RECEPTORS FOR MANY cytokines and growth factors are triggered by the binding of their cognate ligands to assemble into an active multi-subunit complex that frequently comprises a homodimer of the ligand-binding subunit. This results in the activation of intracellular protein tyrosine kinase activities that result in the generation of intracellular signals. Two classes of receptors have been distinguished that act in this way: (1) the receptor tyrosine kinases (RTKs), which have an intrinsic tyrosine kinase activity, and (2) the cytokine receptor (CR) superfamily, which associate noncovalently with intracellular tyrosine kinases.
It has been known for some time that altered forms of RTKs exist that exhibit constitutive, ie, ligand-independent, activity and thus deliver proliferative signals in an uncontrolled manner. Such mutants are frequently oncogenic and have often been identified as the products of retroviral oncogenes; eg, v-erbB is an activated form of the epidermal growth factor receptor, and v-fms is an activated form of the receptor for colony-stimulating factor 1 (CSF-1). Constitutive activation can result from a range of different types of mutations, including point mutations to the intracellular, transmembrane, or extracellular regions; truncations; or fusion with other proteins.
Given the broad similarities in the mechanisms by which RTKs and CRs are triggered and deliver intracellular signals, it is not altogether surprising that mutations to CRs can also result in their constitutive activation and lead to oncogenicity. Nevertheless, there have been far fewer such mutations described to date than in the RTK family; this possibly reflects the lack of intrinsic tyrosine kinase activity of CRs. We will review here the nature and mechanism of action of mutations that have been shown to activate members of the CR family and will discuss the implications of such mutations for the function of normal CRs. We will also consider the potential of activating mutations in CRs to contribute to hematopoietic disorders.
STRUCTURE AND FUNCTION OF MEMBERS OF THE CR FAMILY
CR Structure
The CR family can be defined on a structural basis as comprising receptors that share a common architecture in their extracellular regions. This structure, composed of a repeated fibronectin-like barrel of seven β strands was first predicted by Bazan3 and has been confirmed and extended by solution of the three-dimensional structures of the human growth hormone (hGHR) and prolactin (PRLR) receptors.4 5 We will refer to this structure as a CR module (CRM) and each of two β barrel structures that comprise the CRM as a CR domain (CRD)6; the seven β-strands in the membrane-distal and -proximal CRDs are designated A to G and A′ to G′, respectively. Within the membrane-distal CRD are two pairs of disulfide-linked cysteine residues, whereas the membrane-proximal CRD of nearly all CRs contains a motif with the consensus Trp-Ser-Xaa-Trp-Ser — the so-called WSXWS box. This hallmark of the CR family, along with the CRM structure and lack of an intrinsic tyrosine kinase activity, comprise the three defining features of the family.
Subunit composition of CRs.For the purposes of this review, it is convenient to classify CRs according to their subunit composition. The simplest receptor complexes are homodimers in which two receptor molecules bind one molecule of their ligand. This group is exemplified by the hGH and prolactin receptors,4,5 the erythropoietin receptor (EPOR),7 and the granulocyte-CSF receptor (G-CSFR).8 In this group, each receptor subunit has both a ligand-binding and a signalling function, and as mentioned above, it is believed that ligand-induced dimerization triggers intracellular signalling. As we will discuss below, some of the evidence for this comes from studies of activating mutations.
A second group of CRs comprises two different subunits, one of which — the α subunit — functions primarily in ligand binding, while the other functions to increase the affinity of ligand binding and is also the primary signal transducer. The interleukin-6 (IL-6) receptor thus contains an α subunit (IL-6Rα) that binds IL-6 with low affinity and a β subunit — gp130 — that functions as a signalling subunit.9 The active IL-6R is in fact a higher-order complex comprising two molecules each of IL-6, IL-6Rα, and gp130,10 in which the role of IL-6 and IL-6Rα appears to be exclusively to induce gp130 dimerization.11 This is achieved in part by contacts between IL-6 and each of the two gp130 molecules.12 That the IL-6Rα subunit plays no role in signalling per se is shown by the finding that the cytoplasmic and transmembrane domains are dispensable for IL-6 signalling.9 A second example, which we will refer to extensively below, is that of the receptors for granulocyte-macrophage–CSF (GM-CSF ), IL-3, and IL-5. These three receptors (GMR, IL-3R, and IL-5R), which are discussed in detail by Miyajima et al,13 are each composed of unique ligand-specific α subunits and a shared β subunit (βc ), which appears to be the primary signalling subunit.14-16 (In the mouse, there is also a second, closely related β subunit (βIL-3 ) that functions only in conjunction with the IL-3 receptor α subunit [mIL-3Rα].17 ) It is still unclear whether the α subunits have a direct signalling role. Although their cytoplasmic segments are clearly required for the normal function of these receptors16,18 19 (S. Barry et al, manuscript in press), as yet no signalling molecules have been detected that associate with α subunits, and it appears that, under some circumstances, β subunits are sufficient for signalling (see below). For these receptors, the stoichiometry of the active complex has not been defined, although there is circumstantial evidence suggestive of β subunit dimerization (see below).
The third group of CRs that can be delineated share two characteristics: (1) the active receptor complexes consist of three different types of subunit and (2) one of these subunits is common to several different receptors. One clear example of this group is the IL-2R complex, which comprises a ligand-binding α subunit and two signalling subunits, β and γ (reviewed by Taniguchi and Minami20 ). In this case, the α subunit binds IL-2 with low-affinity, the αβγ complex binds with high affinity and the αβ and βγ complexes bind with intermediate affinity; only complexes containing β-γ heterodimers are capable of signalling. The γ subunit is shared with the receptors for IL-4, IL-7, IL-9, and IL-1521-23 and has been termed γ common (γc ). Another example of a shared signalling subunit is gp130, which, in addition to forming homodimers as part of the IL-6R, is a component of the receptors for leukemia inhibitory factor (LIF ), oncostatin M (OSM), IL-11, and ciliary neurotrophic factor (CNTF ); interestingly, in all of these cases, the LIF receptor is also a shared component.24-26 A further variation on the theme of shared receptor subunits is represented by the IL-4 receptor, of which there appears to be two forms: one containing an IL-4 binding subunit and γc , whereas the other lacks γc but may also contain an IL-13 receptor α subunit.27-29
Signalling by CRs — A Brief Summary
It is not intended here to review intracellular signalling by CRs; this subject has been covered in depth by others.30,31 However, it is important to reiterate that all available evidence suggests that triggering of tyrosine kinases associated with the cytoplasmic portions of the signalling subunits is the critical first step in CR signalling. Of particular importance are the JAK family of kinases,32,33 which are believed to interact with the membrane-proximal regions of CR signalling subunits. Ligand binding and active complex formation result in JAK phosphorylation and activation, probably via transphosphorylation by the JAK molecules associated with each signalling subunit. It is believed that this is the primary event in CR signalling; the activated JAK kinases then phosphorylate tyrosine residues on the receptor subunits and on other intracellular signalling molecules, including adaptors such as Shc as well as receptor-associated latent transcription factors (STATs34 ). The precise roles of the JAK kinases versus other receptor-associated or receptor-activated tyrosine kinases, such as members of the Src family,35 in phosphorylating various substrates is presently unclear. The spectrum of intracellular signal transduction pathways triggered by CRs is similar to that of pathways triggered by RTKs, as might be expected from the basic similarity in their mechanisms of activation and use of tyrosine phosphorylation as a primary signal. Again, delineation of these pathways and how they are activated by CRs is an intensive area of investigation and we will not deal with it further here. However, it is important to realize that the end result of triggering CRs can depend not only on the identity of the CR, but also on the cell type under study, because cells vary with respect to the types and levels of the various intracellular mediators of CR signalling. Thus, a wide range of responses can be elicited that includes cellular proliferation, differentiation, prevention of apoptosis, functional activation of mature cells, and, in some cases, inhibition of cellular functions or even apoptotic cell killing.
ACTIVATING MUTATIONS IN CR SUBUNITS
To date, these have been restricted to three such molecules: the thrombopoietin (TPO) receptor (Mpl or TPOR), the EPOR, and the common β subunit (βc ) of the receptors for GM-CSF, IL-3, and IL-5. In each case, the mutants have been identified as activating by virtue of their ability to induce ligand-independent survival and proliferation of factor-dependent hematopoietic cell lines. The implications of these mutations for normal CR function and their potential to contribute to disease will be considered below. However, we will first review what is known of activating mutations in Mpl, EPOR, and βc .
v-Mpl: A Truncated CR
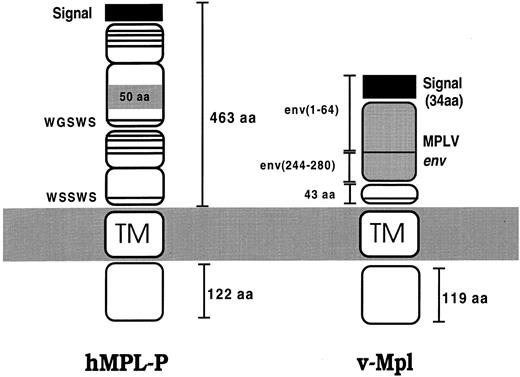
The v-mpl oncogene.The murine v-mpl oncogene encodes a constitutively activated CR that has been transduced by the murine myeloproliferative leukemia virus (MPLV). MPLV is a defective murine retrovirus generated during passage of a cloned Friend murine leukemia virus helper virus. In the presence of helper virus, infection of adult mice with MPLV induces an acute leukemia of short latency characterized by massive proliferation and differentiation of hematopoietic cells representing all the erythroid and myeloid lineages.36 v-Mpl is a 284 residue fusion protein in which the first 100 amino acids are derived from the rearranged Friend-murine leukemia virus (F-MuLV) env region and the other 184 amino acids are encoded by c-mpl.37 c-mpl is expressed predominantly in primitive hematopoietic cells, platelets, and megakaryocytes37-40 and, consistent with this expression pattern, has recently been shown to encode the receptor for TPO (also referred to as Mpl-ligand or megakaryocyte growth and development factor [MGDF ]).41-45
Transformation by v-mpl.Hematopoietic progenitor cells from mice infected with MPLV are growth factor-independent for both proliferation and terminal differentiation. Infection of mice with MPLV results in factor-independent formation of myeloid, erythroid, and megakaryocytic colonies.46,47 In addition, infection by MPLV of several growth factor-dependent hematopoietic cell lines can abrogate the requirement for growth factor.48 Recently, v-mpl has also been shown to transform early B cells and their precursors in vitro. The resulting cells could clone spontaneously in semisolid cultures in the absence of feeder layer or exogenous growth factors and rapidly produced tumors when injected into syngeneic mice.49
Mechanism of v-Mpl signalling.The MPLV-encoded env-mpl fusion gene is responsible for the biological properties of the retrovirus.50 The v-Mpl extracellular domain is composed mostly of F-MuLV env sequences, but with 43 amino acids encoded by c-mpl, whereas the cytoplasmic and transmembrane regions are entirely derived from c-Mpl (Fig 1). Thus, in v-Mpl most of the Mpl extracellular domain has been deleted, generating a truncated receptor. The env-mpl fusion protein most probably functions as a constitutively activated receptor, although the mechanism underlying the constitutive activity of this fusion protein is unclear. Several studies aimed at defining the role of the various fusion protein domains have been performed. Deletion of the WSXWS motif of the env-mpl protein did not abolish MPLV pathogenicity, suggesting that this motif does not have a critical involvement in v-Mpl transport, activation, or signalling.50 This is in contrast to studies on this motif of other CRs51-55 and suggests that, in the fusion protein, the normal role of this motif may be bypassed. Other studies have shown that the membrane-proximal 69 cytoplasmic amino acids are required for transmission of a proliferative signal by v-Mpl.56
Schematic illustration showing the major isoform of Mpl (hMpl-P)38 and the v-Mpl fusion product. Mpl has two CRMs, with the distal one containing an insertion of 50 residues. The v-Mpl product is derived from the MPLV retrovirus and is a fusion protein containing rearranged env sequences fused to truncated Mpl (see text). The conserved cysteine residues and WSXWS motif characteristic of the CR family are indicated by horizontal lines.
Schematic illustration showing the major isoform of Mpl (hMpl-P)38 and the v-Mpl fusion product. Mpl has two CRMs, with the distal one containing an insertion of 50 residues. The v-Mpl product is derived from the MPLV retrovirus and is a fusion protein containing rearranged env sequences fused to truncated Mpl (see text). The conserved cysteine residues and WSXWS motif characteristic of the CR family are indicated by horizontal lines.
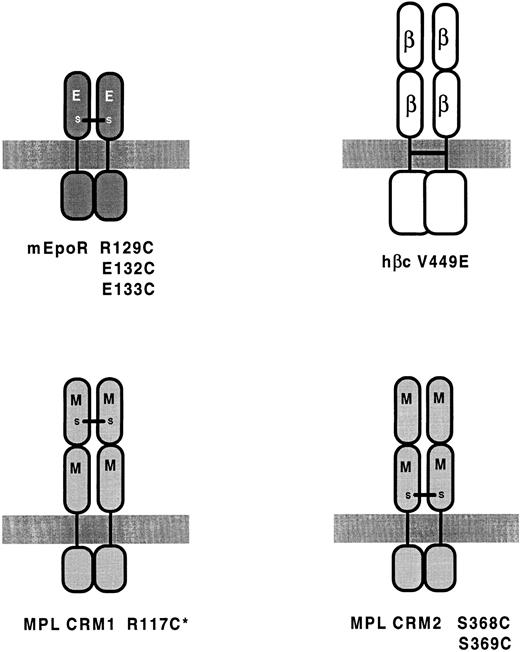
Homodimerization of Mpl induces constitutive activation.Although the mechanism of activation of the v-mpl product has not been determined, it has been shown that Mpl can be activated by cysteine substitutions in a predicted dimer interface domain. Similar substitutions in the corresponding region of EPOR also lead to EPO-independent proliferation (see below). Alexander et al57 used the GH/GHR complex as a prototype for CR complexes and targeted the region of Mpl that aligns with the dimer-interface of each GHR subunit. In GHR, this region is involved in direct inter-subunit interactions that stabilize the receptor dimer.4 Substitution of cysteine for residues in the predicted dimer interface domain in either of the two Mpl CRMs constitutively activated the receptor, presumably through the formation of disulphide-bonded homodimers (Fig 2). Consistent with this, biochemical studies with cells expressing Mpl mutants showed a high molecular weight Mpl-containing complex of dimeric size that reduced to monomeric size on treatment with reducing agent. That Mpl can be activated by homodimerization is consistent with previous studies with G-CSFR-Mpl chimeras, which indicated that homodimerization of the Mpl intracellular domain leads to activation.58 59 Although cysteine substitutions in either CRM lead to activation, cysteine substitution in the N-terminal CRM (Fig 2) led to substantially weaker TPO-independent activity (which was not a result of poorer expression). The investigators suggested that this may be indicative of a more significant contribution by the membrane proximal dimer interface region to Mpl dimerization.
These results suggest that one normal mechanism of Mpl activation in response to ligand involves ligand-induced dimerization mediated, at least in part, by the conserved CR dimer interface domain. Similar experiments indicate that this region, residing within or adjacent to the loop between A′ and B′ β-strands, also appears to function in dimer formation in EPOR (see below). Whether this region mediates receptor-receptor interactions in other cytokine family members is still to be determined. In particular, it will be important to determine which regions are involved in interactions between the subunits of heterodimeric or heterotrimeric receptors.
An oncogenic variant of MPLV (DEL3MPLV) has been described that also appears to be activated by disulphide-linked homodimerization.48 The extracellular portion of the Del3Mpl protein is composed of the first 12 N-terminal amino acids of the env signal peptide fused to the 25 membrane proximal residues of Mpl. This variant of MPLV is as oncogenic as wild-type MPLV. When cysteine 3 of the Del3Mpl protein is mutated to glycine, constitutive activity is lost. Under nonreducing conditions, a high molecular weight band corresponding to a homodimer could be observed, strongly suggesting that this variant of v-Mpl is activated through disulphide-linked homodimerization. It should be noted that the mature env-mpl fusion from wild-type MPLV is devoid of cysteine residues and therefore is probably not activated via this mechanism. The role of the env sequences in activation of v-Mpl is still unclear, but should be resolved by the construction and testing of a truncated version of Mpl lacking all but the membrane-proximal 43 amino acids.
Activating Mutations in the EPOR
Mutations leading to constitutive dimerization.A constitutively active form of the murine EPOR (mEPOR) containing an arginine-to-cysteine substitution at position 129 was isolated as a spontaneous mutant after retroviral transduction and infection of BAF-B03 cells.60 The presence of cysteine at this position and not the loss of the arginine residue was shown to be critical for activation, because the substitution of Glu, Pro, or Ser generated receptors that behaved like wild-type mEPOR.61 This R129C form of EPOR forms disulphide-linked homodimers in the absence of EPO, suggesting that EPOR can be activated by forced dimerization.
R129 of mEPOR is located in the membrane-proximal domain (Fig 2) in a region predicted to correspond to the dimer-interface domain in the GHR homodimer.62 Several other residues within the vicinity of R129 are also within this potential dimer-interface region and some of these have also been targeted for cysteine substitution. Watowich et al62 mutated residues R130, A131, E132, and E133 to alanine or cysteine and tested the resultant mutants for EPO-dependent and EPO-independent activity. Two of these substitutions (E132C and E133C; Fig 2) resulted in ligand-independent proliferation of BAF-B03 and 32D cells, and, like R129C, these two mutants formed disulphide-linked dimers. The other substitutions had no affect on the ability of the receptor to mediate EPO-dependent growth; however, they did not induce constitutive activation or lead to disulphide-linked homodimerization. Thus, disulphide-linked dimerization correlates with constitutive activity, implicating ligand-induced dimerization in receptor activation and signal transduction.
Experiments using truncated forms of the mEPOR in which all or part of the cytoplasmic domain is deleted are consistent with this concept. Truncated forms of mEPOR specifically inhibit EPO-dependent growth of factor-dependent hematopoietic cell lines expressing wild-type mEPO-R.62,63 The dominant negative inhibitory effect of such mutants is also suggestive of ligand-induced homodimerization as a key event in signalling, although these results would also be consistent with a second receptor subunit forming part of the active complex. It has not been possible, using biochemical approaches, to identify ligand-induced dimers of EPOR, possibly due to the low numbers of receptors expressed on the cell surface.62 Cross-linking of EPO bound to a variety of EPOR-expressing cell lines detected several species of different molecular sizes (reviewed by Krantz64 ). Two of the cross-linked polypeptides did not cross-react with EPO-R antiserum, suggesting the possibility that other receptor subunits are part of the ligand activated complex.65 Studies with chimeric receptors, containing the extracellular portion of EPOR, suggested the possibility that other subunits interact with the extracellular domain of EPOR and specify the EPO-specific pattern of protein phosphorylation and gene expression.66 67
Recently, a series of small, related peptide agonists of hEPOR were isolated by screening a random phage display peptide library.68 The structure of one such cyclic peptide with the extracellular domain of EPOR has been determined, showing a peptide dimer bound to a dimer of EPOR.69 The peptide sequence is unrelated to EPO and the arrangement of the EPOR subunits to each other is different to that observed for the two GHR subunits in the GH/GHR complex,4 suggesting that different modes of dimerisation may be capable of receptor activation.
Activation of EPOR by Friend spleen focus-forming glycoprotein (gp55).The replication-defective spleen focus-forming virus (SFFV) contained in the Friend leukemia virus complex encodes a 55-kD glycoprotein (gp55) that is responsible for a rapid and fatal erythroleukemia in infected adult mice.70 SFFV gp55 binds to normal EPOR in the absence of EPO, resulting in constitutive activation and the first stage of Friend erythroleukemia.71,72 The mechanism underlying gp55 activation of EPOR is poorly understood. gp55 bears no amino acid homology to EPO and both the extracellular and transmembrane domains appear to be important for activation of EPOR.73-80 Transport of gp55 to the cell surface is very inefficient and greater than 95% of gp55 is retained in the endoplasmic reticulum, where it interacts with EPOR.81 The presence of this complex within the ER raises the question of whether signalling occurs from the intacellular complex. However, cross-linked complexes of EPOR-gp55 have been detected at the cell suface82; in any case, activated receptors may be internalized very rapidly, making detection of cell surface receptor complexes difficult.
Carboxy-terminal negative regulatory domain of EPOR.EPOR mutants that are truncated at the C-terminus are hypersensitive to EPO. One such mutant was recovered from a subclone that arose after infection of BAF-B03 cells with a retrovirus containing wild-type murine EPOR cDNA.60 This cell line showed increased sensitivity to EPO, with maximal growth occurring at one-tenth of the concentration of EPO required to induce similar growth of cells expressing wild-type EPOR. The EPOR recovered from this cDNA was missing 42 residues at the C-terminus that were replaced by an alanine and a leucine residue. Other C-terminal truncations also lead to hypersensitivity to EPO without affecting receptor surface expression, affinity for EPO, or receptor processing.83 These truncations suggest the presence of a negative regulatory domain (NRD) between residues 196 and 236 of EPOR. This region contains 9 serine residues and 4 tyrosine residues, suggesting that phosphorylation may normally downregulate EPOR responsiveness. This C-terminal segment of EPOR contains a binding site for hematopoietic cell phosphatase (HCP or PTP1C),84,85 suggesting that dephosphorylation events mediated by an associated phosphatase may be involved in the downregulation of EPOR activity. C-terminal truncations also enhance the growth factor response for EGF-R86 and CSF-1 receptor,87 possibly through similar mechanisms. There are now several reports linking C-terminal truncation of the hEPOR to primary familial polycythemia (discussed below).
Summary of Activating Mutations in βc
| Mutant . | Receptor/Domain Affected . | Comment . | Reference . |
|---|---|---|---|
| V449E | hβc Transmembrane domain | Likely to mediate homodimerization | 89 |
| FIΔ | hβc Extracellular domain 4 | 37 aa duplication | 88, 89 |
| HSV | hβc Extracellular domain 4 | Insertion of 11 amino acids | Jones et al (manuscript submitted) |
| Truncation mutants ΔQP, ΔH | hβc Extracellular domain 4 | Truncations removing domains 1, 2, and 3 | 91 |
| I374N, also I374D, I374Q, I374F | hβc Extracellular domain 4 | Point mutation in strand C′ | 89, 90 |
| W358N, L356N | hβc Extracellular domain 4 | Point mutations in strand B′ | 90 |
| Rearranged mβc gene | mβc Extracellular domain | May induce disulfide-linked dimerization | 92 |
| Mutant . | Receptor/Domain Affected . | Comment . | Reference . |
|---|---|---|---|
| V449E | hβc Transmembrane domain | Likely to mediate homodimerization | 89 |
| FIΔ | hβc Extracellular domain 4 | 37 aa duplication | 88, 89 |
| HSV | hβc Extracellular domain 4 | Insertion of 11 amino acids | Jones et al (manuscript submitted) |
| Truncation mutants ΔQP, ΔH | hβc Extracellular domain 4 | Truncations removing domains 1, 2, and 3 | 91 |
| I374N, also I374D, I374Q, I374F | hβc Extracellular domain 4 | Point mutation in strand C′ | 89, 90 |
| W358N, L356N | hβc Extracellular domain 4 | Point mutations in strand B′ | 90 |
| Rearranged mβc gene | mβc Extracellular domain | May induce disulfide-linked dimerization | 92 |
Activating Mutations in βc
Types of activating mutations.The first activating mutation reported in this molecule was a 37 amino acid duplication in the extracellular, membrane-proximal CRD, which occurred spontaneously during retroviral transfer of an hβc cDNA.88 This mutant, termed FIΔ, was identified by virtue of its ability to render murine myeloid FDC-P1 cells factor-independent. Subsequently, several other hβc mutations in or affecting this domain — domain 4 — have been identified as a result of either random mutagenesis or site-directed mutagenesis. Random mutagenesis identified an isoleucine residue (I374) in β-strand C′ as a target for activating mutations89 and, subsequently, other activating substitutions within domain 4 have been identified90; interestingly, all of these are in (predicted) β-strands B′ or C′ (see below and Table 1). It has also been shown that truncation of the extracellular region of hβc can result in activation; for example, a truncation equivalent to that which generated v-Mpl, ie, which leaves only part of domain 4, was shown to allow factor-independent proliferation of FDC-P1 cells.91 However, the critical event appears to be the loss of domain 3, because a truncation that retained all of domain 4, but removed domains 1, 2, and 3, was also activating, whereas removal of only domains 1 and 2 was insufficient to promote factor-independent proliferation.91 Some sequences within domain 4 are required for factor-independent activity, because the most severe truncation tested, which left only 7 residues of domain 4, was inactive. Although an activated truncation mutant of murine βc that lacked all of the normal extracellular domains has been described by Hannemann et al,92 this does not appear to be acting in the same way as the hβc truncations (see also below).
Random mutagenesis also resulted in the isolation of an activating mutation that affects the transmembrane domain of hβc .89 This mutation (V449E) results in the replacement of a valine residue with glutamic acid; as such, it is highly reminiscent of the oncogenic mutation in the neu proto-oncogene that results in a valine to glutamic acid substitution in the transmembrane domain of the Neu/ErbB2 RTK.93 Mutations corresponding to V449E in both murine β subunits have also been constructed and shown to induce factor-independent proliferation of FDC-P1 cells (M. McCormack and T. Gonda, unpublished observations). For reasons outlined below, we believe these mutations fall into a class that may be functionally distinct from the extracellular mutants. The origins and properties of all of the βc mutants discussed in this review are summarized in Table 1.
Mechanism of action of activating mutations.As discussed above, it is more than likely, by analogy with other receptors, that triggering of the normal GM-CSF/IL-3/IL-5 receptors involves dimerization of signalling subunits and activation of intracellular tyrosine kinases. Thus, it is probable, a priori, that activating mutations in βc also result in formation of a complex containing dimerized signalling subunits. This notion fits very easily with the transmembrane mutant V449E (Fig 2) (and its murine homologues), because there is strong evidence that the analogous transmembrane mutation in the Neu/ErbB2 RTK93 induces constitutive receptor dimerization.94,95 This is supported by the existence of activating transmembrane mutants in other RTKs (eg the fibroblast growth factor receptor96 ). It has been suggested that the presence of the hydrophilic residue within the hydrophobic membrane environment allows strong hydrogen bonding between the transmembrane segments94 or induces a conformational change that favors dimer formation.97 Furthermore, the truncated form of murine βc reported by Hannemann et al,92 encoded by a rearranged mβc gene, is also predicted to dimerize. The rearrangement results in a short (34 amino acid), aberrant extracellular domain containing a cysteine residue, which the investigators hypothesized induces the formation of a disulphide-linked mβc dimer.
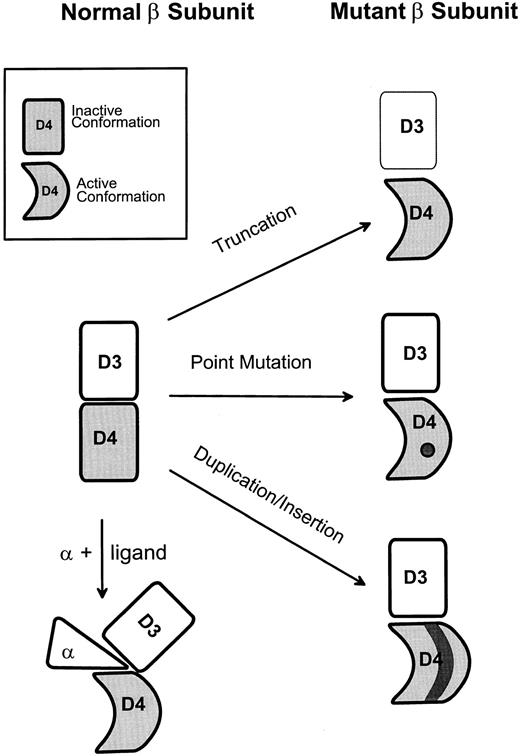
Forced dimerization can also be proposed as a mechanism of activation for the extracellular mutants, ie, these mutations might alter the conformation of the extracellular domain in such a way as to allow constitutive formation of an active receptor complex. In this scenario, the mutations would directly or indirectly affect a structure within the β subunit that normally interacts with another component of the active complex; whether this involves β subunit homodimerization will be considered below. In any case, such a model, illustrated in Fig 3, allows us to rationalize the effects of the various activating mutations as follows. The structural disturbance to domain 4 caused by the insertion of an extra (duplicated) 37 amino acids in the FIΔ mutant may release the β subunit dimerization surface from its normal or inactive conformation. This notion is supported by the fact that, in the HSV mutant (Table 1), insertion of an arbitrary sequence instead of the second copy of the duplication also results in constitutive activation (Jones et al, manuscript submitted). The ability of truncations to activate hβc (and Mpl) may reflect a masking or constraining role for domain 3 in the inactive form of the wild-type β subunit, which would normally be relieved by ligand/α subunit binding. Recent work90 has suggested a related mechanism by which point mutants in the extracellular region (Table 1) are able to confer factor independence and has indicated some of the residues in domain 4 that may be involved. All of the activating extracellular point mutations identified to date affect hydrophobic residues predicted to lie in β-strands B′ or C′; moreover, molecular modelling (Jenkins et al90 and C. Bagley and A. Lopez, manuscript in preparation) predicts that residues in strand B′ normally interact with those in strand C′. Thus, it was suggested that disruption of hydrophobic interactions between strands B′ and C′ may also lead to activation by mimicking the conformational change in domain 4 that is normally induced by ligand and/or α subunit, again allowing domain 4 to assume an active conformation (Fig 3).
A model for activation of βc involving a conformational switch in domain 4. The model postulates that domain 4 can exist in inactive or active conformations and that the active conformation leads to receptor signalling (eg, by allowing association of βc with a second signalling subunit). See text for further explanation.
A model for activation of βc involving a conformational switch in domain 4. The model postulates that domain 4 can exist in inactive or active conformations and that the active conformation leads to receptor signalling (eg, by allowing association of βc with a second signalling subunit). See text for further explanation.
Cell type-specific activity of hβc mutants.A surprising observation that must also be taken into account in any model that attempts to explain the action of the hβc mutants is that they differ in their abilities to signal in a range of factor-dependent cell lines.89,91 Because introduction of the normal human GM-CSF/IL-3/IL-5 receptors into several different cytokine-dependent murine cell lines allows ligand-dependent survival and proliferation (eg, Kitamura et al14 and Sakamaki et al16 ), it might have been expected that constitutively activated forms of hβc would do the same. However, neither the V449E transmembrane mutant nor the I374N or FIΔ extracellular mutants conferred factor-independence on the T-cell line CTLL-2, even though they could function as wild-type β subunits in these cells.89 Moreover, V449E was able to induce factor-independence in BAF-B03 pro-B cells, but none of the extracellular mutants, including point mutants,89,90 FIΔ,89 and the truncation mutants,91 were able to do so. There is a precedent for the first of these findings as a chimeric receptor containing the extracellular domain of the EPOR and the cytoplasmic domain of murine βIL-3 allowed EPO-dependent proliferation in BAF-B03 cells but not in CTLL-2 cells.98 This molecule, like V449E, is also predicted to form a dimer (in the presence of EPO), implying that enforced dimerization (see below) of the intracellular domains of the β subunit may sufficient for activity in BAF-B03 (and FDC-P1) cells but not CTLL-2 cells.
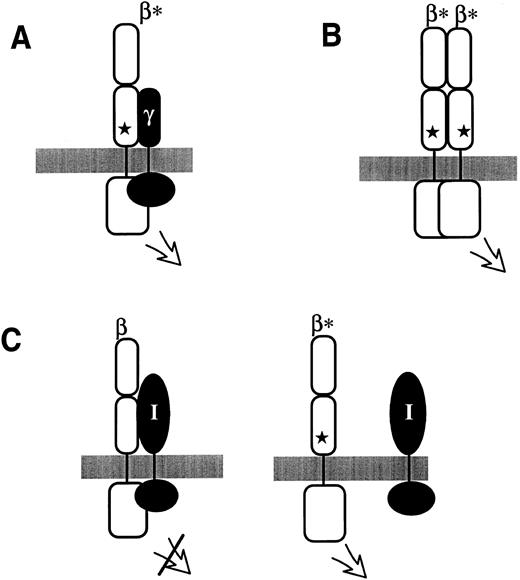
Possible modes by which extracellular mutations in hβc , indicated by a star in each panel, lead to constitutive receptor activity. (A) The extracellular mutation induces constitutive heterodimerization with a putative γ signalling subunit. (B) The extracellular mutation leads to hβc homodimerization. (C) In this model, hβc normally associates with an inhibitory membrane-spanning molecule (I); the extracellular mutations disrupt this association and thus lead to activation. For simplicity, only a single β subunit is shown; however, the inhibitory molecule might normally function in suppressing the activity of a low level of spontaneously forming homodimers or heterodimers.
Possible modes by which extracellular mutations in hβc , indicated by a star in each panel, lead to constitutive receptor activity. (A) The extracellular mutation induces constitutive heterodimerization with a putative γ signalling subunit. (B) The extracellular mutation leads to hβc homodimerization. (C) In this model, hβc normally associates with an inhibitory membrane-spanning molecule (I); the extracellular mutations disrupt this association and thus lead to activation. For simplicity, only a single β subunit is shown; however, the inhibitory molecule might normally function in suppressing the activity of a low level of spontaneously forming homodimers or heterodimers.
We have previously proposed a model which could account for these observations, based on the general notion that the observed differences in activity between cell types reflect the presence or absence of hβc -associated signalling molecules in the various cell types.89 It was suggested that the ability of β subunits (both wild-type and mutant) to function in CTLL-2 cells in the presence of ligand and α subunit reflects the presence of a molecule (X) that associates with the α subunit and is necessary for the function of the receptor complex in these cells. Cell types that normally respond to GM-CSF or IL-3, but not CTLL-2 cells, would contain a molecule (Y), with an overlapping function to X but which associates with the β subunit rather than the α subunit. Thus, β subunit dimers could associate with molecule Y and therefore signal only in cells normally responsive to IL-3 or GM-CSF.
Do extracellular and transmembrane mutants act in the same way?Three alternatives can be envisaged to account for the difference in activity between V449E and the extracellular mutants in BAF-B03 cells (Fig 4): (A) the extracellular mutants may form heterodimers, rather than βc homodimers, with a putative alternate signalling subunit; (B) the difference in activity reflects a quantitative difference between the mutants but that both types constitutively form homodimers; or (C) the extracellular mutations prevent association with a cell-type–specific inhibitory molecule.
The first alternative (Fig 4A) proposes the existence of a third, membrane-spanning signalling subunit, which we termed a γ subunit (by analogy with the IL-2 receptor) and which would be restricted to myeloid cells.89 This would explain why the extracellular mutants, which are postulated to constitutively form βγ dimers, function in FDC-P1 cells and primary murine myeloid cells (M. McCormack and T. Gonda, unpublished observations) but not in lymphoid cells. Although there is presently no direct evidence for such a molecule, there are precedents for one CR signalling subunit associating with alternative partner signalling subunits; in receptor complexes containing gp130, for example, gp130 can either form homodimers (in the IL-6 receptor) or heterodimers with the LIF receptor (as discussed above).
The second possibility (Fig 4B) is that the difference between the mutants is a quantitative phenomenon whereby the signal generated by the extracellular mutant βc homodimers is weaker than that generated by V449E homodimers and that this weaker signal exceeds a threshold required to induce proliferation in FDC-P1 cells but not that for BAF-B03 cells. Different thresholds could reflect qualitative or quantitative variation in receptor-linked signalling molecules, for which again there is a precedent.99 It is more difficult to explain why V449E (and the EPOR-βIL-3 chimeras) should generate a stronger intracellular signal than the extracellular mutants, but one could speculate that the latter may not form functional dimers as efficiently.
Finally, one should consider the possibility that the extracellular mutations reduce the association of an inhibitory molecule that normally blocks receptor activation (Fig 4C). One candidate for such a molecule would be a transmembrane tyrosine phosphatase that would downregulate receptor signalling. A precedent for activation resulting from loss of phosphatase activity exists in the activating D814Y mutation in Kit,100 which leads to the ubiquitin-mediated degradation of the protein tyrosine phosphatase Shp1(PTP1C) (X.-H. Piao and A. Bernstein, personal communication, August 1996). However, at this point in time, we do not favor this model, because it is rather difficult to reconcile with the lack of activity in BAF-B03 cells. One could speculate, though, that cell-type–specificity could result from the presence of different phosphatases in BAF-B03 cells that can act on hβc even in the presence of the mutations.
Currently available data do not allow us to distinguish between these models (or to rule out all others). However, preliminary results suggest the involvement of a positively acting factor, because introduction of a cDNA expression library from FDC-P1 cells into BAF-B03 cells expressing the I374N mutant can complement the latter mutation and confer factor-independence in these cells (B. Jenkins and T. Gonda, unpublished data). Ultimately, discrimination between these three possibilities will require biochemical analysis of β subunit dimerization and/or identification of novel receptor-associated molecules.
Implications for normal receptor function.One outcome of studies of activated βc mutants is the clear implication that enforced β subunit dimerization can result, in some cells at least, in the generation of proliferative signals and thus that hβc dimers may be a component of functional GM-CSF/IL-3/IL-5 receptors. This reinforces the importance of signalling subunit dimerization as a central event in signalling by a wide range of CRs. However, these results need to be considered together with recent work by Muto et al101 that has shown that dimers of normal hβc molecules can be detected by cross-linking or coimmunoprecipitation even in the absence of ligand. The cross-linked dimers, as well as non–cross-linked monomers, underwent tyrosine phosphorylation only in the presence of ligand and α subunit, implying that β subunit dimerization is not sufficient for receptor activation. At first glance, these observations appear inconsistent with the interpretation that the V449E mutation induces hβc dimerization89 and with the ability of ligand-induced dimers of chimeric receptors, containing only β subunit cytoplasmic domains, to transduce proliferative signals.102-104 However, it may be that the physical proximity of two βc molecules is necessary but not sufficient for activation. One might suggest that βc dimers must also adopt a specific conformation to generate an intracellular signal; this could perhaps bring the two cytoplasmic domains and their associated JAK kinases close enough to result in transphosphorylation, βc phosphorylation, and activation.
The notion that a conformational change is required to activate βc is also consistent with the fact that a range of different extracellular mutations in domain 4 can activate hβc (Table 1). As discussed above, the simplest way to interpret these mutations is that domain 4 acts as a conformational switch and that, normally, in the absence of ligand it is constrained in an inactive conformation. The active conformation is presumably induced by ligand plus α subunit or, we suggest, by the activating extracellular mutations (Fig 3). In the GHR, a conformational change involving the F′-G′ loop has been implicated in proliferative signalling (S. Rowlinson and M. Waters, personal communication, September 1996). It is notable that I374 of hβc is predicted to interact directly with residues in strand F′,90 suggesting that mutations at this residue could mimic a conformational change induced by ligand contacting the corresponding loop of domain 4. It is becoming clear that there are crucial contacts between ligand and hβc within domain 4, including the F′-G′ loop,105-107 suggesting that ligand binding may be directly affecting this conformational switch. The requirement for the α subunit, and in particular its cytoplasmic segment16,18 19 (S. Barry et al, manuscript in press), could be interpreted as implying a role for this molecule in achieving or stabilizing an active conformation.
A further implication of the likely presence of β subunit homodimers or, possibly, βγ heterodimers in the active complex and of the ability of extracellular mutations to activate βc is that βc possesses an interactive surface that mediates its association with a second signalling subunit. The location of this structure cannot be predicted from the GHR structure, because the dimer interface in the latter almost certainly corresponds to the α-β interface in the GMR.4,108 However, we would predict that this structure lies within the 45 membrane-proximal residues of domain 4, because these are necessary for the activity of the hβc truncations.91 Interestingly, these residues also include those duplicated in the FIΔ mutant88 and those remaining in the v-mpl product.37
There are also a number of interesting implications that derive from the likely existence of cell-type–specific signalling molecules, which we have inferred from the cell-type–specificity of the activating mutations. First, if model (A) (Fig 4) is correct, ie, if there is an as yet unidentified alternate signalling subunit (a γ subunit) that can interact with βc , it is possible that different signals are elicited by different forms of the normal receptor, eg, by an (αβ)2 form compared to an αβγ or βγ form. This notion is supported by the observation that the types of factor-independent cells generated by introduction of the each class of mutants into primary cells differ substantially (M. McCormack and T. Gonda, unpublished observations). Alternatively, it is conceivable that a normal βγ complex might be a component of a receptor for ligands other than GM-CSF, IL-3, or IL-5 in the same way that gp130 homodimers are found in the IL-6 receptor while gp130 complexes with the LIF receptor α in the receptors for LIF, IL-11, OSM, and CNTF.24-26 Although the phenotype of mice lacking mβ109,110c argues against this latter possibility, it is conceivable that mβIL-3 could also interact with the putative γ subunit and thus mask any phenotype due to an additional ligand.
Implications for the development of therapeutic agents.Some of the ideas discussed above may have ramifications for the development of therapeutic agents that target hβc or intracellular signals generated by hβc . First, the existence of cell-type–specific signalling molecules, such as the putative γ subunit and/or the accessory molecule required for βc homodimer function, raises the possibility of interfering with receptor activity in a restricted range of cell types. Given the commonality of most receptor-activated signal transduction pathways,111 this selectivity may have significant therapeutic implications. Secondly, the identification of the region of hβc that interacts with another signalling subunit (a second β subunit or a γ subunit) may also represent a novel target for potential therapeutics that could interfere with receptor activation. Agents with these activities could potentially be identified by mass screening of low molecular weight compounds for inhibition of any biochemical activity associated with such molecules (such as protein kinase activity) or by identifying peptides, eg, using the phage display technique, capable of binding to and interfering with an hβc dimerization interface. The general feasibility of the latter approach has been borne out by the finding that an IL-6Rα peptide can inhibit IL-6R signalling112 and by the recent isolation (using phage display) of peptides that bind to and activate the EPOR.68 69
It may also be possible to mimic receptor activation, ie, to generate novel receptor agonists, through agents that act outside the cell, as we have discussed elsewhere (Jones et al, manuscript submitted). If the notion that domain 4 of hβc undergoes a conformational switch during receptor activation is correct (Fig 3), the possibility is raised that peptides or other small molecules may be able to bind to critical regions of domain 4 and induce the same conformational change. Again, the recent identification of a peptide EPOR agonist68 69 supports the possibility that this may be achievable. Given the structural and functional conservation within the CR family, successful isolation of such novel agents that act on any one receptor may well establish principles that are applicable to several others.
ACTIVATING MUTATIONS IN CRs — COMMON THEMES
Even with the limited number of CRs for which activating mutations have been identified, it is possible to discern two major themes. The first is the importance of signalling subunit dimerization as the underlying mechanism in most if not all cases, which strengthens the parallels between the CRs and the RTKs. (This will probably remain true even though the mechanism by which extracellular mutations activate hβc is presently unclear, because most alternatives [see above] would require either homodimerization or heterodimerization.) The clearest evidence comes from the disulphide-linked dimers formed by mutants of Mpl and EPOR, and is supported in the case of hβc by the likely constitutive formation of subunit homodimers by the transmembrane mutant V449E (Fig 2). These data also strengthen the case for dimerization being the key event in triggering normal CR signalling.
The second emerging theme, represented by the v-Mpl oncoprotein and certain mutants of hβc , is that of activation by truncation of the extracellular domain. Precisely how these mutations activate the respective receptors has not been fully elucidated, but it is reasonable to suppose that truncation relieves constraints on receptor structure imposed by one or more of the membrane-distal CRDs. We would suggest that this indicates a general mechanism by which ligand binding may act to trigger signalling by normal receptor. Again, it is interesting to note that similar mutations can activate RTKs (eg, v-Kit and v-ErbB), although the mechanisms by which these act are still somewhat unclear.
One important additional inference that may be drawn is that it is likely that activating mutations could be generated and/or occur naturally in other members of the CR family. Given the high degree of structural conservation within this family and the strong conservation of some of the residues that are targets for activating mutations (Table 1 and Jenkins et al90 ), it will be interesting to engineer mutations analogous to those described here.
ONCOGENIC POTENTIAL OF CRs AND EVIDENCE FOR INVOLVEMENT IN HEMATOPOIETIC DISORDERS
The oncogenic potential of constitutively active CR mutants has been clearly shown in the case of Mpl, EPOR and hβc . Mpl was initially isolated as a potently transforming oncogene (v-mpl ) carried by the defective murine myeloproliferative leukemia virus, MPLV (see above). Infection of mice with MPLV in the presence of helper virus induces a lethal myeloproliferative disease characterized by rapid hepatosplenomegaly, erythroblastosis, granulocytosis, thrombocytosis, and polycythemia. Hematopoietic organs show massive infiltration with maturing cells of the erythroid, myeloid, and megakaryocyte lineages.36 The disease is accompanied by the rapid appearance of factor-independent hematopoietic progenitors from several lineages.46,47 FDC-P1 cells expressing another activated Mpl receptor (S368C) have also been shown to be highly tumorigenic in transplanted mice.57 Although these studies indicate that activated Mpl is highly tumorigenic in these assays, it is still unclear whether rearrangement or amplification of c-mpl, the cellular gene encoding Mpl, is involved in the pathogenesis of leukemias. In each of two studies, increased expression of c-mpl was detected in about 50% of patients with AML, but not in patients with myeloproliferative disorders or acute lymphoid malignancies.113 114 Whether this reflects an alteration of the c-mpl gene or an expansion of a cell population that normally expresses c-mpl is unclear.
When the activated EPOR mutant, R129C, is expressed in the nontumorigenic IL-3–dependent cell line BAF-B03, they become factor-independent and form local tumors after injection into syngeneic mice.60 This oncogenicity is also reflected in a mouse model. Growth factor-independent cell lines derived from spleens of mice infected with a recombinant SFFV-derived vector encoding EPOR-R129C lead to the rapid onset of erythroleukemia when transplanted into uninfected mice.115 There is also considerable evidence showing overexpression of the EPOR gene in erythroleukemia cell lines. Insertional activation of the EPOR gene by the SFFV LTR has been observed in two murine Friend erythroleukemia cell lines, T3CL-2116,117 and FCL1.118 The viral gp55 glycoprotein produced from the Friend SFFV is responsible for the constitutive activation of EPOR in these cell lines72; however, it would appear that overexpression of EPOR provides some additional growth advantage leading to development of erythroleukemia. A rearrangement leads to overexpression of an abnormal form of EPOR in the human erythroleukemia cell line TF-1119 and EPOR gene amplification and rearrangement has been observed in another human erythroleukemia cell line, UT-7.118 Despite these documented events in the above cell lines, to date the onset of human erythroleukemia has not been shown to be related to alterations of chromosome 19 where the EPOR gene is located.119,120 A screening of 23 Friend virus-induced murine tumors failed to find any EPOR rearrangements118 and a screening of 10 cases of human erythroleukemia also did not find any acquired mutations in the EPOR gene in leukemic cells.121 Although it has proven difficult to link EPOR mutations to primary leukemias, recent studies have linked mutations affecting the C-terminus of EPOR to primary polycythemias (discussed below).
Several of the activated forms of hβc (FIΔ, I374N, and V449E) have been shown to confer tumorigenicity on the normally nontumorigenic murine myeloid cell line, FDC-P1,88,89 showing their oncogenic capacity. These mutants can also induce factor-independent proliferation of primary hematopoietic cells representing several lineages and promote the generation of tumorigenic cell lines from primary cells (M. McCormack and T. Gonda, unpublished observations). Thus, the possibility is raised that similar mutations in hβc may contribute to myeloproliferative disorders, myeloid leukemias, or other malignancies. A survey that has examined the hβc gene in human leukemias showed no evidence for rearrangements122; however, the study was performed by Southern blotting of genomic DNA and would not have detected point mutations similar to those described in this review. A second survey123 looked for hβc point mutations in AML patients but, again, failed to find any that were not due to simple polymorphisms. However, these investigators examined only the cytoplasmic portion of hβc and thus would have missed any mutations similar to those discussed above, which are all in the transmembrane or extracellular domains. The finding that truncation from the N-terminus can activate hβc91 suggests the possibility that gene rearrangements could activate hβc . However, the requirement for a functional N-terminal signal sequence means that formation of an activated receptor would require an internal deletion removing domain 3 and leaving the N-terminus of hβc intact and fused in frame to domain 4. Alternatively, activation by translocation would require a fusion of domain 4 sequences to the the N-terminal portion of another transmembrane or secretory protein. Such gene rearrangements have not been identified and would probably be very rare events.
Role of CR mutations in primary polycythemias.The term polycythemia is used to describe an increase in the number of circulating red blood cells. Primary polycythemias are due to a defect intrinsic to hematopoietic progenitor and stem cells, whereas secondary polycythemias are driven by extrinsic factors such as increased levels of EPO.124 In primary polycythemias, there is an alteration in the regulation of erythroid progenitors such that they become factor-independent or exhibit enhanced response to growth factors. The classical example of primary polycythemia is polycythemia vera (PV), which is a clonal, malignant, myeloproliferative disorder of the pluripotent stem cell leading to abnormalities of several hematopoietic lineages.125 In 1% to 13% of cases of PV there is a progression to acute leukemia,126 with the phenotype of the leukemic cells usually being myeloid.127 The molecular basis of this disorder is still not well understood; however, we summarize below several studies that suggest a role for CR mutations in this family of diseases.
Studies of EPOR in families with primary autosomal dominant polycythemia suggest that mutations affecting the C-terminal NRD may have a role in these disorders. This group of diseases differs from PV in that there is no observed abnormality in leukocytes or platelets and there have been no reports of leukemic transformation. The clinical condition is mild and patients remain largely asymptomatic. It is a reasonable prediction that altered EPOR may be responsible for this disorder, at least in some families, and there is now good evidence for this. Unique polymorphisms affecting the C-terminus of EPOR have been shown to cosegregate with primary familial polycythemia in several families. In one case, an insertion of a G-residue leads to a change of frame, truncating the receptor 64 residues before the normal C-terminus.128 In a second report, a G to A transition introduces a stop codon and truncates the last 70 amino acids from the receptor.129 Thus, there is a striking similarity between these mutations and the truncation of 40 C-terminal residues from murine EPOR that leads to EPO hypersensitivity (discussed above), suggesting removal of a negative regulatory domain may be the molecular basis for development of polycythemia in these families.
A phenotype resembling PV was generated when a recombinant retrovirus (derived from SFFV) carrying the R129C form of EPOR (SFFVcEpoR) was injected into adult mice.115 In a later study, it was shown that mice infected with SFFVcEpoR showed increases in erythroid, megakaryocyte, and granulocyte-macrophage progenitors.130 Thus, infection with this retrovirus appears to impart a growth advantage on multipotent hematopoietic progenitors, resulting in effects on several lineages. In vitro studies using primary fetal liver cells infected with the SFFVcEpoR retrovirus are consistent with these studies and show that the major effect from expression of ectopic activated EPOR is generation of factor-independent colony-forming unit-erythroid (CFU-E). Effects on growth and differentiation of other progenitors such as burst-forming unit-erythroid (BFU-E), CFU-GM, and megakaryocytic precursors are only seen in the presence of steel factor (SF ).131 Thus, megakaryocyte, erythroid, GM, and multipotent progenitors contain the signalling apparatus that enable them to proliferate and differentiate in response to ectopic expression of an activated CR in vivo. However, normally, EPOR is barely detectable on multipotent progenitors,132 making it an unlikely candidate as the proto-oncogene involved in PV. Consistent with this finding, a number of studies suggest that expression of activated EPOR in a pluripotent stem cell is unlikely to be the molecular mechanism underlying PV. The number of receptors and their affinity for EPO is identical between PV cells and normal erythroid cells,133 and no gross rearrangements or gene amplification have been detected in PV cells.134 Moreover, two recent studies121 135 of PV patients failed to detect point mutations in the EPOR gene. Thus, it seems likely that the defect in PV may involve a molecule other than EPOR that is normally expressed in pluripotent hematopoietic progenitors.
Recently, transgenic mouse models have provided another clue to the molecular mechanism involved in PV. We have generated transgenic mice in which the constitutively activated form of hβc , FIΔ, has been linked to a promoter fragment from the phosphoglycerate kinase (PGK-1) gene (R. D'Andrea and M. A. Vadas, unpublished observations). Peripheral blood analysis of founder animals indicated mild erythrocytosis, and autopsy showed granulocytic and megakaryocytic hyperplasia and vascular congestion in bone marrow. Most transgenic animals were found to have splenomegaly, and spleen histology showed expanded red pulp with large numbers of erythroid/myeloid progenitors and megakaryocytes. This trilineage hyperplasia and splenomegaly are indicative of a pluripotent stem cell defect similar to that seen in PV. Colony assays demonstrated the presence of GM-CSF/IL-3–independent progenitor cells (CFU-M and CFU-GM) in the bone marrow and spleen of these transgenic animals. The numbers of GM-CSF/IL-3–dependent progenitor cells had decreased in the bone marrow and had increased in spleen, suggesting the mobilization of progenitor cells from marrow. Further work will determine whether there is an increase in the number of erythroid progenitors in these mice and whether these progenitors have a reduced requirement for EPO.
Similar results are obtained when hGMRα and hβc are expressed in transgenic mice at all stages of hematopoietic development. In this case, human GM-CSF supports the formation of various colony types from bone marrow of transgenic animals, including GM, eosinophil, mast cell, erythrocyte, megakaryocyte, blast cell, and mixed hematopoietic cell colonies.136 If these mice are injected with hGM for 1 week, increased white blood cell and red blood cell counts are observed. Mice develop splenomegaly due mostly to an increased erythroid component (S. Watanabe and K-I Arai, personal communication, October 1996). These recent studies support the possibility that PV may be due to mutations that cause constitutive activation of a CR normally expressed in multipotent progenitor/stem cells. Because hβc is expressed in multipotent progenitors132 137 and can be activated by several classes of mutation, we suggest that the gene for hβc represents a good candidate for mutations involved in PV. Given the extensive map now available for potential activating mutations in hβc, which are mostly clustered in domain 4 (Table 1), it will be interesting to perform a comprehensive, fine-scale analysis of this region of hβc gene in PV patients.
Our discussion has focussed so far on neoplastic and preneoplastic disorders, which reflect the proliferative actions of cytokines and their receptors. However, it should be remembered that many cytokines also induce activation of mature cells. These are frequently cell types, such as macrophages, neutrophils, mast cells, and eosinophils, that are involved in inflammatory responses. Thus, it is conceivable that activating mutations in certain CRs could result in sustained and inappropriate activation of inflammatory cells and thus lead to a variety of inflammatory conditions.
NOTE ADDED IN PROOF
Gourdou et al138 have recently described an activated form of prolactin receptor generated by deletion of the membrane-proximal cytokine receptor domain.
ACKNOWLEDGMENT
We thank our many colleagues for their contributions to work performed in the authors' laboratories and for many valuable ideas. In particular, we thank Mathew Vadas for support and helpful discussions.
Supported by grants from the National Health and Medical Research Council (NH&MRC) and from the Anti-Cancer Foundation of the Universities of South Australia. T.J.G. is a Senior Research Fellow of the NH&MRC.
Address reprint requests to Thomas J. Gonda, PhD, Hanson Centre for Cancer Research, Institute of Medical and Veterinary Science, Frome Road, Adelaide SA, 5000 Australia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal