Abstract
Alloreactive T cells present in a bone marrow transplant are responsible for graft-versus-host disease (GVHD), but their depletion is associated with impaired engraftment, immunosuppression, and loss of the graft-versus-leukemia effect. We developed a therapeutic strategy against GVHD based on the selective destruction of these alloreactive T cells, while preserving a competent T-cell pool of donor origin. We generated transgenic mice expressing in their T lymphocytes the Herpes simplex type 1 thymidine kinase (TK) suicide gene that allows the destruction of dividing T cells by a ganciclovir treatment. T cells expressing the TK transgene were used to generate GVHD in irradiated bone marrow grafted mice. We show that a short 7-day ganciclovir treatment, initiated at the time of bone marrow transplantation, efficiently prevented GVHD in mice receiving TK-expressing T cells. These mice were healthy and had a normal survival. They maintained a T-cell pool of donor origin that responded normally to in vitro stimulation with mitogens or third party alloantigens, but were tolerant to recipient alloantigens. Our experimental system provides the proof of concept for a therapeutic strategy of GVHD prevention using genetically engineered T cells.
GRAFT-VERSUS-HOST disease (GVHD) is a major complication of allogeneic bone marrow transplantation (BMT) that results in high morbidity and mortality. Donor T cells present in the BMT have been shown to recognize recipient alloantigens and cause GVHD.1 Although innovative strategies for predicting, preventing, or treating GVHD have recently been proposed,2-7 the prophylaxis or treatment of GVHD with current immunosuppressive regimens are only partially successful. In vitro depletion of donor T cells efficiently prevents GVHD. Nevertheless, this method is frequently associated with impaired engraftment,8 immunosuppression causing an increased rate of infection,9 and/or loss of the graft-versus-leukemia (GVL) effect resulting in an increased frequency of leukemia relapse.10
To circumvent these effects, we aimed to develop a strategy allowing the conditional destruction of alloreactive T cells responsible for GVHD, while preserving a T-cell population with a diverse repertoire. The persistence of mature donor-type T cells in the early graft period should permit an enhanced engraftment, limit immunosuppression and may retain the benefit of a GVL effect. The suicide gene system using the Herpes simplex type 1 thymidine kinase (TK) associated with a nucleoside analogue such as acyclovir or ganciclovir (GCV) provides a means to achieve this goal. Cells expressing the TK enzyme can efficiently phosphorylate GCV into a monophosphorylated derivative, a metabolism not otherwise occurring in eukaryotic cells. In turn, GCV monophosphate is converted by cellular kinases into GCV triphosphate that is incorporated into elongating DNA, further causing cell death.11-13 This system combines several advantages. First, TK expression per se does not alter cell function. Second, toxicity is conditioned by the presence of GCV that can be administered by different protocols that vary in onset, dose, and duration. Finally, toxicity is restricted to dividing cells, an obvious advantage for the treatment of pathologies such as GVHD caused by activated T cells.
Previous studies have shown that it is possible to kill dividing TK-expressing cells of transgenic mice in vivo14 or human TK-expressing T cells in vitro.15,16 To test the feasibility and efficacy of this approach in the field of GVHD without turning to viral mediated gene transfer, we developed the transgenic mouse line EpTK in which a TK transgene is expressed in mature peripheral T cells. This was achieved through the use of regulatory sequences that we previously showed to exhibit this specificity of expression in transgenic mice.17 One difficulty with the use of TK transgenic mice is the sterility or hypofertility commonly observed in males, whatever the regulatory sequences used.18,19 This led us to also develop EpΔTK transgenic mice using a truncated variant of TK that retains the functional properties of TK when expressed at sufficient levels, but does not cause sterility.20
In this work, we used EpTK- or EpΔTK-transgenic T cells to induce a lethal GVHD in a model of allogeneic BMT. We show that a 7-day GCV treatment at the time of BMT is sufficient to durably prevent the occurrence of GVHD and does not impair complete donor-type hematologic reconstitution. The treated BMT recipients retain a long-term tolerance to donor and recipient antigens whereas reactivity to mitogens or third party alloantigens is conserved. Furthermore, a late-onset GVHD observed in a minority of initially protected ΔTK-receiving mice could be successfully treated by GCV, suggesting that this approach can also be used as a treatment of ongoing GVHD.
MATERIALS AND METHODS
Mice.C57BL/6 (B6, H-2b, Thy1.2), FVB/N (FVB, H-2q, Thy1.1), DBA/2 (D2, H-2d), and BALB/c (Balb, H-2d) mice were obtained from Iffa Credo (L'Arbresle, France) or CERJ (Le Genest-Saint-Isle, France).
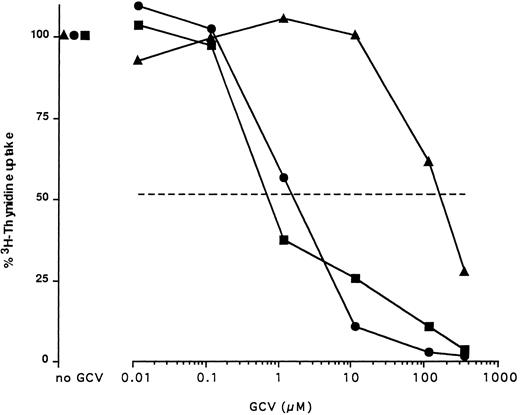
In vitro sensitivity of TK and ΔTK-expressing T cells to GCV. LN cells were cultured for 2 days with ConA in the presence of increasing concentrations of GCV and pulsed overnight with 3H TdR (100% uptake is that observed in the absence of GCV in the culture). The dashed line indicates 50% proliferation. LN cells from EpTK-(▪) and EpΔTK-(•) transgenic mice are compared with cells from a nontransgenic (▴) FVB mouse. Comparable results were obtained in at least five independent experiments.
In vitro sensitivity of TK and ΔTK-expressing T cells to GCV. LN cells were cultured for 2 days with ConA in the presence of increasing concentrations of GCV and pulsed overnight with 3H TdR (100% uptake is that observed in the absence of GCV in the culture). The dashed line indicates 50% proliferation. LN cells from EpTK-(▪) and EpΔTK-(•) transgenic mice are compared with cells from a nontransgenic (▴) FVB mouse. Comparable results were obtained in at least five independent experiments.
Protective effect of a 7-day GCV treatment on the occurrence of GVHD in mice receiving (A) EpTK or (B) EpΔTK transgenic T cells. Irradiation control group (○): Ungrafted irradiated B6 mice (n = 15 in both [A] and [B]). Reconstitution control group (•): Irradiated B6 mice engrafted with FVB BM (n = 15 [A] and 11 [B]). GVHD control group (□): Irradiated B6 mice engrafted with FVB BM plus either FVB nontransgenic T cells and GCV administration or transgenic T cells and PBS administration (n = 25 [A] and 23 [B]). Experimental group (▪): Irradiated B6 mice engrafted with FVB BM plus (A) EpTK or (B) EpΔTK T cells and treated with GCV (n = 13 [A] and 19 [B]). Results are presented as Kaplan-Meier survival curves of cumulative data for each set of 3 independent experiments in both (A) and (B).
Protective effect of a 7-day GCV treatment on the occurrence of GVHD in mice receiving (A) EpTK or (B) EpΔTK transgenic T cells. Irradiation control group (○): Ungrafted irradiated B6 mice (n = 15 in both [A] and [B]). Reconstitution control group (•): Irradiated B6 mice engrafted with FVB BM (n = 15 [A] and 11 [B]). GVHD control group (□): Irradiated B6 mice engrafted with FVB BM plus either FVB nontransgenic T cells and GCV administration or transgenic T cells and PBS administration (n = 25 [A] and 23 [B]). Experimental group (▪): Irradiated B6 mice engrafted with FVB BM plus (A) EpTK or (B) EpΔTK T cells and treated with GCV (n = 13 [A] and 19 [B]). Results are presented as Kaplan-Meier survival curves of cumulative data for each set of 3 independent experiments in both (A) and (B).
Analysis of hematologic reconstitution after BMT. Spleen cells from (A) B6 mice grafted with FVB BM and (B) B6 mice grafted with FVB BM plus EpTK T cells and treated with GCV were analyzed at different times after BMT. (C) is a B6 control mouse. Three-color flow cytometric analyses were performed with a combination of the B-cell–specific B220 MoAb with Thy1.2 and H-2b (recipient type) or Thy1.1 and H-2q (donor type) MoAbs. Monoparametric histograms are depicted in B220+ and B220− gated subsets. Data are representative of reproducible experiments performed at days 22, 51, 70, and 212 after BMT.
Analysis of hematologic reconstitution after BMT. Spleen cells from (A) B6 mice grafted with FVB BM and (B) B6 mice grafted with FVB BM plus EpTK T cells and treated with GCV were analyzed at different times after BMT. (C) is a B6 control mouse. Three-color flow cytometric analyses were performed with a combination of the B-cell–specific B220 MoAb with Thy1.2 and H-2b (recipient type) or Thy1.1 and H-2q (donor type) MoAbs. Monoparametric histograms are depicted in B220+ and B220− gated subsets. Data are representative of reproducible experiments performed at days 22, 51, 70, and 212 after BMT.
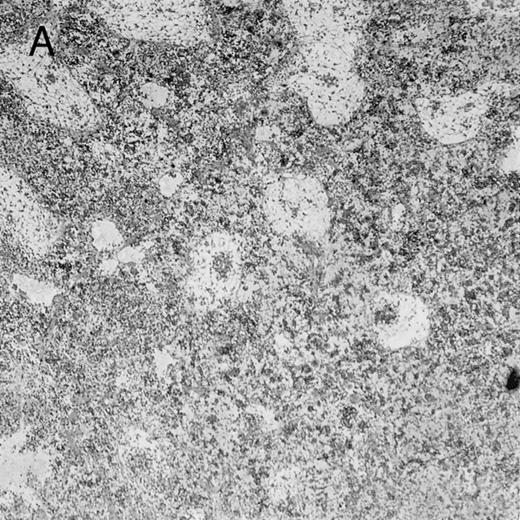
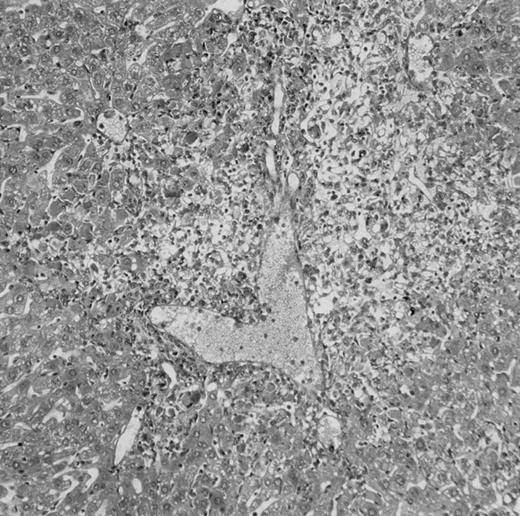
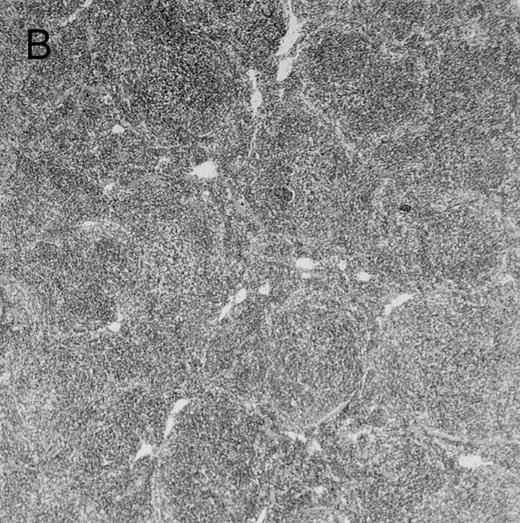
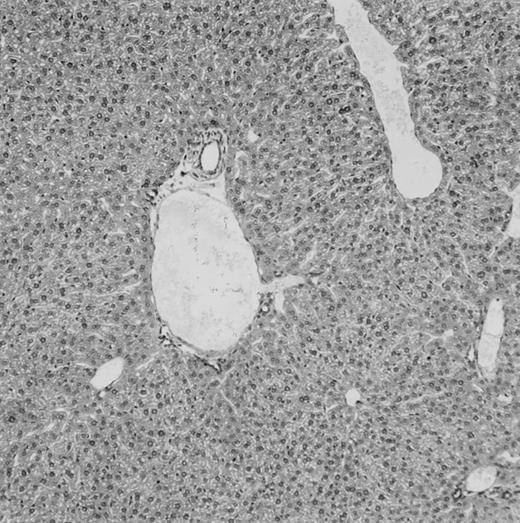
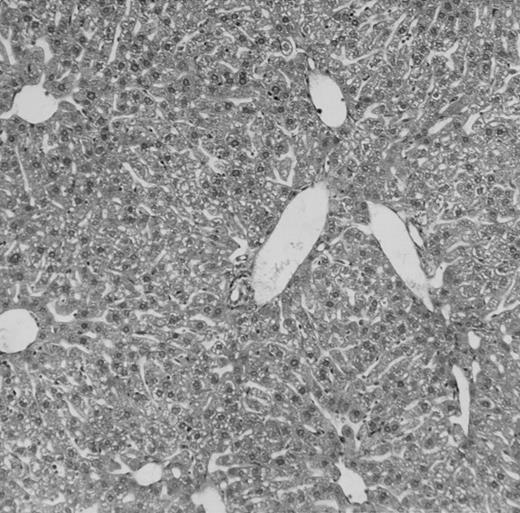
Histologic findings. Organs were collected at different times after BMT and tissue sections were stained with hematoxylin and eosin. Original magnifications for spleen (left) and liver (right) are ×40 and ×100, respectively. (A) B6 mice receiving FVB BM plus EpTK T cells. (B) Control group receiving BM only.
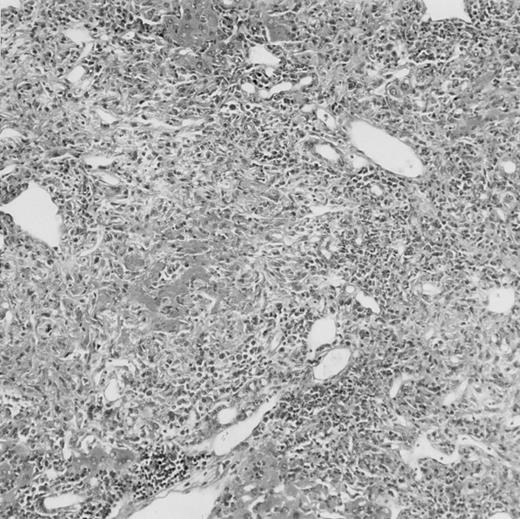
(C) B6 mice receiving FVB BM plus EpΔTK T cells and treated with GCV. (D) B6 mice receiving FVB BM plus EpΔTK T cells, treated with GCV, and developing a late onset GVHD (day 54).
Histologic findings. Organs were collected at different times after BMT and tissue sections were stained with hematoxylin and eosin. Original magnifications for spleen (left) and liver (right) are ×40 and ×100, respectively. (A) B6 mice receiving FVB BM plus EpTK T cells. (B) Control group receiving BM only.
(C) B6 mice receiving FVB BM plus EpΔTK T cells and treated with GCV. (D) B6 mice receiving FVB BM plus EpΔTK T cells, treated with GCV, and developing a late onset GVHD (day 54).
Effect of a Second GCV Administration on the Outcome of Late-Onset GVHD
| . | Mouse . | Clinical Status . | Second GCV Administration . | ||
|---|---|---|---|---|---|
| . | . | Onset . | Clinical Signs . | Days . | Outcome . |
| A | 6.21 | Day 40 | Weight loss, skin lesions, hunching* | − | Died at day 54 |
| 9.25 | Day 116 | Weight loss* | − | Died at day 145 | |
| B | 6.22 | Day 53 | Discrete skin lesions | Days 70 to 77 | Healthy at day 120 |
| 6.23 | Day 40 | Weight loss, skin lesions | Days 54 to 63 | Healthy at day 120 | |
| 9.11 | Day 80 | Weight loss, severe skin lesions, hunching | Days 133 to 140 | Partial recovery, death at day 179 | |
| . | Mouse . | Clinical Status . | Second GCV Administration . | ||
|---|---|---|---|---|---|
| . | . | Onset . | Clinical Signs . | Days . | Outcome . |
| A | 6.21 | Day 40 | Weight loss, skin lesions, hunching* | − | Died at day 54 |
| 9.25 | Day 116 | Weight loss* | − | Died at day 145 | |
| B | 6.22 | Day 53 | Discrete skin lesions | Days 70 to 77 | Healthy at day 120 |
| 6.23 | Day 40 | Weight loss, skin lesions | Days 54 to 63 | Healthy at day 120 | |
| 9.11 | Day 80 | Weight loss, severe skin lesions, hunching | Days 133 to 140 | Partial recovery, death at day 179 | |
This table summarizes the evolution of the 5 GCV-treated mice that presented a GVHD late after BM plus ΔTK T cells transplantation. The second GCV treatment was administered by intraperitoneal injection of 50 mg/kg twice daily, except for mouse 6.23 that was implanted with a miniosmotic pump delivering 50 mg/kg/d.
Abbreviation: −, no second GCV treatment.
GVHD confirmed histologically.
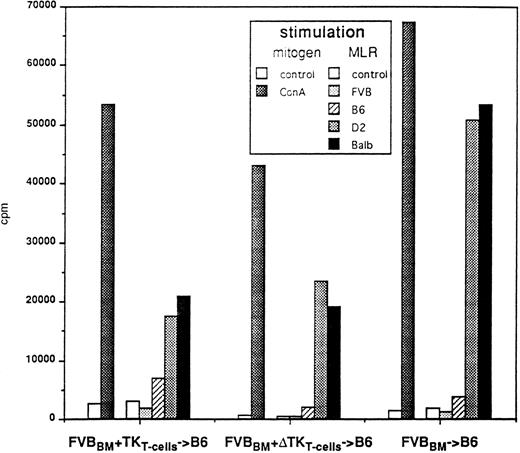
T-cell proliferative responses after GCV treatment. Splenocytes were collected 6 to 8 months post-BMT and cultured in the presence of ConA or in MLR in the presence of donor (FVB), recipient (B6), or third party (D2 or Balb) irradiated splenocytes. Proliferative response of unmanipulated FVB splenocytes to ConA and allogeneic cells were comparable to that obtained with FVBBM → B6 (not shown).
T-cell proliferative responses after GCV treatment. Splenocytes were collected 6 to 8 months post-BMT and cultured in the presence of ConA or in MLR in the presence of donor (FVB), recipient (B6), or third party (D2 or Balb) irradiated splenocytes. Proliferative response of unmanipulated FVB splenocytes to ConA and allogeneic cells were comparable to that obtained with FVBBM → B6 (not shown).
Transgenic mice.EpTK (line 40) and EpΔTK transgenic mice (line 20) were obtained by microinjection and reimplantation of fertilized eggs as described21. These transgenic mice were developed in the FVB background, a strain favorable for transgenesis.22 Briefly, TK coding sequences15 were placed under the transcriptional control of the human CD4 gene promoter (p) and the murine CD4 gene enhancer (E) to achieve specific TK expression in both CD4+ and CD8+ peripheral T cells.17
In vitro sensitivity to GCV.Inguinal lymph node (LN) cells (1 × 105) were cultured in triplicate at 37°C under 5% CO2 in 0.2 mL of RPMI 1640 medium (Flobio, Courbevoie, France) supplemented with 10% fetal calf serum (Flobio), 2 mmol/L glutamine, 5 × 10−5 mmol/L 2-mercaptoethanol, and penicillin/streptomycin/neomycin (GIBCO Laboratories, Grand Island, NY) in round-bottomed 96-well microplates. T lymphocytes were activated by the addition of 3 μg/mL of concanavalin A (ConA) in the presence of increasing concentrations of GCV (Syntex Laboratories, Puteaux, France). After 2 days of culture, cells were pulsed overnight with 1 μCi/well of 3H thymidine (3H TdR) and radioactivity was counted.
Mitogen stimulation and mixed lymphocyte reaction (MLR).Cells were cultured as described above with the addition of 3 μg/mL Con A or using 3 × 105 20-Gy–irradiated splenocytes from different mouse strains. 3H TdR uptake was measured after 2 or 4 days of culture, respectively.
BMT and experimental GVHD.B6 female mice 8 to 10 weeks old were conditioned by 10 Gy 60Co telegammatherapy using a Theratron 780 (Theratronix, Kanata, Canada). Mice were arranged by groups of 15 in a Plexiglas box to ensure correct animal position stability and electronic equilibrium. To obtain homogeneous total body irradiation, we administered a midline dose by two equally weighted parallel opposed beams in an isocentric configuration. The mean dose rate in these experiments was 0.69 Gy/min. Donor BM was collected from femurs and tibias of FVB female mice; cells were then collected under sterile technique by flushing with phosphate-balanced buffer (PBS) and filtration through a nylon Falcon no. 2350 cell strainer (Becton Dickinson, San Jose, CA). B6 recipients were injected intravenously with 107 BM cells 15 to 20 hours after irradiation.
To induce GVHD, spleen and LN cells from TK-transgenic, ΔTK-transgenic, or FVB nontransgenic animals were added to the FVB BM. These cells were recovered from the spleen and the inguinal, brachial, axillary, and mesenteric LN after teasing and filtration and were pooled. To ensure that the same number of peripheral T cells was added to each BMT, a cell aliquot was double-stained with fluorescein isothiocyanate (FITC)-labeled anti-CD3 (hamster IgG, clone 500-A2) and TriColor-labeled anti-B220 (rat IgG2a , clone RA3-6B2) monoclonal antibodies (MoAbs) from Caltag Laboratories (San Francisco, CA). Flow cytometric analysis of 5,000 events was performed extemporaneously on a FACScan analyzer (Becton Dickinson). The number of cells was then adjusted so that each mouse received 107 peripheral CD3+ T lymphocytes, with these latter cells representing an average of 50% to 60% of total added cells.
Administration of GCV.GCV was administered continuously at 50 mg/kg/d for 7 days, a dose previously shown to be efficient for in vivo cell depletion in another model of TK-transgenic mice,20 using a 2001 Alzet mini-osmotic pump (Alza Corp, Palo Alto, CA). These pumps were implanted subcutaneously at the time of BMT under avertin anesthesia21 and delivered 1 ± 0.02 μL/h. A control group received PBS instead of GCV. Mice receiving a second treatment were injected intraperitoneally with GCV at a dose of 50 mg/kg twice daily.
Kaplan-Meier survival curves were established for each group. When mice suffering from advanced stage disease were sacrificed for histological examination, this event was considered as a death in the survival curve. When mice received a second GCV treatment, they were considered in the survival curve until the date of second administration.
Flow cytometric analysis of hematologic reconstitution.Hematologic reconstitution was studied by three-color flow cytometric analysis at different times after BMT. Splenocytes from grafted animals were recovered after collagenase digestion and incubated with a supernatant of anti-Fc receptor 2.4.G2 hybridoma. Cells were then stained with TriColor-labeled anti-B220 (Caltag), biotinylated anti-H-2 Dq (mouse IgG2a , clone KH117; Pharmingen, San Diego, CA) or anti-H-2 Kb (mouse IgG2a , clone AF6-88.5; Pharmingen), and FITC-labeled anti-Thy1.1 (mouse IgG1 , clone OX7; Valbiotech, Paris, France) or anti-Thy1.2 (mouse IgG2b clone 5a-8; Caltag) MoAbs. Biotinylated MoAbs were showed by phycoerythrin (PE)-labeled streptavidin (SAPE; Pharmingen). A total of 20,000 events were analyzed on a FACScan. Splenocytes of FVB or B6 origin were distinguished by the expression of H-2 Dq or H-2 Kb, and by expression of the T-cell–specific allotypic marker Thy1.1 or Thy1.2, respectively.
Pathologic examination.Mice were killed at different times after BMT and autopsied, and tissues were taken for histopathologic analyses. Spleen and liver samples were fixed in Bouin's liquid and embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin.
RESULTS
In vitro GCV sensitivity of T lymphocytes from TK and ΔTK transgenic mice.We verified that dividing T cells from EpTK stimulated by ConA were indeed sensitive to GCV. Fifty percent inhibition of proliferation was obtained at a concentration (IC50 ) of 1 μmol/L, a dosage at least 200-fold lower than that needed to obtain the same effect with nontransgenic cells (Fig 1).
In accordance with previous results,20 T cells from EpΔTK transgenic mice showed a sensitivity to GCV in the same range although slightly inferior to that of T lymphocytes from EpTK mice (Fig 1).
FVB BM-grafted B6 mice: a model of lethal GVHD.We developed a model of GVHD resulting in 100% mortality soon after BMT using FVB mice, a strain not previously used as BM donors in experimental allogeneic BMT. We tested different combinations of recipient irradiation doses, as well as injected BM cell and CD3+ peripheral T-cell numbers. When 10-Gy–irradiated B6 mice were reconstituted with 107 FVB BM cells, we observed prolonged survival, whereas all ungrafted animals died before day 16 (Fig 2). In these conditions, more than 98% of splenocytes were of donor origin (Fig 3A). When 107 CD3+ peripheral T cells from mice of FVB genetic background were added to the FVB BMT, all animals died of GVHD between days 7 and 34 (Fig 2). Similar results were obtained using either PBS-treated mice receiving EpTK or EpΔTK peripheral T cells or GCV-treated mice receiving FVB nontransgenic peripheral T cells. Notably, this observation also indicates that both TK- and ΔTK-expressing T cells in the absence of GCV are fully competent to induce a lethal GVHD. Histopathologic examination of spleen and liver of these animals showed characteristic GVHD lesions such as (1) architecture disruption, necrosis, and congestion in the spleen; (2) hepatic periportal necrosis; (3) mononuclear portal infiltrates; and (4) endothelialitis of portal or centrolobular veinules (Fig 4A). By comparison, B6 mice receiving only FVB BM had a normal histology (Fig 4B).
Prevention of GVHD by genetic immunosuppression.We analyzed whether GCV could prevent GVHD in mice receiving EpTK or EpΔTK CD3+ T cells. We administered GCV using subcutaneous miniosmotic pumps after verifying that mouse GCV plasma concentrations were compatible with those required for killing all dividing TK-expressing cells (data not shown).
Using this delivery mode, we observed that mice receiving a 7-day GCV treatment initiated at the time of transplantation were protected from GVHD. At day 60, the survival rate was 100% in the GCV-treated group receiving EpTK CD3+ T cells, and was still 92% at the end of a 120 day follow-up (Fig 2A). Protected mice were apparently healthy, presented no visible skin lesions, and gained weight comparably to controls receiving only BM (data not shown). Hematologic reconstitution was analyzed by flow cytometry at different times from day 14 to day 212. Splenocytes of GCV-treated animals were of donor H-2q origin in the B-cell (B220+) and non–B-cell (B220−) compartments, the latter containing donor Thy1.1+ T cells but no recipient Thy1.2+ T cells (Fig 3B). Finally, histological analysis in these animals showed nodular architecture in the spleen and no significant hepatocyte necrosis or portal mononuclear cell infiltrates in the liver (not shown) and similar to control animals receiving only BM. Taken together, these data indicate that a GCV treatment, administered at the onset of allogeneic BMT and with a course as short as 7 days, abrogates GVHD and allows a full recovery from the lethal irradiation.
Protection from GVHD was slightly less efficient in the group receiving EpΔTK CD3+ T cells. The survival rate was 79% at day 60 and 67% at day 120 (Fig 2B). However, two different outcomes must be distinguished. On the one hand, a majority of mice behaved as GCV-treated animals receiving EpTK T cells. They were apparently healthy, presented no skin lesions during a 120-day observation period, and showed complete donor-type hematologic reconstitution. There were no histological signs of GVHD in the spleen or liver (Fig 4C).
On the other hand, 5 of 19 mice exhibited signs suggesting the occurrence of a delayed GVHD (Table 1). Clinically, these animals presented weight loss and/or skin lesions on ears, neck, limbs, or abdomen, but only after day 40. In the absence of any further GCV treatment, 2 mice died 14 and 29 days after the occurrence of these signs (Table 1A) with histological signs of severe GVHD (Fig 4D). These results suggest that, in these mice, the 7-day GCV treatment was sufficient to prevent early, but not delayed GVHD.
Effect of a second GCV administration on mice with late-onset GVHD.We tested the hypothesis that this late-onset GVHD was due to a population of EpΔTK alloreactive T cells that had escaped GCV-mediated killing and should therefore still be sensitive to GCV. Indeed, mature peripheral T cells of transgenic origin can persist long after BMT. Using a polymerase chain reaction and transgene specific primers, such cells could still be detected at day 212 in the spleen of seven of eight mice belonging to both groups receiving EpTK or EpΔTK CD3+ T cells (data not shown).
Three mice with late-onset GVHD received a second 7-day course of GCV (Table 1B). Two mice treated soon after the occurrence of GVHD signs fully recovered in 3 weeks with complete disappearance of skin lesions. One mouse that received the second GCV administration more than 50 days after the occurrence of GVHD signs had significant clinical improvement although extensive skin lesions persisted, but died 46 days after the second GCV treatment.
In vitro responsiveness of T cells from protected mice stimulated by mitogen and alloantigen.We tested the ability of T cells from GCV-treated animals to respond to mitogens or alloantigens. Splenocytes from mice grafted with BM plus EpTK or EpΔTK CD3+ T cells and treated with GCV exhibited a strong proliferative response to ConA (Fig 5). In MLR, these mice appeared tolerant to H-2q donor or H-2b recipient antigens, whereas they responded normally to H-2d third party antigens (Fig 5). Altogether, the GCV treatment does not alter proliferative responses in the T-cell compartment and leads to a specific tolerance to recipient alloantigens.
DISCUSSION
At present, T-cell depletion of the BM is the only way to efficiently prevent GVHD in human recipients of allogeneic BMT. Unfortunately, this method results in impaired engraftment and a higher rate of leukemia recurrence by loss of the GVL effect.23,24 In contrast, the presence of contaminating T cells in the BMT permits a good engraftment and potent GVL effect, but leads to a frequent occurrence of GVHD.25 The ideal therapeutic strategy will thus be to design a system allowing specific elimination of alloreactive T cells without affecting T cells with other specificities, including those specific for pathogens or leukemia antigens. Likewise, it should be possible to induce a state of tolerance to the recipient alloantigens and to retain the functional immune system required to fight infections and leukemia recurrence.
Because it is not yet feasible to specifically target T cells based on the specificity of their antigen receptor, we took advantage of the fact that, in the period immediately after BMT, the vast majority of the activated dividing T cells are the alloreactive ones. Indeed, 6% to 12% of donor T cells migrate to and proliferate in a host lymphoid organ during GVHD.26 Furthermore, a high percentage of CD4+ clones generated from freshly isolated T cells activated during GVHD are specific for recipient alloantigens.27 Therefore, killing only the dividing T cells at this period of time should be equivalent to killing specifically alloreactive T cells. To prove the concept, the treatment should prevent the occurrence of GVHD while producing tolerance to recipient alloantigens together with maintenance of mature T cells, preventing the occurrence of severe infections due to immunosuppression. Our experimental system of transgenic mice expressing TK specifically in T lymphocytes allowed us to test this therapeutic strategy.
We indeed demonstrated that a treatment initiated at the time of BMT and with a course as short as 7 days is sufficient to completely prevent the occurrence of GVHD in animals receiving allogeneic BMT and mature T cells expressing TK. Not only were the animals protected from GVHD, but they did not experience apparent infectious complications and they had a normal survival. The persistence of a pool of donor-type Thy1.1+ T cells could be shown by flow cytometry analysis shortly after BMT and confirmed the actual preservation of a mature T-cell compartment despite the GCV treatment. Finally, we could show that these T cells respond normally to mitogen stimulation or third party alloantigens, whereas they do not respond to donor or recipient alloantigens. These experiments demonstrate that most if not all of the allospecific T cells are activated almost immediately after BMT, because they were all killed during the 7-day GCV treatment. This limited destruction of the mature T-cell pool mediated by GCV is of particular interest with regard to the role of T cells in supporting engraftment at the time of BMT. In addition, it was previously shown that a GVL effect can be observed in the absence of GVHD in recipients of non — T-cell–depleted BM.28 It is thus reasonable to assume that the TK/GCV approach may allow effective control of GVHD compatible with favorable conditions of engraftment and maintenance of a GVL effect, an objective that cannot be achieved with classical nonspecific ex vivo T-cell depletion of the BM. For example, an anti–B-cell lymphoma effect mediated by anti-idiotype specific T cells should not be affected by the GCV treatment.29-31 Nevertheless, it should be demonstrated experimentally that it is indeed possible to eliminate GVHD without losing GVL effects or increasing graft rejection. Indeed, it remains possible that the GCV treatment might also eliminate some activated T cells responding to leukemic cells antigens or to residual host lymphoid cells responsible for rejection. In this regard, the timing of GCV ablation may prove to be critical. These aspects are testable with the present experimental model.
In this work, we used two related drug-sensitivity genes, namely TK and a truncated form, ΔTK, initially developed to overcome sterility problems observed in TK-transgenic mice.18,19 Using EpTK or EpΔTK transgenes expressed in mature T cells, we achieved an excellent protection of GCV-treated mice during the first weeks post-BMT. Nevertheless, a minority of mice receiving EpΔTK T cells developed a late-onset lethal GVHD, indicating that some alloreactive T cells can escape GCV treatment under particular experimental conditions. It is unlikely that these cells corresponded to partially activated nondividing alloreactive T cells with a lower avidity T-cell receptor,32 because mice receiving Ep-TK– and EpΔTK-T cell would have behaved identically in this regard. Most probably, it is due to a less efficient GCV-mediated killing in ΔTK- rather than in TK-expressing cells. We previously showed that the properties of ΔTK expressed under the control of an inducible promoter were dependent on its level of expression, a condition not seen with the wild-type complete TK gene.20 We observed in ConA activation experiments that T cells from mice of the EpΔTK transgenic line used in this study were less sensitive to GCV as compared with EpTK. These properties of ΔTK led to the development of a late-onset GVHD that may represent a model of chronic GVHD that should be further characterized. In this system, it was possible not only to prevent GVHD, but also to treat an established disease. However, this later observation was obtained from the treatment of a small number of animals with late-onset GVHD. This point thus deserves further specific investigations, considering its important implications for therapeutics.
Our experimental system should be helpful to evaluate the best possible use of such a strategy for human allogeneic BMT. Gene therapy has now reached the clinical stage. Notably, the TK gene is widely employed in ongoing clinical trials, particularly in the field of anticancer therapy.33 Using retroviral-mediated gene transfer, it is possible to transduce human peripheral T cells with a TK gene and inhibit allogeneic reactivity with GCV in vitro.16 Increasing the level of gene transfer to target cells and optimizing methods of in vitro selection of TK+ T cells to avoid the risk of reinjecting TK− T cells is a major point to be considered regarding the application of this therapeutic strategy to humans. Altogether, there is a rationale for using T-cell–depleted BM together with TK-expressing T cells, a strategy now under evaluation in ongoing clinical trials.33 34 In this regard, several parameters can be analyzed to optimize such a clinical approach. Indeed, the versatility of the TK/GCV system allows the possibility of several different protocols of GCV administration in patients receiving T-cell–depleted BM plus TK-transduced T cells. On the one hand, patients could be treated at the first signs of GVHD, a strategy currently under evaluation in clinical trials. On the other hand, patients could be treated by GCV in a preventive manner as in the present model. This should result in durable protection from GVHD by rapidly killing the alloreactive cells before they expand. Finally, our experimental model should allow us to determine whether it is possible to dissociate the GVHD and GVL effect according to these different modalities of use.
ACKNOWLEDGMENT
The authors thank Eliane Gluckman, Pierre Tiberghien, Jean-Claude Gluckman, François Lemoine, and Gilbert Bensimon for helpful discussions; Bertrand Diquet for pharmacokinetics of GCV; Laurence Lejeune for outstanding technical assistance; and Corinne Pacot for excellent maintenance of our animal facility.
Supported by the Université Pierre et Marie Curie, the Agence Nationale de Recherche contre le SIDA, the Association de Recherche sur les Déficits Immunitaires Viro-Induits, Génopoı̈étic S.A., Assistance Publique-Hôpitaux de Paris, and the Centre National de la Recherche Scientifique.
Address reprint requests to David Klatzmann, MD, PhD, CNRS ERS 107, CERVI, Groupe Hospitalier Pitié-Salpêtrière, 83 boulevard de l'Hôpital, 75651 Paris Cedex 13, France.

![Fig. 2. Protective effect of a 7-day GCV treatment on the occurrence of GVHD in mice receiving (A) EpTK or (B) EpΔTK transgenic T cells. Irradiation control group (○): Ungrafted irradiated B6 mice (n = 15 in both [A] and [B]). Reconstitution control group (•): Irradiated B6 mice engrafted with FVB BM (n = 15 [A] and 11 [B]). GVHD control group (□): Irradiated B6 mice engrafted with FVB BM plus either FVB nontransgenic T cells and GCV administration or transgenic T cells and PBS administration (n = 25 [A] and 23 [B]). Experimental group (▪): Irradiated B6 mice engrafted with FVB BM plus (A) EpTK or (B) EpΔTK T cells and treated with GCV (n = 13 [A] and 19 [B]). Results are presented as Kaplan-Meier survival curves of cumulative data for each set of 3 independent experiments in both (A) and (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4636/3/m_bl_0014f2.jpeg?Expires=1765944263&Signature=VLfBiy~ZYMX1pFI0h3fgXgoHKfrJ5yFDbRfCLr33bPm7kOAjjDLQapSA4sbrZMpkZOKIiEUAJIUZbSVhoaW6J983jn3KOyX9hCpDGa99VSYl6E3wE-dcK6diZPvtPbIP7k8Y88~7cQ0MJ496DD1gyr4SlJXBQdjr730zRpiONCroE66Upq27NKp-rb9JtURgjo8SLAHUSqlP8jioqJu~Pl3l-BhYDI9JZVg9pyuqQ-h1pfIEwEbvFLv2mMX1Hy6XFelBr9boNkBTdOIBiFNJDPukL6VGW6NsMiFhT~KiLz9i67czF8zB0EzwiQx2MLybtWpYXDXfqovurD7QVbsn4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal