Abstract
Familial erythrocytosis (familial polycythemia) inherited as an autosomal dominant trait has recently been reported to be associated with mutations in the gene encoding the erythropoietin receptor (EpoR) in a small number of families. We studied a new kindred with dominantly inherited familial erythrocytosis associated with heterozygosity for a deletion of seven nucleotides between positions 5985 and 5991 in exon 8 of the EpoR gene, resulting in an EpoR peptide that is truncated by 59 amino acids at its C-terminus. A 7-bp direct repeat is present in the normal EpoR gene at the site of this mutation, consistent with the slipped mispairing model for the generation of short deletions during DNA replication. Hypersensitivity to Epo of erythroid progenitors from an affected individual was observed in in vitro methylcellulose cultures, as indicated by more numerous and larger colonies compared with those of a control subject. To study mutant EpoR function, the cDNA encoding the mutant EpoR was synthesized by reverse transcription-polymerase chain reaction of peripheral blood RNA from the proband and stably tranfected into murine interleukin-3–dependent 32D cells. Epo dose-response assays showed that cells expressing the mutant EpoR displayed fivefold to 10-fold increased sensitivity to Epo compared with cells expressing similar numbers of the wild-type EpoR.
THE PROLIFERATION, differentiation, and survival of erythroid progenitor cells are regulated by the glycoprotein hormone erythropoietin (Epo), which exerts its effect on its target cells by binding to the extracellular N-terminal domain of the Epo receptor (EpoR). Erythrocytosis has many diverse causes, often occurring secondary to conditions that result in sustained tissue hypoxia or disorders associated with autonomous, ectopic production of Epo leading to an Epo-mediated increase in red blood cell mass.1 Primary erythrocytosis, on the other hand, is not mediated by an appropriate or inappropriate increase in Epo production but rather is associated with an intrinsic abnormality of hematopoietic progenitor cells resulting in autonomous red blood cell proliferation such as that seen in polycythemia vera.1-3 Erythrocytosis may be familial, occurring secondary to tissue hypoxia associated with high oxygen affinity hemoglobins or abnormalities of 2,3-diphosphoglycerate metabolism, or may occur as a primary disorder, as reported in some families.1-6 Recently, several families with dominantly inherited primary erythrocytosis have been reported in association with mutations in the gene encoding the EpoR.7-13 Six of the eight different human EpoR gene mutations described to date11-13 are associated with familial erythrocytosis (familial polycythemia) and all six result in truncation of the intracellular C-terminal domain of the receptor that is thought to exert a negative effect on receptor function.7-11
We report here a new kindred with familial erythrocytosis associated with a mutation in one allele of the EpoR gene. We determined the structure of the 3′ end of the EpoR gene, the region encoding the C-terminus. Nucleotide sequencing of amplified genomic DNA showed the presence of heterozygosity for a 7-bp deletion in exon 8 of the EpoR gene, causing a frameshift and introduction of a premature termination codon, resulting in synthesis of a truncated EpoR protein in affected individuals. In in vitro cultures, erythroid progenitors of an affected individual displayed hypersensitivity to Epo when compared with those of an unaffected family member. In proliferation assays using murine interleukin-3 (IL-3)–dependent 32D cells transfected with the mutant or wild-type EpoR cDNAs and rendered Epo-dependent, increased Epo sensitivity of cells expressing the mutant EpoR was observed compared with cells expressing similar numbers of the wild-type receptor.
MATERIALS AND METHODS
Case report.We studied a three-generation Caucasian family from New Britain, CT (see Fig 3, top). The proband (III-3), who was first evaluated at 15 years of age because of persistent headaches, had a hemoglobin level of 20.7 g/dL and a hematocrit of 62%. The white blood cell and platelet counts and morphology of the red blood cells in peripheral blood smear were normal. The red blood cell mass had been shown to be elevated in the proband's father (II-4) and paternal aunt (II-1). The plasma Epo level was less than 10 mU/mL in all affected family members and hemoglobin electrophoresis and P50 were normal. The father of the proband also suffered from persistent headaches associated with a hemoglobin level of 22.4 g/dL and he was started on a phlebotomy regimen that provided symtomatic relief. Although hypertension or cardiovascular disease has been previously reported in some individuals with familial erythrocytosis, there was no history of these conditions in this family.14 The results of hematologic studies of the family members are summarized in Table 1.
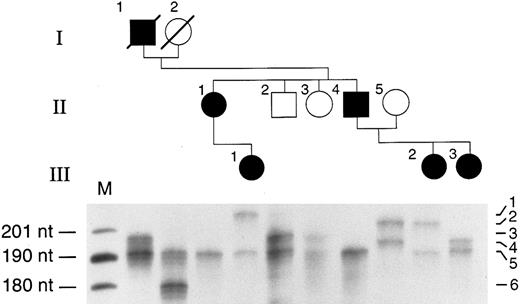
Detection of the mutation by restriction endonuclease digestion of PCR-amplified DNA. The pedigree is shown at the top of the figure. Genomic DNA samples obtained from each family member were amplified using primer pair C and F as shown in Fig 1. The amplification products were digested with Rsa I and fractionated by electrophoresis in a 5% agarose gel. Digestion of the amplification products of the normal allele with Rsa I yields fragments of 84 and 64 bp, whereas the mutant allele yields fragments of 77 bp and 64 bp. None of the unaffected individuals (open symbols) have a 77-bp fragment. All affected individuals in the family (solid symbols) are heterozygous for the mutation.
Detection of the mutation by restriction endonuclease digestion of PCR-amplified DNA. The pedigree is shown at the top of the figure. Genomic DNA samples obtained from each family member were amplified using primer pair C and F as shown in Fig 1. The amplification products were digested with Rsa I and fractionated by electrophoresis in a 5% agarose gel. Digestion of the amplification products of the normal allele with Rsa I yields fragments of 84 and 64 bp, whereas the mutant allele yields fragments of 77 bp and 64 bp. None of the unaffected individuals (open symbols) have a 77-bp fragment. All affected individuals in the family (solid symbols) are heterozygous for the mutation.
Laboratory Test Results
| Family Member . | Hemoglobin (g/dL) . | Hematocrit (%) . | Epo Level (mU/mL) . |
|---|---|---|---|
| II-1 | 19.6 | 60 | 8.9 |
| II-2 | 15.7 | 45 | 11 |
| II-3 | 10.5 | 40 | — |
| II-4 | 22.4 | 66 | 5.3 |
| II-5 | 14.1 | 41 | 13.5 |
| III-1 | 18.6 | 56 | 6.8 |
| III-2 | 17 | 50 | 4.7 |
| III-3 | 20.7 | 62 | 6.2 |
| Family Member . | Hemoglobin (g/dL) . | Hematocrit (%) . | Epo Level (mU/mL) . |
|---|---|---|---|
| II-1 | 19.6 | 60 | 8.9 |
| II-2 | 15.7 | 45 | 11 |
| II-3 | 10.5 | 40 | — |
| II-4 | 22.4 | 66 | 5.3 |
| II-5 | 14.1 | 41 | 13.5 |
| III-1 | 18.6 | 56 | 6.8 |
| III-2 | 17 | 50 | 4.7 |
| III-3 | 20.7 | 62 | 6.2 |
Preparation of genomic DNA and RNA.Peripheral blood from the proband and other affected and unaffected family members was lysed as described previously.15 Genomic DNA was prepared by standard procedures and total RNA was isolated from the hemolysates using TRIzol reagent (GIBCO Life Technologies, Gaithersburgh, MD) according to the manufacturer's protocol. Genomic DNA extraction from sections of paraffin blocks and material from tissue slides containing liver and endometrial tissues of deceased family members I-1 and I-2, respectively, was performed as described previously.16 The final sample pellets of the archival tissues were suspended in 100 μL of H2O and 10 μL was used in subsequent polymerase chain reaction (PCR) amplifications.
Amplification of genomic DNA and cDNA.For each 50-μL PCR amplification, 100 ng of genomic DNA was used in a reaction that contained 10 mmol/L Tris-HCl [pH 8.3], 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.01% gelatin, 200 μmol/L of each dNTP, 5 μmol/L of each primer, and 1 U of Taq polymerase (Expand High Fidelity; Boehringer Mannheim, Indianapolis, IN). The sense oligonucleotide primers were as follows: A, 5′ GGCTCTGAAGCAGAAGATCT 3′ (nucleotides no. 5419-5438, numbering per Noguchi et al17 ); B, 5′ CCAGTGGGCAGTGAGCATGC 3′ (nucleotides no. 5758-5777); and C, 5′ TCCTGCTCATCTGCTTTGGCC 3′ (nucleotides no. 5899-5919). The antisense oligonucleotide primers were as follows: D, 5′ CTGAGAGAGGCCTCGCCAT 3′ (nucleotides no. 6308-6290); E, 5′ GTCATATTGGATCCCTGATC 3′ (nucleotides no. 6250-6231); and F, 5′ GCCAGAGTCAGATACCACAAG 3′ (nucleotides no. 6075-6055). The relative positions of these primers are shown in Fig 1 (top). The denaturing step was performed at 94°C for 1 minute, the annealing step at 58°C for 1 minute, and the extension step at 72°C for 1 minute for 30 cycles. The final extension cycle at 72°C was extended to 7 minutes. Second round PCR amplifications were performed by using 1 μL of the first PCR reaction mix as template. The PCR amplification products were subcloned into the pCRII vector (Invitrogen, San Diego,CA) and nucleotide sequencing was performed for six clones of each PCR product using the dideoxy chain termination method of Sanger using the Sequenase enzyme kit (US Biochemical Corp, Cleveland, OH). Restriction enzyme digests of genomic PCR amplification products were separated in 5% MetaPhor agarose gels (FMC Bioproducts, Rockland, ME). The genomic PCR amplification conditions for EpoR simple sequence repeat polymorphism analysis and the oligonucleotide primer sequences in the region upstream of the translation initiation site of the EpoR gene have been previously described.18
Diagram of exons 7 and 8 of the EpoR gene. The coding sequences are shown as solid boxes, the intervening sequences are depicted as lines, and the 3′ untranslated region as an open box. The lettered arrows indicate the position and orientation of the oligonucleotide primers described in the Materials and Methods. The open arrowhead (▿) above the mutant allele (bottom) indicates the position of the deletion. An Rsa I (R) restriction map of the 177-bp PCR amplification product using primer pair C and F is shown at the bottom.
Diagram of exons 7 and 8 of the EpoR gene. The coding sequences are shown as solid boxes, the intervening sequences are depicted as lines, and the 3′ untranslated region as an open box. The lettered arrows indicate the position and orientation of the oligonucleotide primers described in the Materials and Methods. The open arrowhead (▿) above the mutant allele (bottom) indicates the position of the deletion. An Rsa I (R) restriction map of the 177-bp PCR amplification product using primer pair C and F is shown at the bottom.
For amplification of EpoR transcripts, total RNA was isolated from the peripheral blood lysates of the proband, treated with 2 U of RNase-free DNase (Promega, Madison, WI) for 30 minutes at 37°C, phenol/chloroform extracted, and ethanol precipitated. Ten micrograms of total RNA was heated at 65°C for 5 minutes, cooled to room temperature, and incubated at 42°C for 1 hour in a 25 μL reverse transcription reaction for first-strand cDNA synthesis containing 50 mmol/L Tris-HCl [pH 8.3], 50 mmol/L KCl, 10 mmol/L MgCl2, 10 mmol/L dithiothreitol, 0.5 mmol/L spermidine, 25 U RNasin (Promega), 2 mmol/L of each dNTP, 2.5 μg of random hexamers (Boehringer Mannheim), and 16 U of avian myeloblastosis virus reverse transcriptase (Promega). An aliquot of the reverse transcription product was then amplified directly by PCR as described above using primer A in exon 7 and primer D in exon 8 to distinguish the amplified cDNA product from that resulting from amplification of contaminating genomic DNA. The reverse transcription-PCR (RT-PCR) products were subcloned into the pCRII cloning vector (Invitrogen) and sequenced as described above.
In vitro erythroid colony formation assays.Peripheral blood (15 mL) was obtained in heparin anticoagulant. Mononuclear cells were separated through a Ficoll density gradient and washed twice with phosphate-buffered saline. The cells were diluted to 2.5 × 106/mL and mixed with methylcellulose medium (MethoCult H4531; Stem Cell Technologies, Vancouver, British Columbia, Canada) with the desired concentration of Epo (Amgen, Thousand Oaks, CA) to a final cell density of 2.5 × 105/mL in each plate. Assays were performed in triplicate. The plates were placed in a humidified incubator at 37°C with 5% CO2 and scored for erythroid colony formation at day 15 by two investigators.
Cell lines and reagents.Murine myeloid IL-3–dependent 32D cells originally isolated from long-term bone marrow cultures were previously described19 20 (kindly provided by Dr A. Khanna-Gupta, Yale University, New Haven, CT). Cells were grown in Iscove's modified Dulbecco's medium (IMDM; GIBCO Life Technologies) supplemented with 10% fetal bovine serum and 10% WEHI conditioned medium as a source of IL-3. Both the untransfected 32D cells and the stably transfected pools of cells expressing the mutant or wild-type EpoRs were maintained at a culture density of less than 5 × 105/mL. Recombinant human Epo was purchased from Amgen.
Cloning and expression of the mutant EpoR.The wild-type (WT) human EpoR cDNA cloned in the pRc/CMV(neo) expression vector (Invitrogen) was kindly provided by Dr J.C. Winkelmann (University of Cincinnati College of Medicine, Cincinnati, OH). A 787-bp Stu I-Stu I EpoR cDNA fragment containing exons 7 and 8 from a plasmid containing a mutant cDNA clone from the proband was subcloned into a plasmid containing the wild-type EpoR (LAP37) that had been digested with Stu I and gel purified. The mutant EpoR cDNA was then purified from this plasmid and subcloned into the expression vector pRc/CMV (Invitrogen). The structures of the expression plasmids were confirmed by digestion with multiple restriction enzymes and by DNA sequencing. Plasmid DNAs containing the normal and mutant EpoR cDNAs (10 μg) were transfected into 32D cells by electroporation. Transfected cells were selected in medium containing 0.8 mg/mL G418 for 14 days. Pools of G418-resistant transfected cells were transferred to medium containing 1 U/mL of Epo instead of WEHI-conditioned medium and maintained in 0.4 mg/mL G418, and cells capable of growth in Epo were used in subsequent experiments. Quantitation of the average numbers of wild-type or mutant EpoRs on the stably transfected cells was performed by 125I-Epo binding studies and Scatchard analyses, as described previously.21
Epo dose-response assays.Pools of transfected cells expressing similar numbers of wild-type and truncated EpoR were cultured for 5 days with medium containing IMDM, 10% fetal bovine serum, and the indicated concentrations of Epo. Cells were counted daily and viability was determined by the trypan blue exclusion technique.
RESULTS
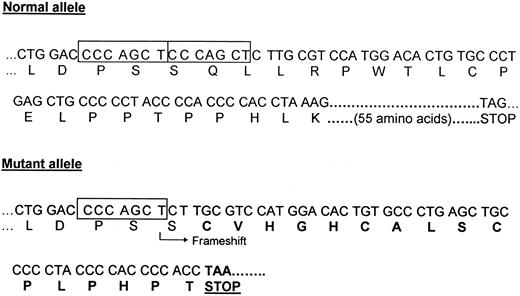
Genetic analyses.Because the first two reported mutations in kindreds with familial erythrocytosis were located in exon 8 of the EpoR gene,7 8 genomic DNA corresponding to exons 7 and 8 of the EpoR gene was amplified by PCR using the primers shown in Fig 1. DNA sequencing of the subcloned genomic amplification products showed the presence of a 7-bp deletion between nucleotides 5985 and 5991 in exon 8 in one allele of the EpoR gene (Fig 2). The same result was obtained by DNA sequencing of individual subclones of cDNA encoding exons 7 and 8 of the EpoR synthesized by RT-PCR of peripheral blood RNA from the proband (data not shown). The presence of a 7-bp difference in size between the normal and the mutant alleles could be visualized by agarose gel electrophoresis of genomic PCR products digested with a restriction endonuclease. This allowed rapid, PCR-based detection of the mutation in each family member. As shown in Fig 1 (bottom), the 177-bp genomic DNA amplification product obtained by PCR using primer pair C and F derived from the normal allele should be digested by the restriction endonuclease Rsa I into 4 fragments of 84, 64, 23, and 6 bp. Digestion by Rsa I of the PCR product derived from the mutant allele results in the presence of a shorter 77-bp fragment (due to the 7-bp deletion shown by ▿), in addition to the 84-bp fragment derived from the normal allele, only in affected individuals of the family. As shown in Fig 3 (bottom), the 7-bp deletion can be visualized by fractionation of the digested PCR product in a 5% agarose gel and is present only in the affected individuals of the family. The deletion results in a shift of the normal reading frame after codon 433 and the introduction of 16 novel amino acids before a premature termination codon is encountered (Fig 4). The truncated receptor is shortened by 59 amino acids at its C-terminal end compared with the normal receptor.
DNA sequence of subcloned PCR products from the EpoR gene of affected individual III-3. Analysis of individual subclones shows the sequences for the normal allele (left) and the mutant allele (right). The 7-bp direct repeat in the normal allele is indicated in boxes. In approximately half of the subclones, a 7-bp deletion in exon 8 between positions 5985 and 5991 was detected. Two of the abnormal amino acids resulting from the frameshift in the mutant allele are indicated in bold letters.
DNA sequence of subcloned PCR products from the EpoR gene of affected individual III-3. Analysis of individual subclones shows the sequences for the normal allele (left) and the mutant allele (right). The 7-bp direct repeat in the normal allele is indicated in boxes. In approximately half of the subclones, a 7-bp deletion in exon 8 between positions 5985 and 5991 was detected. Two of the abnormal amino acids resulting from the frameshift in the mutant allele are indicated in bold letters.
DNA sequence and encoded amino acid sequence of parts of the normal allele (top) and mutant allele (bottom) of exon 8 of the EpoR gene. The position of the 7-bp direct repeat is indicated by the two boxes in the normal allele. The 55 C-terminal amino acids encoded by the normal allele are not shown. The mutant allele (del5985-5991) contains a 7-bp deletion. The arrow indicates the position of the frameshift resulting in an abnormal amino acid sequence (bold letters). A chain termination codon is encountered after the 17th amino acid in the new reading frame.
DNA sequence and encoded amino acid sequence of parts of the normal allele (top) and mutant allele (bottom) of exon 8 of the EpoR gene. The position of the 7-bp direct repeat is indicated by the two boxes in the normal allele. The 55 C-terminal amino acids encoded by the normal allele are not shown. The mutant allele (del5985-5991) contains a 7-bp deletion. The arrow indicates the position of the frameshift resulting in an abnormal amino acid sequence (bold letters). A chain termination codon is encountered after the 17th amino acid in the new reading frame.
Because an apparently identical mutation was identified by Kralovics et al,9 we performed an analysis of the simple sequence repeat polymorphism in the 5′ flanking DNA of the EpoR gene in an attempt to determine the presence or absence of a founder effect. This region of 5′ flanking DNA of the EpoR gene between nucleotides −618 and −420 from the initiator ATG contains a stretch of (GA)n and (GGAA)n repeats that vary in number.18,22,23 The results of this analysis are shown in Fig 5. Both affected siblings in the second generation (II-1 and II-4) inherited allele 5 from their affected father (I-1), whereas the two unaffected siblings in this generation (II-2 and II-3) generation inherited a different allele 5 from their mother. The paternal allele 5 also cosegregates with the disease phenotype in generation III. DNA from the proband of the other affected family9 (kindly provided by Dr J.T. Prchal, University of Alabama, Birmingham, AL) was homozygous for allele 5 (data not shown). Therefore, we cannot exclude a founder effect.
Analysis of simple sequence repeat polymorphism. The pedigree of the affected kindred is shown at the top of the figure. DNA for study was available from two deceased individuals (I-1 and I-2) as well as from the living family members. DNA 5′ to the EpoR gene translation initiation site contains a microsatellite repeat polymorphism18,22 23 and this region of DNA was amplified by PCR and the products fractionated by polyacrylamide gel electrophoresis. Most individuals have two differently sized alleles that correspond to the inherited parental copies of the EpoR gene, one from each parent; two individuals (II-1 and II-4) display only one band, consistent with homozygosity for two alleles of the same size. Six alleles were observed and are numbered in order of decreasing size. The two affected individuals in the second generation (II-1 and II-4) share allele 5 inherited from their affected father (I-1) as well as an allele of the same size from their unaffected mother (I-2). The two unaffected individuals in the second generation (II-2 and II-3) must have inherited allele 5 from their mother (I-2). Thus, allele 5 inherited from the father (I-1) cosegregates with the disease phenotype.
Analysis of simple sequence repeat polymorphism. The pedigree of the affected kindred is shown at the top of the figure. DNA for study was available from two deceased individuals (I-1 and I-2) as well as from the living family members. DNA 5′ to the EpoR gene translation initiation site contains a microsatellite repeat polymorphism18,22 23 and this region of DNA was amplified by PCR and the products fractionated by polyacrylamide gel electrophoresis. Most individuals have two differently sized alleles that correspond to the inherited parental copies of the EpoR gene, one from each parent; two individuals (II-1 and II-4) display only one band, consistent with homozygosity for two alleles of the same size. Six alleles were observed and are numbered in order of decreasing size. The two affected individuals in the second generation (II-1 and II-4) share allele 5 inherited from their affected father (I-1) as well as an allele of the same size from their unaffected mother (I-2). The two unaffected individuals in the second generation (II-2 and II-3) must have inherited allele 5 from their mother (I-2). Thus, allele 5 inherited from the father (I-1) cosegregates with the disease phenotype.
In vitro erythroid progenitor assays.Peripheral blood was obtained for methylcellulose cultures from an affected (III-2) as well as an unaffected family member (II-5) as a control. The results of scoring of burst-forming units-erythroid (BFU-E)–derived colonies at day 15 and at various Epo concentrations are shown in Fig 6. In the absence of added Epo in the culture medium, no BFU-E–derived colonies were detected in the samples from the affected individual as well as from the unaffected individual. At the highest Epo concentration of 1 U/mL, the numbers of BFU-E–derived colonies were similar in the cultures of the affected individual and those of the unaffected family member. However, at relatively low and intermediate Epo concentrations (25 to 50 mU/mL), a significant difference in the number of erythroid colonies was observed. At 25 mU/mL, no colonies were observed in the control plates compared with 17 ± 2.3 colonies in the patient cultures. At 50 mU/mL, a much greater average number of colonies (20 ± 1.6) was seen in the cultures from the individual with erythrocytosis than in the cultures from the unaffected family member. The striking differences in the total numbers of erythroid colonies was accompanied by a significant difference in the size of individual colonies when culture plates from the affected individual and control were compared (data not shown).
Erythroid colony formation assays in two family members. The vertical axis indicates the numbers of BFU-E–derived colonies per 2.5 × 105 cells, expressed as the mean ± standard deviation of assays performed in triplicate. The different final Epo concentrations added to the cultures are shown on the horizontal axis.
Erythroid colony formation assays in two family members. The vertical axis indicates the numbers of BFU-E–derived colonies per 2.5 × 105 cells, expressed as the mean ± standard deviation of assays performed in triplicate. The different final Epo concentrations added to the cultures are shown on the horizontal axis.
Expression and quantitation of EpoR in 32D cells.32D cells stably transfected with the wild-type EpoR cDNA (32DEpoR-WT) or the mutant cDNA from the patient with familial erythrocytosis (32DEpoR-FE) were capable of long-term growth in G418 and Epo. The average number of EpoRs expressed in pools of transfected cells was quantitated by 125I-Epo binding studies and Scatchard analyses (data not shown). The number of high-affinity binding sites for Epo were comparable in 32DEpoR-WT cells (439 ± 145 sites per cell) and in 32DEpoR-FE cells (219 ± 48 sites per cell). The low-affinity binding sites, which are probably not physiologically different, were also similar in 32DEpoR-WT cells (909 ± 30 site per cell) and in 32DEpoR-FE cells (782 ± 311 sites per cell).24 The proliferation assays described below were performed with pools of transfected cells expressing similar numbers of mutant or truncated EpoRs.
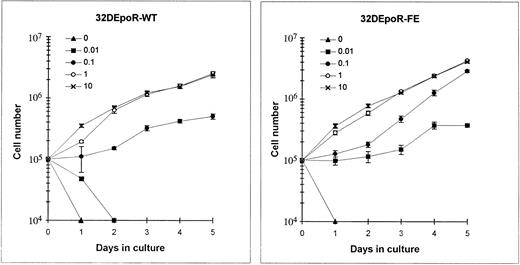
Function of the mutant EpoR.The proliferation of 32D cells expressing the mutant or wild-type EpoRs in various concentrations of Epo was measured by the trypan blue exclusion technique. As shown in Fig 7, 32DEpoR-FE cells displayed increased sensitivity to Epo at 0.1 U/mL, with a sevenfold increase in cell numbers by 5 days of growth. A major difference in the growth curves was particularly observed in cultures at a relatively low (0.01 U/mL) concentration of Epo. At this physiologic concentration of Epo, 32D cells expressing the wild-type EpoR died after 2 days in culture. In contrast, 32D cells expressing the mutant EpoR remained viable with stable cell numbers at 0.01 U/mL for 3 days, followed by proliferation during the next 2 days of culture. At maximal, saturating Epo concentrations, a much less pronounced difference in rate of proliferation was observed. Taken together, these results indicate that cells expressing the mutant EpoR require approximately only about one-fifth to one-tenth the amount of Epo that is required by 32D cells expressing the wild-type EpoR for proliferation. Interestingly, as observed in the growth curves in Fig 7, a relatively low Epo concentration of 0.01 U/mL allows maintenance of cell viability of cells expressing the mutant EpoR but not of cells expressing the wild-type EpoR. A genomic DNA fragmentation pattern typical of programmed cell death was observed in cells expressing the wild-type EpoR cultured in 0.01 U/mL of Epo but not in cells expressing similar numbers of the mutant EpoR (data not shown). This finding suggests that, in contrast to the wild-type EpoR, this mutant EpoR truncated at its C-terminus can support Epo-mediated inhibition of apoptosis at a concentration in culture of only 0.01 U/mL of Epo.
Epo dose-response of 32D cells expressing comparable numbers of wild-type (left) and mutant (right) EpoRs. The cells were cultured for 5 days in medium containing IMDM, 10% fetal bovine serum, and the indicated concentrations of Epo (in units per milliliter). The number of viable cells was measured by the trypan blue dye exclusion technique. Each point indicates the mean ± standard deviation of four independent cultures at each indicated Epo concentration.
Epo dose-response of 32D cells expressing comparable numbers of wild-type (left) and mutant (right) EpoRs. The cells were cultured for 5 days in medium containing IMDM, 10% fetal bovine serum, and the indicated concentrations of Epo (in units per milliliter). The number of viable cells was measured by the trypan blue dye exclusion technique. Each point indicates the mean ± standard deviation of four independent cultures at each indicated Epo concentration.
DISCUSSION
This study describes a new kindred with dominantly inherited familial erythrocytosis with heterozygosity for an Epo receptor gene mutation, including the results of functional studies of the effect of this mutant EpoR in normal human erythroid progenitor cells as well as in a transfected murine hematopoietic cell line. Since the first report by de la Chapelle et al7 of a family with dominantly inherited primary erythrocytosis associated with heterozygosity for a nonsense mutation in the EpoR gene, a total of five additional different mutations of the EpoR gene resulting in the synthesis of a truncated EpoR have been found to be associated with familial erythrocytosis.8-13 The 7-bp deletion, del5985-5991, in exon 8 of one allele of the EpoR identified in this kindred appears to be identical to the mutation reported in abstract form by Kralovics et al9 in an apparently unrelated family. This short deletion occurs within a 7-bp direct repeat in exon 8 of the EpoR. Direct repeats between 2 bp and 8 bp have been found in the immediate vicinity of many short gene deletions associated with various human genetic diseases and provide the basis for a number of models of deletion mutagenesis involving defective recombination, replication, or repair.25 The presence of a 7-bp direct repeat in the normal gene at the site of the deletion in this kindred is consistent with the slipped mispairing model for the generation of short deletions during DNA replication.25 The identification of an apparently identical mutation in an apparently unrelated family raises the interesting question as to whether the two mutations occurred independently in the two families in this region of DNA containing a direct repeat that may constitute a hotspot for replication-based deletion mutagenesis. Comparison of the results of simple sequence repeat polymorphisms in the two different families did not exclude a founder effect (Fig 5 and data not shown).
The function of the mutant EpoR was tested in in vitro erythroid progenitor assays as well as in transfectants of the 32D murine hematopoietic cell line. Two previous studies reported increased Epo sensitivity of endogenous erythroid progenitors of affected individuals with familial erythrocytosis and an EpoR mutation.5,8 In our experiments, the difference in the numbers of BFU-E–derived colonies formed is particularly striking at relatively low added Epo concentrations of 25 and 50 mU/mL (Fig 6). However, in contrast to the report of Juvonen et al,5 who observed significant numbers of erythroid colonies even in the absence of added Epo, a phenomenon characteristic of polycythemia vera progenitors, we did not observe formation of erythroid colonies in the absence of added Epo to the culture medium. The striking increase in numbers of erythroid colonies at relatively low Epo concentrations and the formation of much larger colonies in culture at higher Epo concentrations strongly suggest that increased sensitivity of progenitors to Epo in family members heterozygous for the EpoR mutation plays a role in the development of erythrocytosis. The results of our Epo dose-response experiments (Fig 7) with 32D cells expressing comparable numbers of mutant or wild-type Epo receptors parallel the results of a previous study in which murine IL-3–dependent Ba/F3 cells, transfected with the mutant human EpoR cDNAs from the first two reported kindreds with familial erythrocytosis, displayed increased Epo sensitivity.8 It is important to note that the cells used in the Epo dose-response experiments express either mutant or wild-type EpoRs. Therefore, the actual in vivo situation is probably more complex in heterozygous patients, whose erythroid cells express both mutant and wild-type receptors that are likely to heterodimerize. Moreover, our in vitro erythroid progenitor cultures and Epo-dose response experiments in 32D cells were performed in the presence of serum in the culture medium. This is an important consideration in regards to the recent report by Damen et al,26 who showed that the hyper-responsiveness to Epo of Ba/F3 cells expressing an EpoR lacking its C-terminal 91 amino acids is contingent on the presence of insulin-like growth factor-1 (IGF-1) in fetal calf serum and on activation of MAP kinase due to signal transduction triggered by IGF-1 and possibly other non-Epo growth factors present in serum.
Another significant finding of our Epo dose-response experiments (Fig 7) is that relatively low levels of Epo (0.01 U/mL) appear to be sufficient to inhibit apoptosis in cells expressing the truncated receptor but not in cells expressing the wild-type receptor. Thus, Epo-mediated signals that maintain the viability of the cells are transduced quite efficiently by this truncated mutant receptor but not by the wild-type receptor at quite low Epo concentrations. These results contradict those of the initial report of Nakamura et al27 in which a naturally occurring C-terminal truncated isoform of the human EpoR, lacking all of the amino acid residues encoded by exon 8 (203 amino acids) and predominantly expressed in immature progenitors, did not appear to support the prevention of apoptosis at low Epo concentrations in transfected Ba/F3 cells. These studies implicated the C-terminal domain of the EpoR in mediating signals that prevent apoptosis in response to Epo.27 However, a subsequent analysis of the Ba/F3 cells used in these experiments showed that significant levels of endogenous full-length murine EpoR mRNAs were present and that the truncated receptor isoform was mitogenically inactive.28 More recent studies reported by Zhuang et al29 using an in vitro-derived, truncated EpoR form lacking 154 C-terminal amino acid residues showed that the C-terminal domain of the EpoR is not required to mediate Epo-dependent inhibition of apoptosis and that Epo-induced JAK2 activation plays an essential role in this pathway. Our Epo dose-response assays that show that 10-fold lower concentrations of Epo are sufficient to inhibit apoptosis of 32D cells expressing the truncated EpoR potentially implicate apoptotic response pathway effects in the generation of the erythrocytosis phenotype.
The EpoR gene mutations associated with familial erythrocytosis syndromes are located within exon 8 of the gene that encodes the C-terminal domain of the receptor and result in the synthesis of receptors with C-terminal truncations of 70 to 83 amino acids.7-13 A negative regulatory function for the C-terminus of the EpoR on receptor mitogenic function had been previously suggested by results of experiments with Ba/F3 cells expressing in vitro-generated truncation mutants of the murine EpoR that showed increased Epo sensitivity of cells expressing the truncated EpoRs compared with that of cells expressing the wild-type EpoR.8,30-32 A negative regulator of EpoR function, hematopoietic cell phosphatase (SHP-1), has been shown to interact with the C-terminus of the EpoR32,33 and appears to mediate, at least in part, the downregulation of Epo-mediated activation of JAK2, a nonreceptor tyrosine kinase critical for Epo-induced mitogenesis as well as for inhibition of apoptosis.29,34-36 The apparent importance of SHP-1 as a negative regulator of EpoR mitogenic signalling is also supported by the phenotypes of mice with defective SHP-1 that have markedly increased numbers of splenic colony-forming units-erythroid with increased sensitivity to Epo.37 Because human EpoR mutations associated with erythrocytosis remove the binding site for SHP-1 on the EpoR, it has been postulated that lack of SHP-1 phosphatase activity resulting in prolonged JAK2 tyrosine phosphorylation could, at least in part, play a role in the pathogenesis of dysregulated proliferation of erythroid cells in these syndromes.32 We have recently reported our finding of persistent Epo-induced activation of STAT5, a substrate for JAK2, in 32D cells expressing the truncated EpoR.38 Furthermore, recent work by Damen et al26 showed that hematopoietic cells expressing truncated EpoRs are hyper-responsive to Epo when grown in fetal calf serum not only because the binding site for SHP-1 is lacking but also because MAP kinase is activated by non-Epo growth factors in serum. Thus, the signalling mechanisms responsible for the phenotype of dominantly inherited erythrocytosis and the apparent Epo hypersensitivity observed in in vitro assays of erythroid progenitors from heterozygous affected individuals are likely to be complex because the dominant effect that results in erythrocytosis could be the consequence of the presence of heterodimeric Epo receptors, truncated homodimers, or a combination effect. Regardless, our studies with 32D cell transfectants show that this naturally occurring mutant EpoR mediates proliferation and/or survival of the cells at very low concentrations of Epo. Future studies to determine the specific alterations in signal transduction processes in cells expressing the mutant Epo receptor may provide important insights into the regulation of erythropoiesis.
ACKNOWLEDGMENT
We thank Dr Susan McIntosh for providing clinical information and blood samples of the family members and Dr J.T. Prchal for providing DNA from the proband of the apparently unrelated family affected by the same mutation.9
Supported in part by grants from the Yale institutional Lucille P. Markey Physician Scientist Program (M.O.A.), the National Institutes of Health DK 44058 (B.G.F.), and Veterans Administration Merit Review Award (K.W.H.).
Presented in part at the Biomedicine ‘96 meeting of AFCR/ASCI/AAP, May 1996, and published in part as an abstract (J Invest Med 44:213A, 1996).
Address reprint requests to Bernard G. Forget, MD, Yale University School of Medicine, Hematology Section, WWW 403, 333 Cedar St, New Haven, CT 06520-8021.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal