Abstract
Human parvovirus B19 (B19) IgG was studied retrospectively in 66 allogeneic bone marrow transplantation (BMT) patients using an enzyme-linked immunosorbent assay. Recipient and donor sera had been stored pre-BMT together with sequential sera thereafter. Approximately half of donors and recipients had anti-B19 IgG pre-BMT and thus the relative contributions of donor and recipient immunity to antibody production after transplantation could be assessed. For each patient, a serum taken 2 to 3 years after BMT was also tested and the results show that persistence of B19 antibody depends on prior recipient (P = .0003) but not on donor immunity (P = .8). The findings were similar in both sibling and (VUD) BMT volunteer unrelated donor patients. Analysis of sequential post-BMT sera from 41 of the patients, for whom appropriately timed samples were available, showed primary B19 infection in 3 seronegative individuals, whereas 5 others who were seropositive before BMT underwent recurrent infection. Sequential results from the remaining 33 patients without recent B19 infection showed no evidence for donor antibody transfer and confirmed that antibody persistence depends on prior recipient immunity. B19 IgG levels decreased variably with time and some patients eventually became seronegative. It is concluded that this long-term persistence of B19 antibody post-BMT is most probably due to the existence of long-lived recipient plasma cells.
ALLOGENEIC bone marrow transplantation (BMT) is now widely used to treat a variety of conditions, including hematologic malignancies and some nonmalignant and metabolic disorders.1 Conditioning with chemotherapy or chemoradiotherapy eradicates the diseased marrow, which is then replaced with that of the donor. This procedure also results in ablation of the recipient's immune system, which must be reconstituted. Such immune reconstitution has been the subject of many studies (for reviews see Atkinson2 and Storek and Saxon3 ) but is as yet not fully understood.
Considering, in particular, the reconstitution of the humoral response, after an initial leucopenia the host is repopulated with donor lymphocytes but engrafted donor B cells only commence Ig production slowly, because immune ontogeny must be recapitulated. Thus primary antibody responses to neoantigens do not return to normal for about 1 to 2 years and take even longer in patients with chronic graft-versus-host disease.3 However, where immunity to a particular antigen exists before transplantation in recipient and/or donor, humoral immunity might persist and/or be provided by the donor marrow.
In an attempt to clarify the role of recipient and donor in the above-mentioned situation, we decided to study the persistence of antibody to human parvovirus B19 (B19) after allogeneic BMT. Antibody to B19 is found in approximately half the adult population4 and we expected therefore to identify a substantial number of donor and recipient pairs who were discrepant in their immune status. We report here an analysis of the results for 66 allogeneic BMT recipients.
PATIENTS AND METHODS
Patients.The 66 patients studied had each received an allogeneic BMT at the Hammersmith Hospital during the period from 1984 to 1992. Patients were selected if (1) they had undergone one BMT only and had survived for at least 2 years after transplantation without cytologic and/or hematologic relapse and (2) recipient and donor sera were available before transplantation together with sequential posttransplantation sera, including at least one sample at 2 to 3 years. Fifty patients received marrow from HLA-identical siblings and the remaining 16 individuals received marrow from HLA-matched volunteer unrelated donors (VUD). BMT was performed as previously described.5 6 Prophylaxis with intravenous Ig was not routinely used at the Hammersmith Hospital during the study period. However, 2 patients, D16 and D19 (for patient numbers, see the beginning of the Results), were included in a double-blinded trial of Ig prophylaxis. Further details of the patients' clinical characteristics are shown in Table 1.
Clinical Details of the Patients
| . | Sibling Donor . | VUD . | Total . |
|---|---|---|---|
| No. of patients | 50 | 16 | 66 |
| Sex | |||
| Male | 25 | 10 | 35 |
| Female | 25 | 6 | 31 |
| Age at transplantation | |||
| <20 yr | 4 | 1 | 5 |
| 20-40 yr | 32 | 10 | 42 |
| >40 yr | 14 | 5 | 19 |
| Diagnosis | |||
| Chronic myeloid leukemia | 44 | 16 | 60 |
| Acute lymphoblastic leukemia | 1 | 0 | 1 |
| Acute myeloid leukemia | 4 | 0 | 4 |
| Myelodysplastic syndrome | 1 | 0 | 1 |
| Conditioning regimen | |||
| Cyclophosphamide + total body irradiation | 49 | 15 | 64 |
| Cyclophosphamide + busulphan | 1 | 1 | 2 |
| Graft-versus-host disease prophylaxis | |||
| Cyclosporin + steroids | 2 | 0 | 2 |
| Cyclosporin + methotrexate | 44 | 1 | 45 |
| In vivo or ex vivo T-cell depletion | 4 | 15 | 19 |
| Acute graft-versus-host disease | |||
| Grade 0-I | 31 | 6 | 37 |
| Grade II-IV | 17 | 10 | 27 |
| Unknown/not evaluable | 2 | 0 | 2 |
| Chronic graft-versus-host disease | |||
| Yes | 25 | 11 | 36 |
| No | 19 | 5 | 24 |
| Unknown/not evaluable | 6 | 0 | 6 |
| . | Sibling Donor . | VUD . | Total . |
|---|---|---|---|
| No. of patients | 50 | 16 | 66 |
| Sex | |||
| Male | 25 | 10 | 35 |
| Female | 25 | 6 | 31 |
| Age at transplantation | |||
| <20 yr | 4 | 1 | 5 |
| 20-40 yr | 32 | 10 | 42 |
| >40 yr | 14 | 5 | 19 |
| Diagnosis | |||
| Chronic myeloid leukemia | 44 | 16 | 60 |
| Acute lymphoblastic leukemia | 1 | 0 | 1 |
| Acute myeloid leukemia | 4 | 0 | 4 |
| Myelodysplastic syndrome | 1 | 0 | 1 |
| Conditioning regimen | |||
| Cyclophosphamide + total body irradiation | 49 | 15 | 64 |
| Cyclophosphamide + busulphan | 1 | 1 | 2 |
| Graft-versus-host disease prophylaxis | |||
| Cyclosporin + steroids | 2 | 0 | 2 |
| Cyclosporin + methotrexate | 44 | 1 | 45 |
| In vivo or ex vivo T-cell depletion | 4 | 15 | 19 |
| Acute graft-versus-host disease | |||
| Grade 0-I | 31 | 6 | 37 |
| Grade II-IV | 17 | 10 | 27 |
| Unknown/not evaluable | 2 | 0 | 2 |
| Chronic graft-versus-host disease | |||
| Yes | 25 | 11 | 36 |
| No | 19 | 5 | 24 |
| Unknown/not evaluable | 6 | 0 | 6 |
Serology.All the sera studied had been sent to the Clinical Virology Laboratory for routine diagnostic purposes and the surplus was stored at −20°C until tested for B19 IgG antibody. For this the IDEIA Parvovirus B19 IgG enzyme-linked immunosorbent assay (ELISA; DAKO A/S, Glostrup, Denmark) was used according to the manufacturer's instructions. The antigen in this test is the B19 major capsid protein VP2 expressed in insect cells infected with the relevant recombinant baculovirus. Results were either expressed as absorbance at 450 nm (OD450 ; Figs 1 and 2) or else the OD450 was interpreted as positive, equivocal, or negative using the manufacturer's recommended cutoff so that persistence of antibody at 2 to 3 years post-BMT could be assessed (eg, Table 2). The few sera giving an equivocal result at 2 to 3 years post-BMT (namely those from patients D8, D9, D10, and D12) were further tested to determine whether positive or negative by an immunofluorescence assay for IgG antibody to B197; for this, multiwell glass slides coated with insect cells infected with baculovirus and expressing recombinant B19 minor capsid protein VP1 (Biotrin International, Dublin, Ireland) were overlaid with serum diluted 1 in 10 in phosphate-buffered saline (PBS) and incubated for 45 minutes at 37°C. Thereafter, the slides were washed for 15 minutes with three changes of PBS, allowed to dry, and reincubated with fluorescein isothiocyanate-conjugated F(ab′)2 goat antihuman IgG (Sigma Chemical Co, St Louis, MO) for 45 minutes at 37°C, washed with PBS, and mounted. Positive and negative control sera were included in each test run. Three sera gave weak-specific and the fourth gave moderate-specific fluorescent staining of the VP1 protein aggregates and all sera were therefore deemed positive for IgG to B19.
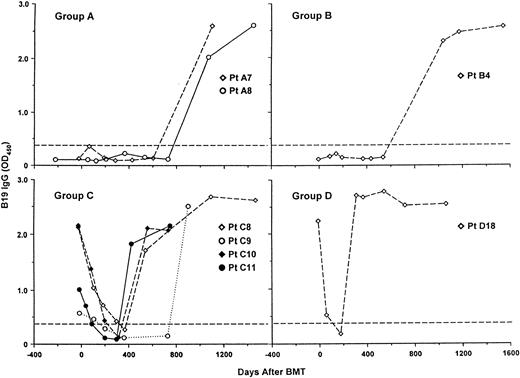
Serologic evidence of primary and recurrent B19 infection in 8 BMT patients. Group A, seronegative recipients with seronegative donors; group B, seronegative recipients with seropositive donors; group C, seropositive recipients with seronegative donors; group D, seropositive recipients with seropositive donors. The dotted line gives the manufacturer's cutoff.
Serologic evidence of primary and recurrent B19 infection in 8 BMT patients. Group A, seronegative recipients with seronegative donors; group B, seronegative recipients with seropositive donors; group C, seropositive recipients with seronegative donors; group D, seropositive recipients with seropositive donors. The dotted line gives the manufacturer's cutoff.
Temporal changes in B19 IgG antibody in 33 BMT patients with no evidence of recent B19 infection. Group A, seronegative recipients with seronegative donors; group B, seronegative recipients with seropositive donors; group C, seropositive recipients with seronegative donors; group D, seropositive recipients with seropositive donors. (Data on patients D13 through D17 were omitted for clarity because they would have superimposed on the data for the 12 patients shown that give an accurate representation of the range of results.) The dotted line gives the manufacturer's cutoff.
Temporal changes in B19 IgG antibody in 33 BMT patients with no evidence of recent B19 infection. Group A, seronegative recipients with seronegative donors; group B, seronegative recipients with seropositive donors; group C, seropositive recipients with seronegative donors; group D, seropositive recipients with seropositive donors. (Data on patients D13 through D17 were omitted for clarity because they would have superimposed on the data for the 12 patients shown that give an accurate representation of the range of results.) The dotted line gives the manufacturer's cutoff.
Persistence of B19 IgG After BMT in Relation to Prior Recipient and Donor Immunity
| . | B19 IgG . | Proportion Seropositive at 2-3 yr Post-BMT . | Years Serum Received Post-BMT . | ||
|---|---|---|---|---|---|
| . | Pre-BMT . | . | Median . | Range . | |
| . | Recipient . | Donor . | . | . | . |
| Group A | − | − | 2/13 | 2 | 2-3 |
| Group B | − | + | 1/15 | 2 | 2-3 |
| Total | 3/28* | ||||
| Group C | + | − | 10/16 | 2 | 2-3 |
| Group D | + | + | 12/22 | 2 | 2-3.5 |
| Total | 22/38* | ||||
| . | B19 IgG . | Proportion Seropositive at 2-3 yr Post-BMT . | Years Serum Received Post-BMT . | ||
|---|---|---|---|---|---|
| . | Pre-BMT . | . | Median . | Range . | |
| . | Recipient . | Donor . | . | . | . |
| Group A | − | − | 2/13 | 2 | 2-3 |
| Group B | − | + | 1/15 | 2 | 2-3 |
| Total | 3/28* | ||||
| Group C | + | − | 10/16 | 2 | 2-3 |
| Group D | + | + | 12/22 | 2 | 2-3.5 |
| Total | 22/38* | ||||
Difference between these two proportions significant (P = .0003).
B19 IgM antibody was detected using the Biotrin Parvo-B19 IgM ELISA (Biotrin International). This is a μ-capture immunoassay and the antigen used is the recombinant capsid protein VP2. Results were calculated in accordance with the manufacturer's instructions.
Statistics.The χ2 test with Yates' correction or Fisher's exact probability test (2-tailed) were used as appropriate to assess the significance of differences between groups.
RESULTS
B19 IgG status before BMT.The IgG ELISA showed that 38 of 66 (58%) recipients and 37 of 66 (56%) donors were seropositive for B19 IgG. On the basis of these results the patients were divided into four groups: group A, seronegative recipients with seronegative donors (nos. A1 through A13); group B, seronegative recipients with seropositive donors (nos. B1 through B15); group C, seropositive recipients with seronegative donors (nos. C1 through C16); and group D, seropositive recipients with seropositive donors (nos. D1 through D22).
Persistence of B19 IgG at 2 to 3 years after BMT.For each of the 66 (50 sibling and 16 VUD BMT) patients, a serum taken between 2 to 3 years after BMT was tested for B19 IgG. The results (Table 2) clearly show that antibody persistence depends on prior recipient and not donor immunity; 22 of 38 (58%) seropositive recipients were seropositive post-BMT as compared with 3 of 28 (11%) seronegative recipients irrespective of the donors' status. In contrast, 12 of 29 (41%) recipients of seronegative donors were seropositive as compared with 13 of 37 (37%) recipients of seropositive donors.
The influence of type of BMT on persistence of B19 IgG was analyzed. Considering first the 50 sibling BMT patients, regardless of donor immune status 16 of 29 (55%) seropositive recipients were seropositive post-BMT as compared with 3 of 21 (14%) seronegative recipients (P = .008), whereas 9 of 21 (43%) recipients of seronegative donors were seropositive as compared with 10 of 29 (34%) recipients with seropositive donors (P = .75). Turning to the 16 VUD BMT patients, regardless of donor immune status 6 of 9 (67%) seropositive recipients were seropositive post-BMT as compared with 0 of 7 (0%) seronegative recipients (P = .01), whereas 3 of 8 (38%) recipients of seronegative donors were seropositive as compared with 3 of 8 (38%) recipients of seropositive donors 38% (P = 1.0). Thus, for both sibling and VUD BMT, B19 IgG antibody persistence depends on prior recipient and not donor immunity.
Primary and recurrent B19 infection.The results presented above might have been confounded by posttransplantation primary or recurrent B19 infection. Indeed, close inspection of Table 2 shows that 2 of 11 BMT recipients in group A (seronegative recipients with seronegative donors) seroconverted for B19 IgG, ie, underwent a primary B19 infection. However, it was not possible to know if B19 infection had occurred in the other three patient groups (B, C, and D) on the basis of a result at a single time point posttransplantation because of the possible persistence of antibody (recipient, donor, or passively acquired from blood products).
Detailed analysis of sequential sera was therefore undertaken in 41 of the 66 patients. Of the remaining 25 patients, 16 were excluded because they were seronegative before BMT who remained seronegative 2 to 3 years later because a preliminary investigation of a representative sample of such seronegatives did not show any change in antibody level except transiently within 200 days of transplant (Fig 2, groups A and B). A further 9 patients from groups C and D were excluded because insufficient sequential serum samples were available. Thus, the sera analyzed were from patients A1 through A8, B1 through B4, C1 through C11, and D1 through D18, respectively. An antibody response indicative of recent B19 infection (ie, a sustained increase in OD450 of greater than 2.0) was found in 8 patients (Fig 1); patients no. A7, A8, and B4 were seronegative before BMT and seroconverted, indicating primary infection, whereas C8, C9, C10, C11, and D18 were seropositive before BMT, lost B19 antibody, and then seroconverted, indicating recurrent infection. In each case but 1 (B4), an accompanying IgM response was recorded (data not shown). In patients A7, A8, B4, and C9, the infection occurred 2 to 3 years after BMT, whereas in patients C8, C10, C11, and D18, a rapid loss of antibody was soon followed by seroconversion at about 1 year after BMT.
As regards the clinical consequences of recent B19 infection,8-11 examination of the hospital case notes showed no evidence of anemia and the hemoglobin level remained within the normal range for all 8 patients (data not shown). Indeed, it is worth noting that in each case infection was only identified during the present retrospective study and not at the time.
Temporal changes in B19 IgG post-BMT in the absence of recent B19 infection.Data for the remaining 33 patients showed no evidence of primary or recurrent B19 infection (Fig 2). Groups A and B (seronegative recipients with seronegative and seropositive donors, respectively) showed very similar patterns. Some patients (A1, A3, A4, B1, B2, and B3) became weakly seropositive but only for the first 200 days after transplantation, whereas the rest remained seronegative throughout. In contrast, the data for groups C and D (seropositive recipients with seronegative and seropositive donors, respectively), although very alike, showed B19 IgG antibody levels that were initially high but that gradually decreased with time. Some patients, eg, C5 and D7, rapidly lost IgG to B19 and became seronegative by 200 days after BMT, whereas, at the other extreme, although antibody levels declined slowly, some patients remained seropositive 700 to 1,000 days post-BMT, eg, C1 and D3, or even longer, eg, C3 and D1. Reference to each patient's clinical details gave no evidence that acute or chronic graft-versus-host disease affected the outcome (data not shown).
Statistical analysis of the data for this subgroup of 33 patients, in whom recent B19 infection had been excluded, showed that antibody persistence depends on prior recipient and not donor immunity; irrespective of donor immune status 50% (12/24) of the seropositive recipients remained seropositive at 2 to 3 years posttransplantation as compared with 0% (0/9) of seronegative recipients (P = .01), whereas 30% (4/13) of recipients of seronegative donors remained seropositive as compared with 40% (8/20) of recipients of seropositive donors (P = .7).
DISCUSSION
The main aim of this study was to investigate the relative contributions of pre-existing donor and recipient humoral immunity to persistence and duration of specific IgG production after BMT. Wahren et al12 were the first to study this problem by analysis of a small number of donor and recipient pairs who were discrepant in their immune status for various viruses. They concluded that transfer of donor immunity and persistence of recipient antibody may both occur but antibody was only detected beyond 1 year after BMT in a few cases.
The above-noted observations have since been clarified and extended by others. Looking first at humoral immunity to a virus that always persists after primary infection, eg, human cytomegalovirus (CMV), endogenous viral antigens are available in the CMV seropositive recipient for presentation to the repopulating donor lymphocytes and thus CMV antibody continues to be produced regardless of the serologic status of the donor.13,14 If the donor is also CMV seropositive, memory cells specific for CMV are present in the donor marrow and a rapid secondary response is seen,14-16 whereas if the donor is CMV seronegative, a slower primary antibody response occurs.14
In contrast, with viruses that do not normally persist after primary infection, such as measles, mumps, and rubella, although humoral immunity may be detected for several years after allogeneic BMT,17 it eventually disappears, presumably because of a lack of endogenous or exogenous antigenic stimulation. However, because almost all adults have either been infected with or vaccinated against measles, mumps, and rubella and hence antibody to these viruses is widespread, these viruses are not the best choice to investigate the individual contributions of donor and recipient immunity after BMT. We therefore chose B19 for this purpose because it infects only about 50% of the population4 and is an example of a nonpersisting virus.
Persons with pre-existing immunity to B19 have IgG antibody to both VP1 and VP2.18 However, care must be taken in the choice of an anti-VP2 assay because only those in which the antigen retains its native conformation will detect a long-lived IgG response.18-21 We used an assay with VP2 in the native confirmation18 as our standard test and further tested any sera giving equivocal results using an anti-VP1 assay. In the event, as anticipated, 58% of our donors and 56% of our recipients were found to be seropositive pre-BMT and we were readily able to divide the patients into four reasonably sized groups on the basis of donor and recipient immunity. It was therefore possible to distinguish between the relative duration and contribution of donor and recipient immunity to specific antibody production.
Considering first the role of donor immunity, there was no evidence for transfer of B19 antibody by donor cells after BMT (Table 2). Indeed, examination of sequential sera (Fig 2) shows no difference in the time course between groups A and B (seronegative recipients with seronegative and seropositive donors, respectively) and it must be presumed that the observed brief weak seropositivity is due to antibody passively acquired from blood and blood products at the time of BMT and immediately after BMT. Furthermore, comparison of groups C and D (seropositive recipients with seronegative and seropositive donors, respectively) shows that there is no difference in antibody persistence after BMT regardless of donor antibody status. Ljungman et al17 previously found that there was no influence of donor seropositivity on the probability of becoming seronegative to mumps, which is in agreement with our results. However, these investigators concluded that donor immunity did contribute to production of specific antibodies early after BMT; the reason for this discrepancy is not readily apparent but may be explained by qualitative differences in the definition of immunity. Finally, Lum et al22 reported, from a study of 6 patients in which donor and recipient immunity was discrepant, that donor antibodies to measles and diphtheria can be transferred. However, these investigators analyzed only one serum sample taken within 1 year of BMT and did not investigate either sequential or long-term changes in antibody, and the findings are therefore difficult to evaluate.
On the other hand, as shown in the present work, B19 IgG antibody persistence after BMT clearly depended on prior recipient immunity (Table 2 and Fig 2). These results cannot be explained on the basis of passively administered Ig or other blood products because the half-life of IgG in the blood is a mere 21 days and persistence of antibody was assessed 2 to 3 years after transplantation, long after the immediate posttransplantation period when such therapy is routinely administered. Moreover, all patients who had suffered a relapse to their original leukemic state and/or received a second transplant and hence might require blood products were excluded from the study. Another possibility might be continued antigenic stimulation by B19 persisting long after primary infection, resulting in antibody persistence in the same way as for CMV.13-16 This seems unlikely because, although there are a few case reports describing persistence of B19 in immunocompetent individuals with symptoms attributable to this infection,23,24 there is no evidence for viral persistence in the absence of such symptoms.24 In addition, although antibody persisted after BMT, it tended to decrease with time and some patients eventually became seronegative (Fig 2), whereas the opposite would be expected in the presence of continued antigenic stimulation. The most likely explanation seems to be that the persistent antibody is of recipient origin; this conclusion is supported by a recent report that a substantial proportion of serum IgG remains of recipient origin several years after BMT in a surprisingly high proportion of patients.25 The explanation for this unexpected persistence may well be the presence of long-lived recipient plasma cells that have survived high-dose irradiation and chemotherapy and that disappear only gradually by attrition. Such a hypothesis runs counter to the current dogma that plasma cells are short-lived. However, evidence supporting a brief life span is surprisingly scarce (for review see Ahmed and Gray26 ). In fact, one study has shown the existence of some long-lived plasma cells depending on the isotype produced and the site of production,27 and an early report indicates a very long life span for a small proportion of plasma cells.28
Because B19 IgG persists for different times in different patients, there were clearly other factors involved besides prior recipient immunity. However, no risk factor leading to rapid loss of antibody could be identified in this investigation. In particular, there was no difference in outcome between sibling and VUD BMT patients. Neither was there any evidence that acute or chronic graft-versus-host disease affected the outcome. A much larger study is needed to define possible risk factors more accurately. In fact, there is as yet no general agreement on the influence of chronic graft-versus-host disease on loss of antibody. Lum et al29 found it to be a significant risk factor, whereas Ljungman et al,17 in agreement with our findings, did not; the explanation may lie in the relative proportion of severe graft-versus-host disease in the three different investigations, because the patients of Lum et al29 included a significant number of HLA-mismatched donors, whereas those of Ljungman et al17 and the series reported here had few or none.
Considering primary and recurrent B19 infection in the patients under study, the incidence was 20% (8/41) over a period of 2 to 3 years. However, this is certainly an overestimate because 16 patients seronegative pre-BMT were excluded from analysis as they remained seronegative. Transmission of B19 from blood or blood products seems unlikely as an explanation for this apparently high incidence because such transmission has been described in only two case reports.30 31 In any case, 4 patients (A7, A8, B4, and C9) were infected 2 to 3 years after BMT and did not receive any transfusions at that time. However, 4 patients (C8, C10, C11, and D18) who were all seropositive pre-BMT rapidly lost antibody followed by seroconversion at about 1 year after BMT, and these recurrent infections may have been associated with receiving blood or blood products or just possibly with reactivation of persistent virus.
In conclusion, the present investigation has clearly identified the recipient as the continuing source of B19 antibody after BMT and suggested the presence of persisting long-lived recipient plasma cells.
ACKNOWLEDGMENT
This work was submitted by H.A.A. as part of the requirements for the degree of MSc in Infectious Diseases (University of London). The authors are most grateful to E. Whittaker for excellent secretarial assistance, to Dr R. Szydlo for information from the Hammersmith Hospital bone marrow transplant database, and to Dr B.J. Cohen for helpful advice.
Address reprint requests to K.N. Ward, MB, BChir, PhD, Department of Infectious Diseases, Royal Postgraduate Medical School, Du Cane Road, London W12 0NN, UK.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal