Abstract
The development of inhibitory antibodies to factor VIII in patients affected by a mild form of hemophilia A (factor VIII < 0.05 IU/mL) is considered a rare event. In this study, we evaluated the relationship between genotype and anti-factor VIII antibody formation in a patient with mild hemophilia A. Mutation analysis showed that a missense mutation in the factor VIII gene leading to replacement of Arg593 by Cys in the A2 domain of factor VIII was associated with hemophilia A in this patient. The anti-factor VIII antibodies present in the patient's plasma were characterized using metabolically labeled factor VIII fragments expressed in insect cells. The anti-factor VIII antibodies, composed of subclasses IgG2 and IgG4, reacted with both the fragment corresponding to the factor VIII heavy chain and the A2 domain. The Arg593 → Cys substitution was introduced into the cDNA encoding the A2 domain of factor VIII and the resulting construct was expressed in insect cells. Strikingly, the metabolically labeled A2 domain carrying the Arg593 → Cys mutation was not recognized by the anti-factor VIII antibodies present in the plasma of the patient. These data indicate that the anti-factor VIII antibodies are exclusively directed against exogenous factor VIII. This strongly suggests that the Arg593 → Cys substitution results in recognition of wild-type factor VIII as nonself and is thereby related to the formation of anti-factor VIII antibodies after factor VIII replacement therapy in this particular patient.
HEMOPHILIA A is a sex-linked hereditary bleeding disorder caused by a deficiency or dysfunction of clotting factor VIII.1 Patients affected by the severe form of this disease (factor VIII activity <0.01 IU/mL) suffer from spontaneous bleeding in joints, muscles, and soft tissue. In moderate (factor VIII activity 0.02 to 0.05 IU/mL) or mild (factor VIII activity 0.05 to 0.4 IU/mL) hemophilia, bleeding may occur after minor trauma or surgery. Bleeding can be prevented or arrested by the administration of factor VIII concentrate.
Factor VIII consists of a series of homologous domains and circulates in plasma as a metal-ion–linked heterodimer.2,3 The heavy chain (molecular weight 90,000 to 200,000), composed of the A1-A2-B domains, is heterogeneous due to limited proteolysis within the B-domain. The light chain (molecular weight 80,000) consists of the A3, C1, and C2 domain. A serious complication of factor VIII replacement therapy is the development of inhibitory antibodies directed against factor VIII. Immunoblotting and immunoprecipitation assays with inhibitor plasmas have shown that human anti-factor VIII antibodies bind predominantly to epitopes within the A2 and/or C2 domains.4-7 Using a series of active recombinant human/porcine factor VIII hybrids, a major epitope of factor VIII-inhibiting antibodies has been localized to amino acid sequence Arg484-Ile508 in the A2-domain of factor VIII.8 The epitope of antibodies directed against the C2 domain of factor VIII consists of a common core of amino acid residues Val2248 to Ser2312.9
The reported cumulative incidence of inhibitor development in patients with severe hemophilia A is about 20%, varying from 5% to 52%.10,11 Patients with mild or moderate hemophilia are at a much lower risk of inhibitor development than are severely affected patients. In a prospective study of inhibitor incidence among 1,306 hemophilia A patients, only 6% of the inhibitors were found in patients with factor VIII ≥0.03 IU/mL.12 In severely affected hemophiliacs, the inhibitor risk is strongly associated with nonsense mutations and large deletions in the factor VIII gene. Only a limited number of patients with a missense mutation in the factor VIII gene develop an inhibitor.13 The risk of inhibitor development in patients with a missense mutation is in the same range for severe or moderate/mild hemophilia (2 of 61 and 3 of 202, respectively).14-16 Missense mutations in the factor VIII gene may affect the structure of endogenously circulating factor VIII, thereby predisposing for inhibitor development upon administration of factor VIII concentrate. Limited information is available on the epitopes of antibodies found in mildly or moderately affected hemophilia A patients.
In the present study, we evaluated antibody formation in a patient affected by mild hemophilia A. We showed that the genetic defect associated with hemophilia in this patient was a missense mutation in the factor VIII gene, leading to substitution of Arg593 by Cys in the A2 domain of factor VIII. The anti-factor VIII antibody was characterized by immunoprecipitation with metabolically labeled recombinant factor VIII fragments. Our data provide evidence for a relation between the Arg593 → Cys substitution in the A2 domain of factor VIII and antibody formation in this particular patient.
MATERIALS AND METHODS
Materials.DNA-modifying enzymes, restriction enzymes, Grace's insect medium, fetal calf serum, and antibiotics were obtained from GIBCO (Breda, The Netherlands). The serum-free medium EX-CELL 401 was purchased from JRH Biosciences (Sera Lab Ltd, Crawley, UK). Oligonucleotide primers, protein G Sepharose-4FF, CNBr-activated Sepharose-4B, and Gelatin Sepharose-4B were obtained from Pharmacia-LKB (Woerden, The Netherlands). The Taq track sequencing system was obtained from Promega (Madison, WI). Pfu-polymerase was purchased from Stratagene (Cambridge, UK). Radioactive chemicals were obtained from Amersham ('s Hertogenbosch, The Netherlands). The plasmid pAcGP67B and the Baculogold Baculovirus Autographa californica DNA were purchased from Pharmingen (San Diego, CA). Spodoptera frugiperda (Sf-9) insect cells and Trichoplusia ni High Five insect cells were obtained from Invitrogen (Leek, The Netherlands). Characterization of monoclonal antibodies (MoAbs) CLB-CAg 9 and CLB-CAg 117 has been described previously.17 Mouse MoAbs directed against human IgG1, IgG2, IgG3, IgG4, and IgM were obtained from the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service and coupled to CNBr-activated Sepharose-4B.
Coagulation assays.Blood was collected for coagulation assays by venapuncture in tubes containing 9 vol blood and 1 vol 3.2% (wt/vol) trisodium citrate. The blood sample was centrifuged for 10 minutes at 12,000g. Plasma was collected and immediately stored at −70°C in 0.5- to 1.0-mL aliquots. Factor VIII procoagulant activity was assayed by a one-stage clotting assay.18 Inhibitor titers were measured in plasma using the Bethesda assay essentially as described previously.19
Molecular genetic studies.Blood was collected by venapuncture in EDTA for isolation of peripheral blood lymphocytes. Chromosomal DNA was extracted from the lymphocytes by Qiagen columns (Qiagen GMBH, Hilden, Germany) according to the manufacturer's instructions. The 26 exons of the factor VIII gene with the bordering intron regions were amplified by polymerase chain reaction (PCR) using oligonucleotide primers that will be described elsewhere (manuscript in preparation). PCR products were analyzed by single-stranded conformation polymorphism (SSCP)20 and PCR products displaying an aberrant SSCP pattern were analyzed by direct sequencing.
Plasmid constructions.The previously described plasmid pCLBBPVdB695 has been used as template in the construction of plasmids encoding fragments of factor VIII.21 Reaction conditions for PCR were 5 minutes at 95°C; 2 minutes at 50°C; 3 minutes at 72°C; 37 cycles of 45 seconds at 95°C, 90 seconds at 50°C, and 3 minutes at 72°C; and 5 minutes at 65°C in the presence of 1 mmol/L dNTPs, Pfu-polymerase reaction buffer, 50 pmol of each oligonucleotide primer, and 2.5 U Pfu-polymerase. The plasmid pCLB-GP67-A2 encoding the A2 domain of factor VIII was constructed by amplifying a fragment using oligonucleotide primers A2-1 (5′ ATTCCATGGGATCAGTTGCCAAGAAGCAT 3′; nucleotide position 1174-1191 of factor VIII, sense) and A2-2 (5′CTTGCGGCCGCGGAGAATCATCTTGGTTCAATGGC 3′; nucleotide position 2262-2277 of factor VIII, antisense). The amplified fragment was purified, digested with Nco I and Not I and cloned into plasmid pAcGP67B. In the resulting construct, designated pCLB-GP67-A2, cDNA encoding amino acid sequence Ser373-Arg740 of factor VIII is fused to that encoding the leader peptide of the acidic glycoprotein GP67. The pCLB-GP67-90K plasmid encoding amino acids Ala1-Arg740 of the heavy chain of factor VIII was constructed by amplifying a 548-bp fragment using oligonucleotide primers 90K-1 (5′TCTCCATGGGTGCCACCAGAAGATACTAC 3′; nucleotide position 58-75 of factor VIII, sense) and 90K-2 (5′ ACATACTAGTAGGGCTCC 3′; nucleotide position 577-594 of factor VIII; antisense). The 548-bp PCR product was purified and digested with Nco I and Nde I fragments. The plasmid pCLB-BPVdB695 was digested with Nde I (nucleotide position 461 of factor VIII) and Kpn I (nucleotide position 1811 of factor VIII), resulting in a 1,351-bp fragment. The plasmid pCLB-GP67B-A2 was digested with Kpn I and Not I and the resulting fragment was cloned into the pAcGP67B plasmid together with the Nco I-Nde I fragment and the Nde I-Kpn I fragment. The resulting plasmid was termed pCLB-GP67-90K. The pCLB-GP67-80K plasmid, encoding amino acids Glu1648-Tyr2332 of the light chain fragment of factor VIII, was constructed by amplifying a fragment using oligonucleotide primers 80K-1 (5′ GCCCCATGGGGGAAATAACTCGTACTACTC 3′; nucleotide position 5002-5020 of factor VIII; sense) and 80K-2 (5′ CTGTACTGTCACTTGTCTCCC 3′; nucleotide position 5659-5679 of factor VIII; antisense). The PCR product was purified and digested with Nco I and Nde I. The pCLB-BPVdB695 plasmid was digested with Nde I (nucleotide position 5521 of factor VIII) and Not I. The resulting Nco I-Nde I and Nde I-Not I fragments were cloned together into the pAcGP67B plasmid, yielding pCLB-GP67-80K. The plasmid pCLB-GP67B-A2-R593C encoding the A2 domain of factor VIII in which the Arg593 is replaced by Cys was constructed by site-directed mutagenesis using overlap extension. Oligonucleotide primer R593C-1 (5′ GAGAATATACAATGCTTTCTCCC 3′; nucleotide position 1822-1844 of factor VIII; sense) was used together with oligonucleotide primer A2-2 to amplify a part of the A2 domain. Oligonucleotide primer R593C-2 (5′ GGGAGAAAGCATTGTATATTCTC 3′; nucleotide position 1822-1844 of factor VIII; antisense) and oligonucleotide primer A2-1 were used to amplify a fragment corresponding to the amino terminus of the A2 domain. Both amplified fragments were purified and used as template in a PCR using primer A2-1 and A2-2 to amplify a fragment that was purified and digested with Nco I and Not I and cloned into the pAcGP67B plasmid, yielding pCLB-GP67B-A2-R593C. The nucleotide sequence of all constructs was verified by oligonucleotide sequencing.
Expression and metabolic labeling of selective domains of factor VIII in insect cells.Recombinant baculoviruses expressing the various recombinant factor VIII fragments were obtained after transfection of Sf-9 cells according to the instructions of the manufacturer. High Five cells were infected with recombinant baculoviruses at a multiplicity of infection of 7. The cells were maintained in culture medium consisting of 25% (vol/vol) Grace's insect medium and 75% (vol/vol) of EX-CELL 401 medium supplemented with 2.5% fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. At 24 hours postinfection, the cells were pulse-labeled with [35S]methionine (50 μCi/mL; specific activity, >800 Ci/mmol) for 24 hours in a similar culture medium lacking methionine. Medium of metabolically labeled cells was collected in an equal volume of 2 times concentrated immunoprecipitation buffer (IPB). IPB consists of 50 mmol/L Tris (pH 7.6), 1 mol/L NaCl, 1.2% (vol/vol) Triton X-100, 0.1% (vol/vol) Tween-20, 1.0% (wt/vol) bovine serum albumin, 35 mmol/L EDTA, 10 μg/mL soybean trypsin inhibitor, 10 mmol/L benzamidine, and 5 mmol/L N-ethylmaleimide.
Immunoprecipitation.Immunoprecipitation was performed essentially as described previously.7 Conditioned media were precleared by incubation for 2 hours at room temperature with Gelatin Sepharose 4B and two successive incubations with protein G Sepharose 4FF. Specific adsorption was performed overnight at 4°C by adding preformed complexes of protein G Sepharose 4FF and 30 μL of patient plasma. MoAbs CLB-CAg 9 (1 μg/mL) directed against the A2 domain of factor VIII and CLB-CAg 117 (1 μg/mL) directed against the light chain of factor VIII were used to monitor the expression of the factor VIII fragments. Plasma of several healthy donors was used to demonstrate the specificity of the adsorption.
For antibody subclass typing, specific adsorption was performed using αIgM, αIgG1, αIgG2, αIgG3, or αIgG4 covalently linked to CNBr-activated Sepharose-4B. Immunoprecipitates were extensively washed with IPB and finally with 20 mmol/L Tris-HCl (pH 7.6). Bound protein was eluted by boiling for 5 minutes in sodium dodecyl sulfate (SDS) sample buffer and analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. After electrophoresis, gels were fixed in 30% methanol and 10% acetic acid and treated with 20% diphenyloxazol in acetic acid for 30 minutes. Finally, gels were incubated in H2O and dried and autoradiography was performed.
RESULTS
Patient.Patient A, born in 1961, was affected by mild hemophilia A with a factor VIII level of about 0.20 IU/mL. His HLA-identical twin brother, patient B, also suffered from mild hemophilia A, with a similar factor VIII level. In 1980, patient A developed an inhibitor after a period of extensive perioperative replacement therapy. He had frequent hematomas and spontaneous muscle bleeding episodes, irresponsive to factor VIII administration, that could be controlled by Feiba (Immuno AG, Vienna, Austria). Concomitant with an increase in the titer of the inhibitor, the level of circulating factor VIII decreased to less than 0.01 IU/mL (Table 1). Apart from the inhibitor, no further abnormalities could be detected when extensive laboratory evaluations and immunopathologic tests were performed. After 15 months, the level of the inhibitor decreased to less than 1 BU/mL and remained at a low level. During the next 12 years there were only five bleeding episodes, all successfully treated with factor VIII concentrate. In 1992, the patient received extensive perioperative factor VIII replacement therapy again. In 1994, the in vivo recovery of immuno-affinity purified factor VIII concentrate was decreased (0.52 IU/mL after the administration of 50 IU/kg) and the factor VIII half-life was rather short (5.5 hours). This suggested that antibodies directed against factor VIII were present 14 years after the first clinical signs of the inhibitor. Strikingly, the patient had a normal response after 1-deamino-8-D-arginine vasopressin (DDAVP) administration (0.3 μg/kg) when this was tested 1 week later. His factor VIII increased from a baseline level of 0.15 IU/mL to 0.55 IU/mL. Plasma samples from 1992 onwards have been used in the present study to characterize the anti-factor VIII antibodies by immunoprecipitation.
Patient Characteristics Over Time
| Time . | Factor VIII Activity (IU/mL) . | Inhibitor (BU/mL) . | Half-Life . | Bleeding Pattern . |
|---|---|---|---|---|
| . | . | . | (h) . | . |
| Before 1980 | 0.20 | 0.0 | Mild | |
| 1980 | 0.01 | 22 | 2-3 | Severe muscle hematomas |
| 1981 | 0.08 | 1.2 | Mild | |
| 1992 | 0.14 | 0.7 | Mild | |
| 1994 | 0.14 | 0.7 | 5.5 | Mild |
| 1995 | 0.14 | 0.4 | Mild |
| Time . | Factor VIII Activity (IU/mL) . | Inhibitor (BU/mL) . | Half-Life . | Bleeding Pattern . |
|---|---|---|---|---|
| . | . | . | (h) . | . |
| Before 1980 | 0.20 | 0.0 | Mild | |
| 1980 | 0.01 | 22 | 2-3 | Severe muscle hematomas |
| 1981 | 0.08 | 1.2 | Mild | |
| 1992 | 0.14 | 0.7 | Mild | |
| 1994 | 0.14 | 0.7 | 5.5 | Mild |
| 1995 | 0.14 | 0.4 | Mild |
Plasma levels of factor VIII and inhibitor were determined as described in the Materials and Methods. The factor VIII antigen levels (measured by an enzyme-linked immunosorbent assay using two different MoAbs directed towards the light chain of factor VIII) were 0.08 IU/mL in the plasma samples of 1992, 1994, and 1995. No cross-reactivity of the inhibitor against porcine factor VIII was observed in 1992.
Epitope mapping of antibodies directed against factor VIII. Recombinant factor VIII fragments corresponding to the factor VIII heavy chain (90K), the A2 domain (A2), and the factor VIII light chain (80K) were expressed in High Five cells and metabolically labeled as described. Binding of anti-factor VIII antibodies to the metabolically labeled factor VIII fragments was assessed by immunoprecipitation and analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. Lane 1, MoAb CLB-CAg 9 (90K, A2) or CLB-CAg 117 (80K); lane 2, anti-factor VIII antibodies present in the patient's plasma (sample from 1992); and lane 3, control plasma. Immunoprecipitation of the metabolically labeled factor VIII heavy chain gave rise to a nonspecific band of approximately 40 kD. Molecular weight markers are given on the right of the figure.
Epitope mapping of antibodies directed against factor VIII. Recombinant factor VIII fragments corresponding to the factor VIII heavy chain (90K), the A2 domain (A2), and the factor VIII light chain (80K) were expressed in High Five cells and metabolically labeled as described. Binding of anti-factor VIII antibodies to the metabolically labeled factor VIII fragments was assessed by immunoprecipitation and analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. Lane 1, MoAb CLB-CAg 9 (90K, A2) or CLB-CAg 117 (80K); lane 2, anti-factor VIII antibodies present in the patient's plasma (sample from 1992); and lane 3, control plasma. Immunoprecipitation of the metabolically labeled factor VIII heavy chain gave rise to a nonspecific band of approximately 40 kD. Molecular weight markers are given on the right of the figure.
Patient B developed a transient inhibitor around the same time as patient A. His inhibitor was lower (peak, 9.0 BU/mL) and the clinical picture was less severe. He had received factor VIII replacement therapy on about 5 occasions before he developed an inhibitor.
Characterization of the anti-factor VIII antibodies.To characterize the antibodies against factor VIII present in the plasma of patient A, immunoprecipitation analysis was performed with metabolically labeled factor VIII fragments expressed in insect cells. These experiments showed the presence of antibodies in the patient's plasma directed against the heavy chain of factor VIII (Fig 1). Further analysis indicated that these antibodies bound to the carboxyterminal part of the heavy chain, the A2 domain. In contrast, no detectable binding of the antibodies to the metabolically labeled light chain of factor VIII was observed, whereas MoAb CLB-CAg 117 reacted readily with this fragment. A plasma sample of patient B from 1993 was analyzed in a similar manner but no antibody binding to metabolically labeled domains of factor VIII could be detected upon immunoprecipitation analysis (data not shown).
Subclass determination was performed by immunoprecipitation using subclass specific antibodies. The anti-factor VIII antibodies present in the plasma of patient A consisted of subclasses IgG2 and IgG4 (Fig 2). We next examined the reactivity of the anti-factor VIII antibodies in plasma samples of patient A from 1992 to 1995. The amount of metabolically labeled A2-domain reactive with the anti-factor VIII antibodies was found to decrease, indicating a decline in the antibody concentration in plasma over time (Fig 3).
Subclass determination of anti-factor VIII antibodies. Binding of anti-factor VIII antibodies to metabolically labeled A2 domain was performed by immunoprecipitation using subclass-specific MoAbs. Immunoprecipitations were analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. The results of a plasma sample of patient A from 1992 are shown. Lane 1, total IgG; lane 2, IgG1; lane 3, IgG2; lane 4, IgG3; and lane 5, IgG4. Molecular weight markers are given at the right of Fig 2. No binding to αIgM was found (data not shown).
Subclass determination of anti-factor VIII antibodies. Binding of anti-factor VIII antibodies to metabolically labeled A2 domain was performed by immunoprecipitation using subclass-specific MoAbs. Immunoprecipitations were analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. The results of a plasma sample of patient A from 1992 are shown. Lane 1, total IgG; lane 2, IgG1; lane 3, IgG2; lane 4, IgG3; and lane 5, IgG4. Molecular weight markers are given at the right of Fig 2. No binding to αIgM was found (data not shown).
Binding of anti-factor VIII antibodies over time. Immunoprecipitation analysis of antibodies binding to the A2 domain of factor VIII, with plasma samples of patient A from various time points. Lanes 1 through 4, plasma of patient A from 1992, 1993, 1994, and 1995; and lanes 5 and 6, plasma of patient B from 1993 and 1995. Some nonspecific binding of a protein of approximately 100 kD was observed in this particular experiment. A molecular weight marker is given on the right of the figure.
Binding of anti-factor VIII antibodies over time. Immunoprecipitation analysis of antibodies binding to the A2 domain of factor VIII, with plasma samples of patient A from various time points. Lanes 1 through 4, plasma of patient A from 1992, 1993, 1994, and 1995; and lanes 5 and 6, plasma of patient B from 1993 and 1995. Some nonspecific binding of a protein of approximately 100 kD was observed in this particular experiment. A molecular weight marker is given on the right of the figure.
Relation of the genetic defect associated with hemophilia A and antibody formation.To study a relation between the genotype of hemophilia A and antibody formation in this patient, we screened the factor VIII gene for a genetic defect by SSCP. An aberrant migration pattern was observed for the fragment corresponding to exon 12 of the factor VIII gene. Direct sequencing showed a point mutation (C → T) that predicted replacement of Arg593 by Cys in this patient, in agreement with a previous report.22
We hypothesized that this mutation could be related to the epitope of the antibody. To address this issue, we introduced the Arg593 → Cys in the construct that encodes for the A2 domain. The resulting construct, termed pCLB-GP67-A2-R593C, was expressed in insect cells. Immunoprecipitation of the metabolically labeled modified A2 domain, termed A2R593C, with MoAb CLB-CAg 9 showed that A2R593C was present in the conditioned medium in a similar concentration as the wild-type A2 domain (Fig 4, lanes 1 and 2). Human antibodies, present in the plasma of two other hemophilia A inhibitor patients and one spontaneous inhibitor patient, also bound to both wild-type and modified A2 domain (data not shown). However, the antibodies present in the plasma of patient A did not react with the modified A2 domain. This observation provides evidence that the anti-factor VIII antibodies react solely with wild-type factor VIII and are not directed towards the mutant factor VIII synthesized by the patient. Thus, in this particular patient, our data suggest a link between the missense mutation causing factor VIII deficiency and antibody development.
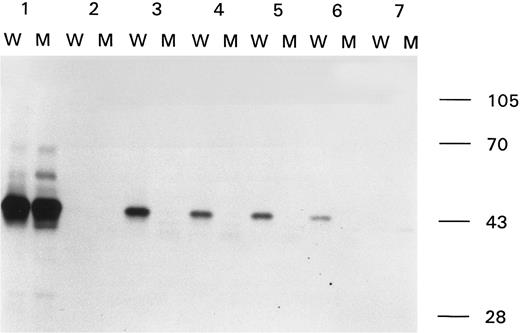
Binding of anti-factor VIII antibodies to wild-type and modified A2 domain. Immunoprecipitation analysis of antibody-binding to the wild-type A2 domain (W) and the A2 domain with the Arg593 → Cys substitution (M). Sample 1, MoAb CLB-CAg 9; sample 2, control plasma; samples 3 through 6, plasma of patient A from 1992, 1993, 1994, and 1995; and sample 7, plasma of patient B from 1993. At the right side of the figure the molecular weight marker is depicted.
Binding of anti-factor VIII antibodies to wild-type and modified A2 domain. Immunoprecipitation analysis of antibody-binding to the wild-type A2 domain (W) and the A2 domain with the Arg593 → Cys substitution (M). Sample 1, MoAb CLB-CAg 9; sample 2, control plasma; samples 3 through 6, plasma of patient A from 1992, 1993, 1994, and 1995; and sample 7, plasma of patient B from 1993. At the right side of the figure the molecular weight marker is depicted.
DISCUSSION
We report a patient affected by mild hemophilia who developed anti-factor VIII antibodies that appeared to be exclusively directed to wild-type A2 domain when analyzed by immunoprecipitation. The antibodies did not bind to the A2 domain containing the Arg593 → Cys substitution associated with hemophilia A in this patient.
Inhibitor development in patients with mild hemophilia is relatively rare. Thirty-two cases concerning inhibitor development in moderate or mild hemophilia have been published.11,12 23-38 In some of these patients inhibitor formation is reported to be related to a period of intensive factor VIII replacement therapy. In patient A, antibody formation was indeed preceded by a period of intensive factor VIII replacement therapy. The low frequency of inhibitor formation in patients with mild and moderate hemophilia may result from tolerance to administrated factor VIII, induced by endogenously synthesized factor VIII. Our data indicate that a missense mutation that alters the immunogenic determinants of the endogenously synthesized factor VIII may predispose to antibody development upon initiation of factor VIII replacement therapy. This suggests that wild-type factor VIII is recognized as nonself by the immune system of this patient.
The Arg593 → Cys mutation in the factor VIII gene has been identified in other patients, but it has not been associated with inhibitor development.14 15 This and the milder clinical picture of the twin brother illustrates the importance of additional factors besides the genotype.
It should be noted that immunoprecipitation was performed with plasma samples obtained 12 to 15 years after the inhibitor was first observed. At this point, the inhibitory activity had decreased considerably according to the Bethesda assay. Because the factor VIII specificity of inhibitors may change over time,39 it cannot be excluded that the antibodies present in the tested samples differ in epitope specificity from the antibodies present at the time of the initial inhibitor detection. An antibody with a different epitope could explain the decrease in circulating factor VIII levels to less than 0.01 IU/mL in 1980, which is unlikely to result from the anti-factor VIII antibodies described in this report. We assume that the antibodies binding to the A2 domain of factor VIII are the cause of the slight inhibition of factor VIII activity in the Bethesda assay, present in the plasma samples from 1992 to 1995. However, the inhibitory nature of the antibodies could not be ascertained, because neutralization experiments using wild-type A2 domain and modified A2 domain were unsuccessful, most likely due to the very low inhibitor titer. It appears that this patient has had the following sequence of immunologic events. After the initial loss of immune tolerance involving shared epitopes, the patient subsequently developed immune tolerance to all but the foreign epitope. This observation is reminiscent of the sequence of events in posttransfusion purpura (PTP). In PTP, an immune response is elicited by alloantigens present on platelets in transfused blood products. The transient thrombocytopenia observed in these patients is thought to result from reactivity of the antibodies with autologous platelets.40
The Arg to Cys mutation at amino acid position 593 in factor VIII may change the conformation of the A2 domain of factor VIII.41 Furthermore, we cannot exclude that the baculovirus-expressed A2R593C domain has an altered structure due to an S-S bound peptide. This would then provide an alternative explanation for lack of binding of the patient's antibody to the mutated A2 domain. However, gross alterations in the mutant A2 domain seem unlikely, because the mutant A2 domain is bound by antibodies present in the plasma of two other hemophilia A inhibitor patients and one spontaneous inhibitor patient.
Two other missense mutations leading to a new cysteine residue at amino acid position 2105 and 2229 of factor VIII have been associated with inhibitor development in mild and moderately severe hemophilia A.15,42 Furthermore, as in our patient, a discrepant response to factor VIII concentrate and DDAVP has been reported in two brothers with mild hemophilia caused by an Arg2150 → His mutation.33 Together with the findings in the present report, these observations indicate that certain missense mutations in the factor VIII gene may predispose to inhibitor formation. Our findings may provide a molecular basis for the increased risk of inhibitor formation after factor VIII replacement therapy in certain families affected by mild hemophilia A.43
Aveolar soft part sarcoma. A 25-year-old woman presented with an 18-month history of an enlarging thigh mass. At the time of removal of this mass, miltiple bilateral pulmonary metatases were present; other sites of spread were absent. The diagnosis of alveolar soft part sarcoma was confirmed by the elctron microscopic identification of unique membrane-bound, intracellular rhomboid crystals seen in longitudinal (A) and cross-sectional (B) views. (Original magnification [A] × 171,000 and [B] × 251,000.) (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)
Aveolar soft part sarcoma. A 25-year-old woman presented with an 18-month history of an enlarging thigh mass. At the time of removal of this mass, miltiple bilateral pulmonary metatases were present; other sites of spread were absent. The diagnosis of alveolar soft part sarcoma was confirmed by the elctron microscopic identification of unique membrane-bound, intracellular rhomboid crystals seen in longitudinal (A) and cross-sectional (B) views. (Original magnification [A] × 171,000 and [B] × 251,000.) (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)
ACKNOWLEDGMENT
We thank Prof Dr R.C. Aalberse for his continuous support and Dr D.H. Joziasse for advice concerning expression of the factor VIII fragments in insect cells. We are grateful to Dr M.J.S.H. Donath, Dr Y. van den Eijnden-Schrauwen, Dr O.D. Christophe, Dr P.J. Lenting, and Dr K. Mertens for critical reading of the manuscript.
Address reprint requests to Jan Voorberg, PhD, Department of Blood Coagulation, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands.




![Aveolar soft part sarcoma. A 25-year-old woman presented with an 18-month history of an enlarging thigh mass. At the time of removal of this mass, miltiple bilateral pulmonary metatases were present; other sites of spread were absent. The diagnosis of alveolar soft part sarcoma was confirmed by the elctron microscopic identification of unique membrane-bound, intracellular rhomboid crystals seen in longitudinal (A) and cross-sectional (B) views. (Original magnification [A] × 171,000 and [B] × 251,000.) (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4371/3/m_bl_0062f1.jpeg?Expires=1764956153&Signature=zjjq77A5kRP33U-ds37GWSTf7-2zZdsJT9sSWuuPnls7XxbllRG6D9yxt8i1EQ5CeMnJwCfXeYAPHg72X0mBUuHhUJ7q6molhKa1Ur26SRuT96sYx8mHmS~ivbtBZVEDW9UQ8ISuSPz7vaXbegywwPXG8q1f0OkWeVbrCiff6N5QjQLmg-VtQGbqQc4i1bAZfWT6VF6sNGCoixuEVv0s2gbrHwLAKhAEQJLGd0VMfQDaZlPTxHMuHpb-uc8OCF4UGCA54QEL-o9KwJauVAddpZeDnxJPhBJjc3entWTJPoYgxHcC8ITdnDt7ZaTrl8ZXrDdyONNcGzOojXXL~jYgYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal