Abstract
Thrombin activation requires assembly of a prothrombinase complex of activated coagulation factors on an anionic phospholipid surface, classically provided by activated platelets. We have previously shown that anionic phosphatidylserine is exposed by rat vascular smooth muscle cells (VSMCs) undergoing apoptosis after serum withdrawal. In this study, using a chromogenic assay, we have shown thrombin generation by apoptotic VSMCs expressing c-myc (VSMC-myc) with an area under the thrombin-generation curve (AUC) of 305 ± 17 nmol⋅min/L and a peak thrombin (PT) of 154 ± 9 nmol/L. The thrombin-generating potential of the apoptotic VSMC-myc cells was greater than that of unactivated platelets (P = .003 for AUC; P = .0002 for PT) and similar to calcium-ionophore activated platelets (AUC of 332 ± 15 nmol⋅min/L, P = .3; PT of 172 ± 8 nmol/L, P = .2). Thrombin activation was also seen with apoptotic human VSMCs (AUC of 211 ± 8 nmol⋅min/L; PT of 103 ± 4 nmol/L) and was inhibited by annexin V (P < .0001 for AUC and PT). VSMC-myc cells maintained in serum generated less thrombin than after serum withdrawal (P = .0002 for AUC and PT). VSMCs derived from human coronary atherosclerotic plaques that apoptose even in serum also generated thrombin (AUC of 260 ± 2 nmol⋅min/L; PT of 128 ± 4 nmol/L). We conclude that apoptotic VSMCs possess a significant thrombin-generating capacity secondary to phosphatidylserine exposure. Apoptotic cells within atherosclerotic plaques may allow local thrombin activation, thereby contributing to disease progression.
A GROWING BODY OF evidence implicates thrombin in vascular disease. Thrombin is a serine protease that specifically cleaves fibrinogen to fibrin, thereby stabilizing newly formed clot. Thrombin directly activates platelets to adhere, aggregate, and release the contents of their α granules1 and is thereby a mediator of the intravascular coagulation seen in acute myocardial infarction and unstable angina pectoris. Other cellular effects of thrombin may enhance neointima formation, including increased vascular smooth muscle cell (VSMC) proliferation2,3 and migration,4 possibly through upregulation of tissue-type plasminogen activator5 and the urokinase receptor.4 Leukocyte adhesion and diapedesis, which are thought to be important in atherogenesis, may also be enhanced by a thrombin-mediated increase in P-selectin expression6 and gap junction formation7 in endothelial cells. Thrombin induces monocyte chemoattractant protein-1 production by VSMCs8 and is therefore chemotactic to monocytes.9 Thrombin upregulates plasminogen activator inhibitor-1,5,10 thereby decreasing fibrinolysis, and endothelin,11 both of which have been associated with vascular disease. The thrombin receptor, which mediates these cellular effects, is highly expressed in atherosclerotic lesions12 and in the neointima produced by vascular injury.13 Fibrin, an end product of thrombin activity, has been shown within atherosclerotic plaques.14 15
The production of active thrombin from its inactive precursor prothrombin requires the assembly of a prothrombinase complex incorporating activated coagulation factor X on an anionic phospholipid surface, classically provided by activated platelets.16 Negatively charged phospholipids, such as phosphatidylserine, are normally restricted to the inner cytoplasmic leaflet of the lipid bilayer of the platelet membrane, but are exposed on the outer surface after activation.17 The mechanism underlying this exposure of phosphatidylserine is incompletely understood, but is invariably accompanied by the appearance of small microparticles of membrane at the platelet surface that may be the site of assembly of the prothrombinase complex.18,19 Several lines of evidence using phosphatidylserine-specific phospholipases,20,21 artificial phospholipid vesicles,16,20,22,23 and annexin V, which specifically binds exposed phosphatidylserine,24 have confirmed that phosphatidylserine exposure is required for prothrombinase activity. Recent reports have also shown that lymphocytes undergoing apoptosis lose membrane phospholipid asymmetry with consequent phosphatidylserine exposure.25
We have previously described the production of rat and human VSMC cell lines stably expressing the proto-oncogene c-myc or the adenovirus E1A gene after retrovirus-mediated gene transfer.26,27 These VSMCs undergo reproducible, high rates of apoptosis after serum withdrawal, as documented by their characteristic morphology on electron microscopy and time-lapse videomicroscopy and by DNA fragmentation patterns. We have also documented phosphatidylserine exposure in these apoptotic VSMCs using radiolabeled annexin V28 and shown that the phosphatidylserine exposure partly mediates their phagocytosis by adjacent VSMCs. Recent studies have demonstrated that human coronary atherosclerotic plaques, which are frequently associated with areas of thrombosis, have high rates of apoptosis of both VSMCs and macrophages.29 30 We have therefore investigated whether apoptotic VSMCs provide a surface for the generation of active thrombin as an additional consequence of their phosphatidylserine exposure.
MATERIALS AND METHODS
Materials.Reptilase reagent (Batroxobin) was obtained from Pentapharm Ltd (Basel, Switzerland) and 20 BU was reconstituted in 0.5 mL distilled water. Chromogenic substrate S2238 was obtained from Chromogenix AB (Mölndal, Sweden), reconstituted to 4 mmol/L, and stored at 4°C. Calcium Ionophore A23187 was obtained from Sigma (St Louis, MO) and reconstituted in dimethyl sulfoxide at 400 μmol/L. Russel's Viper Venom was obtained from Diagnostic Reagents Ltd (Thame, Oxon, UK) and the stock solution of 0.1 mg/mL in immidazole buffer was diluted 1 in 60 before use. Human recombinant annexin V was a kind gift of Dr John Tait (University of Washington, Seattle, WA). All other chemicals were obtained from Sigma.
Cell culture.VSMCs were isolated from thoracic aortic explants of 6-week-old Sprague-Dawley rats. Normal human VSMCs were derived from the media of coronary arteries of patients undergoing transplantation for nonischemic cardiomyopathy. Patients were from the University of Washington Medical Center transplantation program between 1987 and 1994. Plaque VSMCs from primary human coronary artery plaques were obtained from patients who underwent directional coronary atherectomy (Simpson Coronary Atherocath; Devices for Vascular Intervention Inc, Redwood City, CA) in the cardiac catheterization laboratory of the University of Washington Medical Center between 1990 and 1992. Isolation of cells from these tissues and histology of the tissues of origin were as described before.31 Cells were cultured in M199 medium containing 10% fetal calf serum (FCS; GIBCO BRL, Life Technologies Inc, Gaithersburg, MD), 20 mmol/L HEPES (Flow, McLean, VA) and equilibrated with 95% air and 5% CO2 . Subconfluent cells were analyzed immunocytochemically to confirm smooth muscle cell origin (α-actin positive, vimentin-positive, von Willebrand factor-negative, desmin-negative, and smooth muscle myosin-negative). Cells at passage 5 were used for experiments and retrovirus infections.
Production of retrovirus-infected cell lines.The retrovirus constructs used to create VSMC cell lines constitutively expressing c-myc and adenovirus E1A (12S) were based on the pDORneo and pBabepuro retrovirus vectors32 and contained full-length cDNAs of each species.27 VSMCs were infected with retrovirus vectors as previously described.26 31 Twenty-four hours after infection, resistant cells were selected in 500 μg/mL (rat cells) or 750 μg/mL (human cells) of G418 (Geneticin; GIBCO). Resistant cell populations were used for experiments not less than 6 weeks after infection. Cells were maintained in medium containing antibiotics at all times.
Time-lapse videomicroscopy.Cells were prepared for videomicroscopy as previously described.31 Briefly, cells were maintained in medium containing 10% FCS, washed three times in medium containing 0% FCS, and then cultured in this latter medium. Flasks were gassed with 95% air and 5% CO2 every 24 hours and sealed. An Olympus OM-70 microscope (Olympus, Tokyo, Japan) was enclosed in a plastic environment chamber and maintained at 37°C by an external heater. The time-lapse equipment consisted of a Sony 92D CCD camera (Sony, Tokyo, Japan) with a Panasonic 6730 time-lapse video recorder (Panasonic, Osaka, Japan). Films were analyzed for morphology of apoptosis and cell death rates as previously described,31 using an observer blind to cell type and treatment conditions. Apoptotic cell death events were scored midway between the last appearance of normality and the point at which the cell became fully detached and fragmented, an interval of typically 60 to 90 minutes. Each individual cell culture was analyzed in duplicate as a minimum (plaque VSMCs, n = 8; normal coronary VSMCs, n = 8).
Platelets and activated platelets.Blood was obtained from one of the authors (P.D.F.) by collection into 0.109 mol/L sodium citrate. Platelet-rich plasma was obtained by centrifugation at 150g for 8 minutes and the platelet count and volumes were measured using a Coulter Model STKS (Coulter, Hialeah, FL). Platelets were washed twice in buffer C (PBS, 0.05% human albumin, and 3 mmol/L sodium azide) and recovered by centrifugation at 1,000g for 5 minutes. Platelets were finally resuspended to a concentration of 375 × 109/L, giving a final concentration of 50 × 109/L in the thrombin generation assay. Activation was achieved by vortexing the resuspended platelets with a final concentration of 4 μmol/L calcium ionophore A23187 and 0.5 mmol/L calcium chloride.
Apoptotic bodies.To generate apoptotic VSMCs, near-confluent VSMC cultures were washed three times in medium with 0% FCS and incubated in this medium for 24 hours. Culture flasks were tapped repeatedly to dislodge apoptotic bodies that were recovered in the supernatant medium. The bodies were counted with a hemacytometer using a 1 in 2 dilution in 0.025% trypan blue. On all occasions, the bodies excluded trypan blue. The apoptotic bodies were thereafter treated exactly as the platelet-rich plasma, being washed twice in buffer C, recovered by centrifugation at 1,000g for 5 minutes, and finally resuspended in buffer C at a concentration of 16.8 × 109/L, except where otherwise stated. The final concentration was rechecked on the hemacytometer. This gave a concentration in the thrombin generation assay of 2.24 × 109/L, calculated to give an equivalent surface area to 50 × 109/L platelets. These calculations assume an average platelet volume of 8 fL (as confirmed on the Coulter Counter), an average apoptotic body radius of 5 μm (based on previous studies31 ), and both platelets and apoptotic bodies to be spheres.
Defibrinated plasma.Plasma from healthy donors was pooled (1 L) and defibrinated by adding 1 part reptilase to 200 parts plasma and incubating at 37°C for 10 minutes. The fibrin clot was pelleted by centrifugation at 2,000g for 10 minutes. The remaining plasma was filtered through sterile 0.2-μm filters to remove any remaining platelet microparticles and stored at −20°C in 1-mL aliquots.
Thrombin generation assay.Phospholipid-dependent thrombin generation was measured using the assay as previously validated and described.33-35 Briefly, 10 μL Russel's Viper Venom was added to 200 μL defibrinated, 0.2 μm filtered plasma followed by 40 μL of the suspension of freshly prepared platelets or apoptotic bodies. Exactly 30 seconds later, 50 μL 0.1 mol/L calcium chloride was added and the reaction was incubated at 37°C in a waterbath. At 30-second intervals, 10-μL aliquots were removed and added to tubes containing 465 μL buffer B (0.05 mol/L Tris-HCl, 0.1 mol/L sodium chloride, 0.5% bovine albumin, 20 mmol/L sodium EDTA) and 25 μL of S2238 (a chromogenic substrate specific for thrombin) at a final concentration of 20 μmol/L. After 4 minutes at 37°C, the reaction was stopped by the addition of 300 μL of 1 mol/L citric acid. Three hundred microliters of the resulting solution was transferred to a microtitre well plate and the absorbance at 405 nm was read in a Titertek Multiskan MCC plate reader (ICN Biomedicals Ltd, Thame, Oxon, UK). Conversion of the results to nanomoles per liter of thrombin was calculated from a previously derived standard curve using pure human α-thrombin.34
Hirudin and annexin V studies.The effect of hirudin on the cleavage of S2238 was studied by preparing a 1.5-fold molar excess. Hirudin (100 U) was dissolved in 100 μL 0.1 mol/L calcium chloride, and 50 μL of this solution was added in place of the 0.1 mol/L calcium chloride in the thrombin generation assay. The effect of annexin V was studied by adding 2.3 μL of a stock solution (217 μmol/L) to 97.7 μL of the resuspended apoptotic bodies to give a final concentration of 5 μmol/L. The resulting mixture was incubated at room temperature for 30 minutes before the thrombin generation assay.
Thrombin generation assay for monolayers.The thrombin generation assay as described relies on the detachment of apoptotic bodies into the supernatant medium. To determine thrombin generation by nonapoptotic VSMCs that do not detach required adaptation of the assay for use with monolayers. VSMCs were grown in 6-well plates until confluent. Cells were then gently washed and incubated at 37°C either with medium and 0% FCS or with control medium containing 10% FCS. After serum deprivation, time-lapse videomicroscopy shows a sequence of changes beginning with cell retraction, followed within a few minutes by intense membrane activity with blebbing and apoptotic body formation.31 The first cells undergo these changes about 15 minutes after the removal of serum, with a stochastic recruitment of cells thereafter. We chose an incubation period of 4 hours. Counting of the recovered supernatant on the hemacytometer over this period showed that no apoptotic bodies had detached.
After 4 hours of incubation, the plates were transferred to a waterbath at 37°C. The supernatant medium was gently removed and stored for subsequent cell counting on the hemacytometer, and each well was gently washed once with 5 mL PBS, pH 7.4. Twenty microliters of Russel's Viper Venom was added to 480 μL of defibrinated 0.2-μm filtered plasma, followed by 100 μL of 0.1 mol/L calcium chloride. Thirty seconds later, this activated plasma was transferred to each well with continuous gentle swirling to ensure complete coverage of the monolayer. At 30-second intervals, 10-μL aliquots were removed and added at 37°C to tubes containing 490 μL of the buffered chromogenic substrate S2238 as described above. The chromogenic reaction was stopped after 4 minutes at 37°C by the addition of 300 μL of 1 mol/L citric acid, and the absorbance was read as before.
Statistical analysis.Results are quoted as the mean ± SEM. Comparisons between groups were performed using Student's unpaired t-test assuming equal variances on the untransformed data, in which a prior F-test showed similar variances, and on logarithmically transformed data elsewhere as stated. Calculations were performed using the Microsoft Excel statistics package (Microsoft Corp, Seattle, WA).
RESULTS
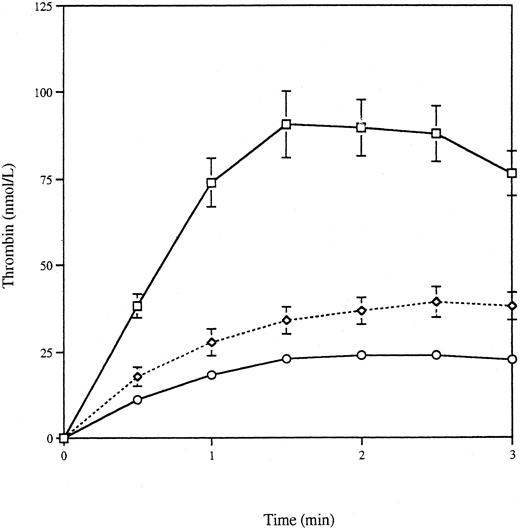
Thrombin generation by apoptotic VSMCs.Apoptosis was induced by serum deprivation of a rat VSMC cell line expressing human c-myc (VSMC-myc), a gene product known to enhance apoptosis, and the apoptotic bodies produced over 24 hours were recovered by centrifugation. Previous studies have shown that 78.2% ± 9.2% of VSMC-myc cells undergo apoptosis over 24 hours after serum withdrawal, as assessed by time-lapse videomicroscopy.27 Figure 1 shows the thrombin generation curve produced by 2.24 × 109/L apoptotic bodies, along with the curves generated by 50 × 109/L platelets and calcium ionophore activated platelets and buffer C alone. These concentrations were chosen, as discussed in the Materials and Methods, to approximate the surface area of platelets and apoptotic bodies in the assay. Thrombin generation was complete by 3 minutes, and for further statistical comparison the area under the curve to 3 minutes (AUC) and the peak thrombin concentration were calculated, as recorded in Table 1. Thrombin generation by the apoptotic bodies was significantly greater than that seen with buffer C alone (P < .0001 for logarithmically transformed AUC and peak thrombin, two-tailed t-test) or with platelets (P = .003 for AUC and P = .0002 for peak thrombin, two-tailed t-test) and of similar magnitude to that seen with activated platelets (P = .3 for AUC and P = .2 for peak thrombin, two-tailed t-test). Table 1 also shows a clear correlation between the concentration of apoptotic bodies and thrombin generation (r = .965 for AUC and r = .978 for peak thrombin).
Thrombin generation curves for platelets and VSMCs. Apoptosis was induced in VSMC-myc cells by serum deprivation for 24 hours. The detached apoptotic bodies were washed and resuspended to give a final concentration in the thrombin generation assay of 2.24 × 109/L. Thrombin generation was measured for 6 minutes (□) and compared with 50 × 109/L unactivated (○) or calcium-ionophore activated (⋄) platelets or buffer C alone (▵).
Thrombin generation curves for platelets and VSMCs. Apoptosis was induced in VSMC-myc cells by serum deprivation for 24 hours. The detached apoptotic bodies were washed and resuspended to give a final concentration in the thrombin generation assay of 2.24 × 109/L. Thrombin generation was measured for 6 minutes (□) and compared with 50 × 109/L unactivated (○) or calcium-ionophore activated (⋄) platelets or buffer C alone (▵).
Thrombin Generation by Apoptotic VSMCs and Platelets
| . | No. of Experiments . | AUC (nmol⋅min/L) . | Peak Thrombin (nmol/L) . |
|---|---|---|---|
| Buffer C | 8 | 43.8 ± 3.4* | 26.3 ± 2.5* |
| Platelets | 14 | 220.8 ± 14.8* | 98.6 ± 7.4* |
| Activated platelets | 15 | 331.5 ± 15.1† | 172.5 ± 7.8† |
| Apoptotic VSMC-myc cells (2.24 × 109/L) | 8 | 304.6 ± 16.9 | 154.5 ± 9.0 |
| Apoptotic VSMC-myc cells (1.32 × 109/L) | 2 | 267.0 ± 5.4 | 130.9 ± 11.1 |
| Apoptotic VSMC-myc cells (0.73 × 109/L) | 2 | 170.0 ± 3.2 | 75.8 ± 1.0 |
| Apoptotic VSMC-myc cells (0.11 × 109/L) | 2 | 90.4 ± 0.4 | 39.2 ± 0.7 |
| Apoptotic human E1A cells | 2 | 211.0 ± 8.5‡ | 102.8 ± 4.1‡ |
| . | No. of Experiments . | AUC (nmol⋅min/L) . | Peak Thrombin (nmol/L) . |
|---|---|---|---|
| Buffer C | 8 | 43.8 ± 3.4* | 26.3 ± 2.5* |
| Platelets | 14 | 220.8 ± 14.8* | 98.6 ± 7.4* |
| Activated platelets | 15 | 331.5 ± 15.1† | 172.5 ± 7.8† |
| Apoptotic VSMC-myc cells (2.24 × 109/L) | 8 | 304.6 ± 16.9 | 154.5 ± 9.0 |
| Apoptotic VSMC-myc cells (1.32 × 109/L) | 2 | 267.0 ± 5.4 | 130.9 ± 11.1 |
| Apoptotic VSMC-myc cells (0.73 × 109/L) | 2 | 170.0 ± 3.2 | 75.8 ± 1.0 |
| Apoptotic VSMC-myc cells (0.11 × 109/L) | 2 | 90.4 ± 0.4 | 39.2 ± 0.7 |
| Apoptotic human E1A cells | 2 | 211.0 ± 8.5‡ | 102.8 ± 4.1‡ |
Apoptotis was induced by serum starvation of rat VSMCs expressing c-myc or normal human coronary VSMCs expressing the adenovirus gene E1A. Apoptotic cells were suspended to give an assay concentration of 2.24 × 109/L (except where otherwise stated), giving approximately the same surface area as 50 × 109/L unactivated or calcium-ionophore–activated platelets. Defibrinated plasma was activated with Russel's Viper Venom, and the thrombin generated after the addition of platelets or apoptotic VSMCs was measured in a chromogenic assay. The table shows the area under the thrombin generation curves (AUC) and the peak thrombin concentration. Values are mean ± SEM.
P < .005 compared with apoptotic VSMC-myc cells.
P = not significant compared with apoptotic VSMC-myc cells.
P < .0001 compared with buffer C.
To exclude the possibility that the observed thrombin generation was restricted to rat VSMCs or VSMCs expressing c-myc, human VSMCs expressing the adenovirus gene E1A, another pro-apoptotic gene product, were also studied. Human VSMC-E1A cells (11.7% ± 1.2%) undergo apoptosis over 24 hours after serum withdrawal (M.R.B., unpublished observations). Both the AUC and the peak thrombin for apoptotic human VSMC-E1A cells were significantly greater than for buffer C (P < .0001, one-tailed t-test), but were significantly less than for the VSMC-myc cells in 0% serum (P = .04 for AUC and P = .03 for peak thrombin, two-tailed t-test; Table 1). These results suggest that it was the process of apoptosis per se, rather than the species of origin of the VSMCs or the pro-apoptotic gene product present, that was responsible for thrombin generation.
In these experiments, thrombin generation was measured by cleavage of the chromogenic substrate S2238, which may not be completely specific for thrombin. The addition of 50 U of hirudin, which antagonizes thrombin by directly binding to the active site, reduced the AUC for apoptotic VSMC-myc cells (1.32 × 109/L) from 267.0 ± 5.4 nmol⋅min/L to 15.2 ± 0.8 nmol⋅min/L (P = .0005, one-tailed t-test), and the peak thrombin from 130.9 ± 11.1 to 6.3 ± 0.5 (P = .001 for logarithmically transformed data, one-tailed t-test). This experiment confirms that it is thrombin that is generated on the surface of the apoptotic VSMCs.
Effect of annexin V on thrombin generation by apoptotic VSMC-myc cells.To test whether the thrombin generated by apoptotic VSMCs was dependent on exposure of phosphatidylserine, experiments were repeated in the presence of annexin V, a specific ligand for phosphatidylserine with a kd for platelets of 7 nmol/L.36 Apoptotic VSMC-myc cells were produced and harvested as before and resuspended to give a final concentration in the assay of 2.24 × 109/L. Aliquots of the apoptotic VSMC-myc cells were incubated with and without 5 μmol/L annexin V at room temperature for 30 minutes immediately before the thrombin generation assay. Figure 2 shows that incubation with annexin V reduced the AUC from 332.7 ± 5.8 nmol⋅min/L to 128.3 ± 6.3 nmol⋅min (n = 4, P < .0001, one-tailed t-test) and the peak thrombin concentration from 171.0 ± 3.5 nmol/L to 69.5 ± 10.7 nmol/L (P < .0001, one-tailed t-test). Annexin V had a similar effect on thrombin generation by platelets and activated platelets (data not shown).
Effect of annexin V on thrombin generation by apoptotic VSMC-myc cells. Apoptotic VSMC-myc cells were collected and resuspended as before and then incubated at room temperature for 30 minutes with and without 5 μmol/L annexin V. Thrombin generation curves are shown for apoptotic cells (□) and annexin V-treated apoptotic cells (⋄).
Effect of annexin V on thrombin generation by apoptotic VSMC-myc cells. Apoptotic VSMC-myc cells were collected and resuspended as before and then incubated at room temperature for 30 minutes with and without 5 μmol/L annexin V. Thrombin generation curves are shown for apoptotic cells (□) and annexin V-treated apoptotic cells (⋄).
Thrombin generation by nonapoptotic VSMC-myc cells.To determine whether the thrombin-generating capacity of the VSMC cell lines studied was dependent on apoptosis and not merely a property of transfected VSMCs, the thrombin generation assay was adapted to study nonapoptotic VSMC-myc cells growing in monolayers, as described in the Materials and Methods. Over a 24-hour period, the rates of apoptosis were 78.2% ± 9.2% for VSMC-myc cells in 0% FCS and 4.6% ± 0.7% for those in 10% FCS.27 Figure 3 shows that the VSMC-myc cells in 0% medium generated significantly more thrombin than the VSMC-myc cells maintained in 10% medium, with an AUC of 209.3 ± 19.0 nmol⋅min/L (n = 6) and 87.3 ± 10.0 nmol⋅min/L (n = 6), respectively (P = .0002, one-tailed t-test). Peak thrombins were 93.9 ± 9.8 nmol/L and 39.6 ± 4.2 nmol/L, respectively (P = .0002 for logarithmically transformed data, one-tailed t-test). For wells with no coating cells, the AUC was 55.9 ± 1.0 nmol⋅min/L and the peak thrombin was 25.1 ± 0.8 nmol/L (n = 4). These values were less than those for VSMC-myc in 10% serum (P = .036 for AUC and P = .028 for peak thrombin, two-tailed t-test assuming unequal variances). Thrombin activation is thus not simply an intrinsic property of the transfected VSMCs; apoptosis is also required.
Thrombin generation by monolayers of serum-starved and serum-maintained VSMCs. VSMC-myc cells were grown in monolayers in 6-well plates. Once confluent, they were washed and then incubated with 0% or 10% serum for 4 hours. After a second washing, the thrombin-generating reaction was performed in each well. Curves are shown for 0% serum (□) and 10% serum (⋄) incubated VSMCs and in the absence of cells (○).
Thrombin generation by monolayers of serum-starved and serum-maintained VSMCs. VSMC-myc cells were grown in monolayers in 6-well plates. Once confluent, they were washed and then incubated with 0% or 10% serum for 4 hours. After a second washing, the thrombin-generating reaction was performed in each well. Curves are shown for 0% serum (□) and 10% serum (⋄) incubated VSMCs and in the absence of cells (○).
Effect of serum deprivation on thrombin generation.To show that removal of serum itself did not induce phosphatidylserine exposure and thrombin generation independently of apoptosis, we induced VSMC apoptosis in the presence of serum. We have previously shown that VSMCs derived from atherosclerotic human coronary plaques undergo a high rate of apoptosis in culture even in the presence of serum.31 We therefore studied a human coronary plaque VSMC line expressing adenovirus E1A (HASMC-66 E1A) that has an apoptotic rate of 7.11% ± 0.8% over 24 hours in 10% serum, increasing to 73.4% ± 6.8% on serum withdrawal (M.R.B., unpublished observations). Figure 4 shows that human plaque-derived VSMCs undergoing apoptosis in 10% serum generated significantly more thrombin than buffer C alone. The respective AUCs were 261.2 ± 2.3 nmol⋅min/L (n = 4) and 43.8 ± 3.4 nmol⋅min/L (n = 8), and the respective peak thrombins were 128.0 ± 3.5 nmol/L and 26.3 ± 2.5 nmol/L (P < .0001 for logarithmically transformed AUC and peak thrombin). The corresponding values for the bodies generated in 0% serum were 303.1 ± 26.1 nmol⋅min/L and 158.8 ± 18.6 nmol/L (n = 3), which were not significantly different from the results obtained with bodies generated in 10% serum, showing that the thrombin generation does not simply result from serum withdrawal. These experiments also show that apoptotic human VSMCs derived from coronary artery plaques possess a significant thrombin-generating capacity.
Thrombin generation by apoptotic VSMCs generated in 0% and 10% serum. VSMCs derived from human coronary atherosclerotic plaques (HASMC-66 E1A), which apoptose even in the presence of serum, were grown to confluence. Thrombin generation was measured for apoptotic HASMC-66 E1A cells generated in 0% (□) and 10% (⋄) serum. For comparison, the thrombin-generation curve for buffer C is also shown (○).
Thrombin generation by apoptotic VSMCs generated in 0% and 10% serum. VSMCs derived from human coronary atherosclerotic plaques (HASMC-66 E1A), which apoptose even in the presence of serum, were grown to confluence. Thrombin generation was measured for apoptotic HASMC-66 E1A cells generated in 0% (□) and 10% (⋄) serum. For comparison, the thrombin-generation curve for buffer C is also shown (○).
DISCUSSION
The aim of this study was to test the hypothesis that VSMCs undergoing apoptosis could provide a surface for the assembly of a prothrombinase complex through their exposure of phosphatidylserine. We studied VSMC cell lines derived from rat aortas and from human coronary arteries and coronary atherosclerotic plaques transfected to induce stable expression of pro-apoptotic gene products. In these cells, reproducible and high rates of apoptosis can be induced, usually by the withdrawal of serum survival factors.
We found that apoptotic bodies derived from rat VSMC-myc cells by serum withdrawal generated active thrombin, as measured by cleavage of S2238 and inhibited by hirudin. To assess whether the amount of thrombin generated by apoptotic bodies was physiologically relevant, platelets and calcium ionophore-activated platelets were also studied. Activation of platelets is known to result in phosphatidylserine exposure: collagen and thrombin together cause the exposure of 100,000 sites per platelet, and calcium ionophore A23187 causes the exposure of 200,000 sites per platelet as measured by annexin V binding.36 Because platelets and apoptotic bodies differ in size, the concentrations used in the assay were adjusted in an attempt to equate the total surface area present. Given that platelet activation is accompanied by the appearance of surface microparticles that may be the major site of phosphatidylserine exposure and the assumptions underlying the calculations, this must be regarded as an approximation at best. We found that apoptotic VSMC-myc cells generated significantly more thrombin than unactivated platelets and a similar amount to platelets maximally activated by calcium ionophore A23187. The correlation between the concentration of apoptotic VSMCs and the amount of thrombin generated further suggests that the thrombin generation resulted from a specific property of the apoptotic cell membrane.
We showed that apoptotic human VSMCs expressing E1A also had a significant thrombin-generating capacity, although less than that of rat VSMC-myc cells. This may reflect a difference in the number of exposed phosphatidylserine sites, because we have previously shown that apoptotic rat VSMC-myc cells express twice as many annexin V binding sites as apoptotic VSMCs expressing E1A.28 The reason for this difference is not clear, but may reflect differing potencies of the pro-apoptotic gene products, just as the variation in the procoagulant potency of different platelet agonists has been shown to result from a difference in phosphatidylserine exposure.37 We showed that phosphatidylserine exposure was required for thrombin generation as annexin V, a protein with a very high affinity for phosphatidylserine at physiologic calcium concentrations, significantly reduced thrombin generation by apoptotic VSMCs.
To demonstrate that apoptosis was necessary for the thrombin-generating capacity of apoptotic VSMC-myc cells and that it was not just an intrinsic feature of the transfected cells required the modification of the thrombin generation assay for use with monolayers. The adapted assay allowed the comparison of thrombin activation by monolayers with different rates of apoptosis. Serum-starved VSMC-myc cells generated significantly more thrombin than cells maintained in 10% serum. There was a small difference between the latter and the nonspecific reaction that occurred in the absence of any cells. The relative contribution to this of the nonapoptotic VSMC-myc cells and of the small number of cells that undergo apoptosis in 10% serum is not known. However, it is clear that there was a large increase in thrombin-generating capacity after the induction of apoptosis by serum withdrawal. We have shown significant thrombin production by apoptotic human coronary plaque VSMCs produced in the presence of serum, thereby excluding the possibility that serum contains some factor that inhibits cell-associated thrombin generation on cells and that serum withdrawal per se accounts for our results.
Taken together, our experiments show that VSMCs possess a significant thrombin-generating capacity when they undergo apoptosis. To our knowledge, this is the first direct demonstration of thrombin activation by apoptotic cells. Our data are consistent with a recent study that showed a decreased clotting time in plasma exposed to irradiated endothelial cells, nutrient-deprived HeLa cells, and a temperaturesensitive murine lymphocyte cell line undergoing apoptosis.38 This effect was abolished by annexin V and so was also phosphatidylserine-dependent. Thrombin generation was not measured, but our results suggest that it was thrombin production on the surface of the apoptotic cells that induced clot formation. Ultrastructural study showed binding of the annexin V to the plasma membrane, but not to internal cellular membranes, showing that thrombin generation does not result from a loss of membrane integrity with formation of the prothrombinase complex on the internal plasma membrane. Similarly, we showed exclusion of trypan blue by all the apoptotic cells that were studied in the thrombin generation assay. These data do suggest that all apoptotic cells may have a thrombin-generating capacity by virtue of phosphatidylserine exposure. Classically, apoptosis has been viewed as a mechanism of silent cell removal without signaling sequelae. However, the experiments described here suggest that apoptotic VSMCs could provide a site for the local generation of thrombin.
The most obvious site in the vasculature for thrombin generation dependent on apoptosis is the atherosclerotic plaque. Tissue factor activity has been documented in plaques,39 is expressed by plaque VSMCs,40 and could allow the activation of factor X and subsequent thrombin generation on an anionic phospholipid surface. Although a number of studies have shown the presence of apoptotic VSMCs in plaques,29,30 current methodologies do not allow an accurate quantitation of their frequency, so it is not possible to extrapolate directly from our data to the situation in the arterial wall. Nonetheless, VSMCs derived from atherosclerotic plaques maintain a high rate of apoptosis in culture31 and it may be relevant that we have shown significant thrombin generation in these VSMCs. Our data allow speculation that apoptosing VSMCs provide a catalytic surface for the activation of thrombin within the atherosclerotic plaque. This could in turn contribute to the deposition of fibrin seen in plaques,14,15 although the classical view has been that this results from the incorporation of mural thrombi into the plaque.41 However, it should be noted that the very phosphatidylserine exposure that allows thrombin generation may also mediate the recognition and phagocytosis of apoptotic VSMCs by other VSMCs, therefore removing exposed phosphatidylserine and limiting thrombin production.28 Further work will be required to determine whether apoptotic cells in plaques do indeed provide a catalytic site for the activation of thrombin.
P.D.F. is a British Heart Foundation (BHF ) Clinical PhD student, C.D.B. is a Medical Research Council Clinician Scientist, P.L.W. is a BHF Professor, and M.R.B. is a BHF Clinician Scientist.
Address reprint requests to Dr Paul D. Flynn, University of Cambridge Department of Medicine, Box 157, Level 5, Addenbrooke's Hospital, Hills Road, Cambridge CB2 2QQ, UK.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal