14 Moreover, the hypoxic induction of HIF-115 and genes such as erythropoietin can be reduced or abolished by exposure of cells to a variety of protein kinase inhibitors.16-20 In view of these findings, recent experiments implicating the tyrosine kinase c-src in the induction of VEGF mRNA by more severe oxygen deprivation (anoxia) are potentially of great interest.21 We therefore examined the role of c-src and related kinases in the hypoxic induction of erythropoietin, and other genes (VEGF and Glut-1) in which HIF-1 has been implicated. Our results indicate that c-src does not have an obligatory or central role in this response to hypoxia.
MATERIALS AND METHODS
Materials.Fetal calf serum and cell culture medium were purchased from GIBCO (Paisley, UK). [α-32P]guanosine triphosphate (400 Ci/mmol) and [γ-32P]adenosine triphosphate (ATP; 3,000 Ci/mmol) were purchased from Amersham International (Amersham, UK). RNA polymerases, DNase, RNase A, and T1 were purchased from Boehringer Mannheim (Lewes, UK). Other chemicals were purchased from Sigma (Poole, UK).
Plasmids.pSG5 SrcK+ and pSG5 SrcK− were provided by S. Courtneidge and have been described by Twamley-Stein et al.22 pSG5 SrcK+ expresses c-src, whereas pSG5 SrcK− expresses an interfering (dominant negative) mutant c-src, bearing the single substitution (Lys 295 → Met) in the kinase domain of c-src. pcDNA-1 Csk was provided by H. Hanafusa and has been described by Sabe et al23; the plasmid expresses c-src kinase (csk), a negative regulator of src family tyrosine kinases. The HIF-1–activated reporter plasmid p(PGK24)3TKGH contained three copies of the oligonucleotide P24, which contained the HIF-1 site from the mouse phosphoglycerate kinase-1 5′ enhancer located 10 bp 5′ to the TATA box of the herpes simplex virus thymidine kinase (TK) promoter linked to growth hormone.3 Plasmid pBSα- contains a full-length α1 -globin gene with 1.4 kb of 5′ flanking sequence.
Cell culture.Immortalized mouse fibroblasts were provided by P. Soriano. The wild-type, src−, and csk− variants were originally isolated from normal or transgenic mouse embryos bearing targeted disruptions of the c-src gene (src−) or the csk gene (csk−), and were transformed by infection with a retrovirus, transducing SV40 large T antigen.24 25 The human hepatoma cell line, Hep3B, was obtained from the European Collection of Animal Cell Cultures (PHLS, Centre for Applied Microbiology and Research, Salisbury, UK). The murine fibroblasts were grown in Dulbecco's minimum essential medium supplemented with glutamine (2 mmol/L), penicillin (50 U/mL), streptomycin sulfate (50 μg/mL), and 10% fetal calf serum. Hep3B cells were maintained in minimum essential medium with Earle's salts and the same supplements.
Transfection and experimental conditions.Transfection was performed by electroporation using a 1-mF capacitor array charged at 375 V. Stable transfectants of Hep3B cells were created by the electroporation of cells with linearized plasmids pSG5 SrcK+, pSG5 SrcK−, pcDNA-I Csk, or pcDNA1/Neo (control), plasmids pSG5 SrcK+ and pSG5 SrcK− being cotransfected with pcDNA1/Neo to provide a selectable marker. Stable integrants were selected using G418 at 400 μg/mL; clones were derived from these pools, and grown in similar medium.
Transient transfection of Hep3B cells was performed using approximately 107 cells in a 1-mL cuvette with 25 μg p(PGK24)3 TKGH, 25 μg pBSα-, and 20 μg of the expression plasmid pSG5 SrcK+, pSG5 SrcK−, pcDNA-I Csk, or pcDNA1/Neo (control). Pools of transfected cells were split for parallel normoxic and hypoxic incubation, dishes being exposed to hypoxia for 16 hours commencing approximately 24 hours after transfection.
Experiments were performed on cells approaching confluence, with care being taken that comparative experiments between different cells were performed on cultures of similar density. Hypoxic incubation was in an atmosphere of 0.1% or 1% oxygen, 5% carbon dioxide with the balance as nitrogen, in a NAPCO 7301 incubator (Precision Scientific, Chicago, IL). Anoxia was achieved by placing the cells in a GasPak (Becton Dickinson, Cockeysville, MD) anaerobic jar, with oxygen being removed by the catalytically activated combination of oxygen with hydrogen (GasPak Anaerobic System). Anoxic conditions were monitored with methylene blue indicator strips. For experiments involving a short exposure to hypoxia, medium was pre-equilibrated for 12 hours at the appropriate level of oxygenation (control or hypoxia) and added to the culture at the start of the experiment.
In vitro src kinase assay.The immunoprecipitation of src and assay of tyrosine kinase activity was performed using modifications of the method described by Gould and Hunter.26 In brief, cells were rinsed with ice-cold phosphate-buffered saline, and cell lysate was prepared by scraping cells into modified RIPA buffer (50 mmol/L Tris-HCl [pH, 7.4]; 1% NP 40; 0.25% sodium deoxycholate; 150 mmol/L sodium chloride; 1 mmol/L EGTA; 1 mmol/L phenylmethylsulfonyl fluoride; 1 mmol/L sodium orthovanadate; and 1 μg/mL each of aprotonin, leupeptin, and pepstatin) at 4°C. Src was immunoprecipitated from the lysate (200 μg of protein) using 2 μL of anti-src monoclonal antibody 327 (Oncogene Science, Cambridge, MA) by mixing for 2 hours at 4°C followed by the addition of 50 μL of protein-A-Sepharose CL-4B precoated with rabbit antimouse immunoglobulin G and mixed for a further 1 hour. The immunocomplexes were collected by centrifugation at 4°C, then washed three times with RIPA buffer and once with kinase buffer (100 mmol/L PIPES, pH 6.8; 20 mmol/L manganese (II) chloride; 100 mmol/L sodium (IV) orthovanadate) and then resuspended in 25 μL of kinase buffer.
Kinase reactions were then performed in a final volume of 20 μL of kinase buffer by adding 0.01 mg of acid-activated enolase as substrate and 5 μCi of [γ-32P] ATP (3,000 Ci/mmol). The reaction was incubated for 10 minutes at 30°C and terminated by the addition of 2× sodium dodecyl sulfate (SDS) sample buffer and heating at 95°C for 5 minutes. The samples were analyzed with 10% SDS polyacrylamide gel electrophoresis and autoradiography. Radiolabeled substrate was quantified using a Phosphor Imager (Molecular Dynamics, Kent, UK). All assays were performed in parallel with normoxic controls.
Nuclear extract and electrophoretic mobility-shift assay.Nuclear extract was prepared using a modification of the protocol described by Semenza and Wang.2 For electrophoretic mobility-shift assay (EMSA), a double-stranded oligonucleotide probe derived from the mouse erythropoietin 3′ enhancer27 was used. The oligonucleotide, 5′-GCCCTACGTGCTGCCTCGCATGGC-3′, was labeled using [γ-32P]ATP (3,000 Ci/mmol; Amersham) and T4 polynucleotide kinase, then annealed with 4× molar excess of the complementary strand. Binding reactions were performed in a 20-μL volume that contained 50 mmol/L KCl, 1 mmol/L MgCl2 , 0.5 mmol/L EDTA, 5 mmol/L dithiothreitol, 5% (vol/vol) glycerol, and 0.25 μg of sonicated poly (dI-dC). Nuclear extract (5 μg) was incubated with this mixture for 5 minutes at room temperature before probe (≈0.1 ng) was added. Incubation was continued for a further 10 minutes before electrophoresis (12.5 V/cm) at 4°C using 5% polyacrylamide in 0.3× TBE (30 mmol/L Tris, 30 mmol/L boric acid, 0.06 mmol/L EDTA, pH 7.3 at 20°C).
RNase protection assay.Measurement of mRNA levels by RNase protection assays was performed using probes to murine VEGF12 and Glut-1,12 rat β-actin,12 and human erythropoietin,9 VEGF,9 and Glut-16 as previously described. RNA was prepared using a modified single-step acid/guanidinium thiocyanate/phenol/chloroform extraction method (Rnazol B; Cinna/Biotecx Laboratories, Houston, TX) and dissolved in hybridization buffer (80% formamide; 40 mmol/L piperazine-N,N′-bis[2-ethanesulfonic acid]; 400 mmol/L sodium chloride; 1 mmol/L EDTA, pH 8). RNA concentration was determined by measurements of absorbance at 260 nm. The quantities of RNA analyzed in each assay were as follows: VEGF 20 μg, Glut-1 10 μg, erythropoietin 50 μg, and β-actin 0.5 μg and from transient transfections 10 μg. Each sample was assayed for β-actin mRNA as an internal control for RNA extraction and sample processing. To accommodate the differences in mRNA abundance between the test genes and β-actin, separate hybridizations were performed using 0.5 μg of RNA from each sample, and the β-actin riboprobe. After RNAse digestion, the samples were combined and then processed and electrophoresed together. Autoradiography was performed at −70°C for 12 to 48 hours; protected species were quantitated in a Phosphor Imager.
Statistical analysis of measurements made on parallel cell cultures was by Student's two-tailed paired t test. When normalized data are given, the statistical calculation was made on the raw counts.
RESULTS
Previous studies that showed an increase in src activity in hypoxia, have exposed cells to severe hypoxia.21,28 In contrast, most studies of HIF-1 and HIF-1–dependent gene activation have used more moderate hypoxia, typically an atmosphere containing 1% oxygen.2 29 As a first step in determining whether c-src activation might underlie the regulation of HIF-1, we examined changes in src activity in mouse fibroblasts exposed to 1% oxygen or more severe hypoxia. The results of immune complex assays for src activity are summarized in Fig 1. In keeping with previous reports, severe hypoxia (0.1% oxygen, or catalyst-generated anoxia) produced a clear activation of src, which was maximal at 60 minutes. In contrast, exposure of cells to 1% oxygen did not lead to significant changes in src activity at any of the time points studied.
The src kinase activity in fibroblasts exposed to hypoxia. Transformed embryonic murine fibroblasts, described in the Methods, were exposed to different oxygen tensions (21%, 1%, 0.1 %, and anoxia [0%]) for 30, 60, and 90 minutes. The results are expressed as the ratio of activity to that of normoxic cells cultured in parallel and are the means ± SE of 3 (30 and 90 minutes) or 5 (60 minutes) independent experiments. An increase in activity was seen when cells were exposed to severe hypoxia (0.1% oxygen or anoxia), but not 1% oxygen; significantly different from normoxic activity, *P < .05.
The src kinase activity in fibroblasts exposed to hypoxia. Transformed embryonic murine fibroblasts, described in the Methods, were exposed to different oxygen tensions (21%, 1%, 0.1 %, and anoxia [0%]) for 30, 60, and 90 minutes. The results are expressed as the ratio of activity to that of normoxic cells cultured in parallel and are the means ± SE of 3 (30 and 90 minutes) or 5 (60 minutes) independent experiments. An increase in activity was seen when cells were exposed to severe hypoxia (0.1% oxygen or anoxia), but not 1% oxygen; significantly different from normoxic activity, *P < .05.
This result suggested that HIF-1 activation and src kinase activation might reflect different responses to hypoxia, and that src kinase activation might not be necessary for HIF-1 activation. To investigate this further, we examined the induction of HIF-1 DNA-binding activity, and the induction of HIF-1–responsive genes, in cells with manipulated src kinase activity.
HIF-1 activity was first determined by electrophoretic mobility shift assays of nuclear extracts prepared from the wild-type (src+) murine fibroblasts and src− fibroblasts derived in a similar manner from mice bearing a targeted disruption of the c-src gene. As has been reported in many other cell lines, exposure of wild-type murine fibroblasts to an atmosphere of 1% oxygen resulted in the rapid induction of HIF-1. Exposure of src− cells to 1% oxygen resulted in an identical induction of HIF-1 DNA-binding activity (Fig 2).
An electrophoretic mobility shift assay demonstrating HIF-1 in nuclear extracts from src wild-type and src-deficient cells. An electrophoretic mobility shift assay demonstrating the presence of binding activity induced by hypoxia (HIF-1) to radiolabeled oligonucleotide E24, which contains the HIF-1–binding site from the murine erythropoietin 3′ enhancer. Nuclear extracts were prepared from embryonic mouse fibroblasts lacking src (src−) or wild-type cells possessing src (src wt) exposed to normoxia (21% oxygen) or hypoxia (1% oxygen) for 1 (1h) and 2 hours (2h). The inducible species and the constitutive species are indicated. The time course and binding of the inducible species does not differ between wild-type and src− cells.
An electrophoretic mobility shift assay demonstrating HIF-1 in nuclear extracts from src wild-type and src-deficient cells. An electrophoretic mobility shift assay demonstrating the presence of binding activity induced by hypoxia (HIF-1) to radiolabeled oligonucleotide E24, which contains the HIF-1–binding site from the murine erythropoietin 3′ enhancer. Nuclear extracts were prepared from embryonic mouse fibroblasts lacking src (src−) or wild-type cells possessing src (src wt) exposed to normoxia (21% oxygen) or hypoxia (1% oxygen) for 1 (1h) and 2 hours (2h). The inducible species and the constitutive species are indicated. The time course and binding of the inducible species does not differ between wild-type and src− cells.
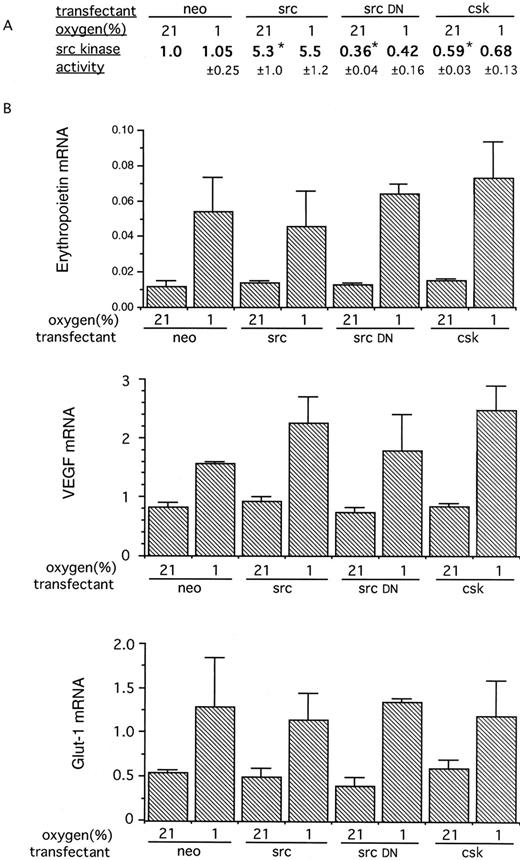
HIF-1–dependent, hypoxia-inducible, gene expression was next assayed in Hep3B cells transfected with genes designed to increase or reduce src kinase activity; both stable and transient transfection experiments were performed. Four types of stable transfectant were produced: Hep3B/neo (control), bearing the expression vector pcDNA neo without DNA insert; Hep3B/src (src overexpression), bearing pSG5 SrcK+, Hep3B/srcDN (src kinase inactive interfering mutant), bearing pSG5 SrcK−; and Hep3B/csk (overexpression of the src− regulator csk), bearing pcDNA-1 Csk. After electroporation, transfectants were selected in G418, and experiments were performed on pooled transfectants and clonal populations derived from the same pool. Experiments performed on pools of these transfectants showed that the anticipated manipulations of src kinase activity had been achieved, with normoxic kinase activity (normalized to the control pool Hep3B/neo) being Hep3B/src, 0.45; Hep3B/src DN, 0.39; and Hep3B/csk, 0.55. No difference in the normoxic or hypoxic expression of erythropoietin mRNA or VEGF mRNA was observed (data not shown). More detailed experiments were performed on clonal populations derived from each pool. Results are shown in Fig 3. Immune complex src kinase assays showed similar perturbations of kinase activity to those in the original pools. In normoxia, the Hep3B/src cells had approximately fivefold greater src kinase activity than the Hep3B/neo control cells, whereas activity was reduced twofold to threefold in Hep3B/srcDN and Hep3B/csk cells. As with the mouse fibroblasts, no further changes in src kinase activity were observed when the transfected Hep3B cells were exposed to 1% oxygen.
(A) Src kinase activity in Hep3B cells stably transfected with plasmids encoding src, a kinase inactive mutant src, or csk. The stably transfected cells were assayed for src kinase activity in parallel with control cells transfected with the neomycin resistance plasmid alone (neo). Cells were exposed to hypoxia (1%) or normoxia for 60 minutes. The results are expressed as the ratio of activity to that of the normoxic control and are the means ± SE of 3 independent experiments. Alterations in src kinase activity consistent with the overexpression of the src gene, src-dominant negative gene, and the csk gene were seen. Significant differences in src kinase activity from normoxic control transfectants (neo) are indicated (*P < .05). Exposure of cells to 1% oxygen produced no significant changes in src kinase activity in any of the transfectants. (B) RNase protection analysis of expression of genes induced by hypoxia in the Hep3B stable transfectants. The cells were exposed in parallel to 21% oxygen or 1% oxygen for 4 hours. The results are expressed as the ratio of the mRNA of interest to that of the actin control (×100) and are the means ± SE of 3 independent experiments. Despite the substantial alterations in src kinase activity, no significant differences were seen in the normoxic expression or hypoxic induction of any gene.
(A) Src kinase activity in Hep3B cells stably transfected with plasmids encoding src, a kinase inactive mutant src, or csk. The stably transfected cells were assayed for src kinase activity in parallel with control cells transfected with the neomycin resistance plasmid alone (neo). Cells were exposed to hypoxia (1%) or normoxia for 60 minutes. The results are expressed as the ratio of activity to that of the normoxic control and are the means ± SE of 3 independent experiments. Alterations in src kinase activity consistent with the overexpression of the src gene, src-dominant negative gene, and the csk gene were seen. Significant differences in src kinase activity from normoxic control transfectants (neo) are indicated (*P < .05). Exposure of cells to 1% oxygen produced no significant changes in src kinase activity in any of the transfectants. (B) RNase protection analysis of expression of genes induced by hypoxia in the Hep3B stable transfectants. The cells were exposed in parallel to 21% oxygen or 1% oxygen for 4 hours. The results are expressed as the ratio of the mRNA of interest to that of the actin control (×100) and are the means ± SE of 3 independent experiments. Despite the substantial alterations in src kinase activity, no significant differences were seen in the normoxic expression or hypoxic induction of any gene.
The cells were assayed for expression of erythropoietin, VEGF, and Glut-1 mRNAs — genes known to be inducible in hypoxia by mechanisms including the activation of HIF-1. Despite large differences in src kinase activity, no differences in the normoxic expression of these genes were observed between the different transfectants (Fig 3B). As expected, all genes were inducible in 1% oxygen, but again there were no differences in hypoxic gene expression between the transfectants (Fig 3B).
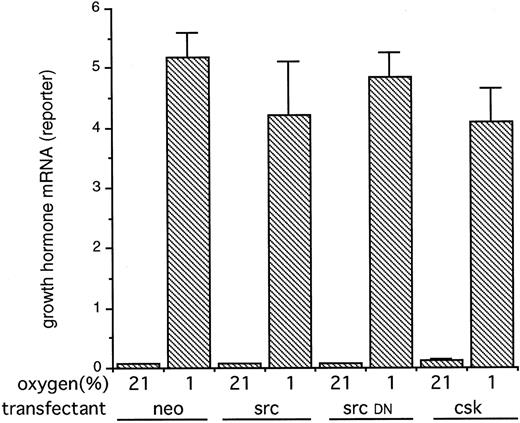
Because it could be argued that transient expression of the c-src and csk genes could produce larger or uncompensated alterations in src activity that were not achieved in the stable transfectants, we also tested the action of the expression plasmids in transient cotransfection assays. In these experiments, the HIF-1–dependent reporter construct p(PGK24)3TKGH was cotransfected with either pSG5 SrcK+ (wild-type src), pSG5 SrcK− (src kinase inactive interfering mutant), or pcDNA-1 Csk (overexpression of the src− regulator csk) or pcDNA neo (control). Exposure of these transfected Hep3B cells to 1% hypoxia produced a substantial induction of growth hormone reporter gene expression as described previously. However, the cotransfection with genes designed to increase or decrease src kinase activity had no effect on the normoxic or hypoxic expression of this HIF-1–dependent reporter construct (Fig 4).
The expression of a HIF-1–dependent reporter gene in Hep3B cells transiently cotransfected with plasmids encoding src, a kinase-inactive mutant src or csk. Hep3B cells were cotransfected with the HIF-1 dependent plasmid p(PGK24)3TKGH, the transfection control plasmid pBSα− and either a control plasmid (neo) or plasmids encoding src (src), a kinase-inactive mutant src (src DN), or csk (csk). Twenty-four hours after electroporation, cells were exposed to normoxia (21%) or hypoxia (1%) for 16 hours. Results are expressed as the ratio of reporter growth hormone mRNA to control α-globin mRNA and are the means ± SE of 3 independent experiments. No significant differences in reporter gene expression were seen between control and transfections designed to manipulate src kinase activity.
The expression of a HIF-1–dependent reporter gene in Hep3B cells transiently cotransfected with plasmids encoding src, a kinase-inactive mutant src or csk. Hep3B cells were cotransfected with the HIF-1 dependent plasmid p(PGK24)3TKGH, the transfection control plasmid pBSα− and either a control plasmid (neo) or plasmids encoding src (src), a kinase-inactive mutant src (src DN), or csk (csk). Twenty-four hours after electroporation, cells were exposed to normoxia (21%) or hypoxia (1%) for 16 hours. Results are expressed as the ratio of reporter growth hormone mRNA to control α-globin mRNA and are the means ± SE of 3 independent experiments. No significant differences in reporter gene expression were seen between control and transfections designed to manipulate src kinase activity.
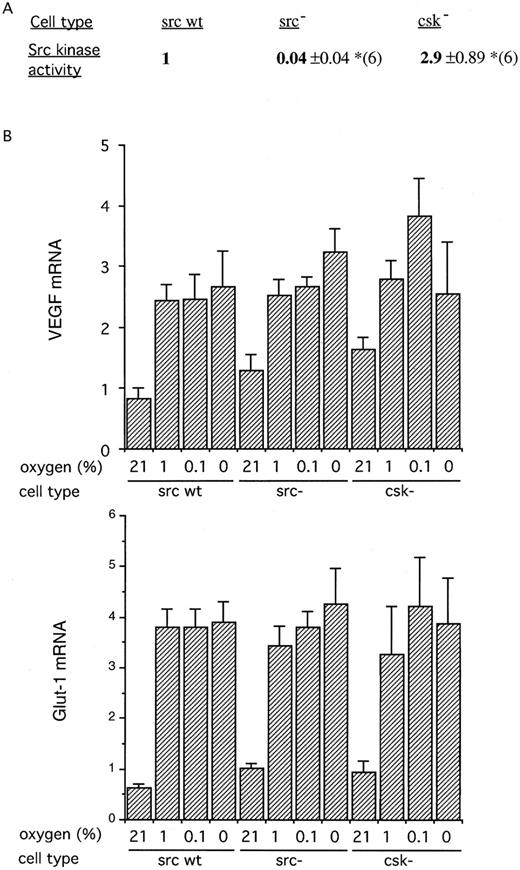
Taken together, these results indicate that src kinase is unlikely to be important in the regulation of HIF-1. HIF-1 has been implicated in the regulation of VEGF mRNA, yet anoxic induction of VEGF mRNA was reported to be severely reduced in src− cells.21 We therefore hypothesized that there might be different processes contributing to the hypoxic induction of VEGF mRNA, including HIF-1 activation at 1% oxygen and a distinct src kinase–dependent mechanism at more severe degrees of hypoxia. To address this, we compared the induction of VEGF and Glut-1 at different severities of hypoxia, including anoxia, in wild-type (src+), src−, and csk− mouse fibroblasts. As expected, the src− cell showed essentially zero activity in immune complex assays of src activity. In keeping with the src-inhibitory function of the csk gene, csk− cells showed approximately threefold greater src activity than the wild-type fibroblasts (Fig 5A).
(A) Src kinase activity in fibroblasts derived from mice deficient in src (src−) or csk (csk−). The results are expressed as the ratio of activity to that wild-type fibroblast cells (src wt) and are the means ± SE of 6 independent experiments. As would be anticipated, the cells deficient in src had negligible src kinase activity, while those deficient in the regulatory csk had significantly increased activity. (*P < .05). (B) RNase protection analysis of gene expression. The cells were exposed in parallel to varying oxygen concentrations for 4 hours (21%, 1%, 0.1%, and anoxia). Results are expressed as the ratio of the mRNA of interest to that of the actin control (×100) and are the means ± SE of 5 independent experiments. Despite the substantial differences in src kinase activity between the cells, no significant differences were seen in the expression of VEGF or Glut-1 at any oxygen tension.
(A) Src kinase activity in fibroblasts derived from mice deficient in src (src−) or csk (csk−). The results are expressed as the ratio of activity to that wild-type fibroblast cells (src wt) and are the means ± SE of 6 independent experiments. As would be anticipated, the cells deficient in src had negligible src kinase activity, while those deficient in the regulatory csk had significantly increased activity. (*P < .05). (B) RNase protection analysis of gene expression. The cells were exposed in parallel to varying oxygen concentrations for 4 hours (21%, 1%, 0.1%, and anoxia). Results are expressed as the ratio of the mRNA of interest to that of the actin control (×100) and are the means ± SE of 5 independent experiments. Despite the substantial differences in src kinase activity between the cells, no significant differences were seen in the expression of VEGF or Glut-1 at any oxygen tension.
After exposure to all severities of hypoxia or anoxia, both VEGF and Glut-1 mRNAs were induced, but there were no consistent changes in gene expression between the cell lines to correlate with the large differences in src kinase activity. Apart from an apparent increase in VEGF mRNA in normoxic csk− fibroblasts, which was not statistically significant, there were no differences between the wild-type, src−, and csk− fibroblasts in the expression of either gene, at any level of hypoxia (Fig 5B).
DISCUSSION
The recognition of a highly conserved and widely acting mechanism of oxygen-regulated gene expression that operates through the activation of HIF-130,31 has generated considerable interest in the underlying processes of oxygen sensing and signal transduction. Evidence of a possible role for protein phosphorylation in these events has been obtained through exposure of cells to protein kinase inhibitors. The broadly active inhibitor, staurosporine, compounds with selectivity for protein kinase C, and others, such as genistein, with activity against tyrosine kinases, can all reduce hypoxic induction of erythropoietin in hepatocytes or hepatoma cells (Jelkmann et al,16 Kurtz et al,17 Faquin et al,18 Eckardt et al,19 Gess et al,20 and our unpublished observations, April 1996). In keeping with these results, and with the involvement of HIF-1 in the transcriptional response to hypoxia, protein kinase inhibitors, including both the serine/threonine kinase inhibitor 2-aminopurine, and the tyrosine kinase inhibitor genistein, prevent the activation of HIF-1 by hypoxia.15
A variety of protein kinase activities can be induced by hypoxia or anoxic stress,32 including the tyrosine kinase c-src.21,28 The abrogation of anoxic induction of VEGF mRNA by genistein, and alterations in the anoxic induction of VEGF mRNA in response to genetic manipulations of src kinase activity, have led to the recent proposal that c-src activation has a critical role in the oxygen-dependent regulation of VEGF mRNA.21 As VEGF is one of the genes regulated, at least in part, by the system involving activation of HIF-1,4,5 12 we examined the possible role of c-src activation in this system.
We assayed HIF-1 DNA-binding activity and the expression of VEGF and that of two other genes induced by hypoxia for which there is functional evidence of regulation by HIF-1 (erythropoietin and Glut-1). Two series of cell types with manipulated src activity were used: fibroblast cell lines derived from homologous recombinant mouse strains, and transfected Hep3B cells, a cell type that shows regulated erythropoietin gene expression,33 in addition to the more widely expressed VEGF and Glut-1 genes. Our results confirmed that c-src can be activated by severe hypoxia, but provided no support for the involvement of c-src in HIF-1 activation, or in the regulation of any of these genes by hypoxia. We also considered the possibility that responses to anoxia, rather than hypoxia, might be affected by c-src manipulation, but were unable to detect any difference in the induction of VEGF and Glut-1 mRNA at any level of hypoxia.
In addition to assaying the effects of overexpression and underexpression of c-src itself, we studied the effects of overexpression and underexpression of csk. Csk is a negative regulator of c-src, which operates through phosphorylation of the c-terminal regulatory domain of c-src.34 In keeping with this, we found that csk− mouse fibroblasts had increased c-src activity and Hep3B cells overexpressing csk had reduced c-src activity. Regulation by csk is common to a number of members of the nonreceptor tyrosine kinase family such as fyn and yes.34 Again, we found no changes in hypoxic gene regulation in the csk-manipulated cells, indicating that it is also unlikely that these molecules are involved in the observed response to hypoxia and able to provide a compensatory transduction mechanism in the src− cells.
Our results are therefore in conflict with those of Mukhopadhyay et al21 in respect to the involvement of c-src in the hypoxic regulation of VEGF mRNA. In that report, several src− lines were used that showed a variable reduction in the induction of VEGF mRNA by hypoxia. The reasons for this variation are unclear, but presumably reflect variation in a property other than src kinase activity. However, our finding of normal hypoxic gene regulation in a single src− cell line is a clear result, which itself provides strong evidence against a role of src kinase in this type of gene regulation. This conclusion is further supported by the experiments on the csk− cells and transfected Hep3B cells. In a subsequent report, Mukhopadhyay et al35 have reported that v-src cotransfection, but not hypoxia, activated a 2.6-kb VEGF promoter sequence, a result which also implies that v-src induction and hypoxia operate on VEGF mRNA by different mechanisms. It may also be relevant that, as expected from the involvement of c-src in cell proliferation, we found that manipulations of c-src activity had effects on growth rate. We have previously found that cell density before and during the hypoxic exposure can affect gene induction by hypoxia (Maxwell et al30 and unpublished results, April 1996), so that there is the potential for indirect association with changes in gene regulation to arise from effects on growth. In the current experiments, we took care to ensure that comparison was made between cells grown to equivalent densities.
Whatever the explanation, our results indicate that c-src activation is not critical for hypoxic induction of HIF-1, or for the hypoxic induction of genes encoding erythropoietin, VEGF, and Glut-1. Alternative explanations must therefore be sought for the action of protein kinase inhibitors on the induction of these genes by hypoxia.
ACKNOWLEDGMENT
The authors thank S. Courtneidge for providing plasmids pSG5 SrcK+ and pSG5 SrcK−, H. Hanafusa for providing plasmid pcDNA-1 Csk, and P. Soriano for providing src- and csk-deficient fibroblasts. We would like to thank Lynn Nicholls for excellent technical assistance.
Supported by the Wellcome Trust. J.M.G. was a Medical Research Council Training Fellow.
Addresss reprint requests to Peter J. Ratcliffe, MD, Room 420, Institute of Molecular Medicine, John Radcliffe Hospital, Headington, Oxford OX3 9DU, UK.

![Fig. 1. The src kinase activity in fibroblasts exposed to hypoxia. Transformed embryonic murine fibroblasts, described in the Methods, were exposed to different oxygen tensions (21%, 1%, 0.1 %, and anoxia [0%]) for 30, 60, and 90 minutes. The results are expressed as the ratio of activity to that of normoxic cells cultured in parallel and are the means ± SE of 3 (30 and 90 minutes) or 5 (60 minutes) independent experiments. An increase in activity was seen when cells were exposed to severe hypoxia (0.1% oxygen or anoxia), but not 1% oxygen; significantly different from normoxic activity, *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/2/10.1182_blood.v89.2.503/4/m_bl_0014f1.jpeg?Expires=1769128209&Signature=BU~dC6mYDupRteyvZ~8oTWYMMBtBonY1SxuHtyKG0jzzd8GKoTjclmSsw6CIgqYRkDqk2jv80iX5EYCKH~meEXAAPT0R7SiP9hePMZofkCvfQxX1xi-PC74UtDy5-XmfL-f5YkH~NxbY219Ig39O0HOlJHm3pmIGln5KFKPwlNCCTV2GCE-tFpx6a-twTd4YjvmuospUtDnpNngGgawrCjQOClVqRdbcTMPMRTN9cL1dBxRhUrkv~aq5sU5aS-8H1fAV~7LM3838S8s3j2EnmBrl6kuOUQKqq-bqPGvK-7pO8eP2TzjPnhoeGf67wjLq~96c9IuBTgR45-MxvAc7DA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal