Abstract
We have established a clonal cell culture system that supports the proliferation of committed natural killer (NK) cell progenitors of mice to investigate the pathway and cytokine regulation of NK cell development. Day 14 fetal thymocytes cultured in methylcellulose with interleukin-7 (IL-7), IL-15, and steel factor (SF ) formed diffuse colonies that could not be classified to known colony types. Single-cell origin of the colonies was established by micromanipulation of the colony-forming cells. Cells in the colonies are very blastic, showing no cytoplasmic differentiation, and express Ly5, Thy-1, and CD25 but not myeloid, B, mature T, or NK cell markers. The cells lack T, B, and myeloid potentials but can differentiate to mature NK cells in fetal thymus organ culture, suggesting that the colonies consist of NK committed progenitors. Examination of the minimal cytokine requirement for the NK colony formation showed that IL-7 and SF are indispensable for the formation of immature NK cell colonies. Both IL-2 and IL-15 increased the frequency of colonies. In contrast to IL-2, IL-7, and IL-15, IL-4 strongly inhibited the formation of the colonies. This quantitative clonal culture will provide a useful means to examine the mechanism of NK cell development.
NATURAL KILLER (NK) cells are large granular lymphocytes that appear to be distinct from T or B lymphocytes in both morphology and function. NK cells mediate the killing of certain tumor cells and virally infected cells in non–major histocompatability complex (MHC)-restricted manners.1,2 They are also known to mediate the rejection of donor cells in bone marrow transplantation.3 Although the importance of NK cells has been established in various immune responses, their ontogeny and regulation of development are largely unknown.
NK cells and T lymphocytes share a number of features, including surface markers, growth factor requirements, and functions.4-6 Recently several groups of investigators reported that NK cells are also related to T cells in their developmental pathways. In humans, NK/T common precursors have been reported to be present in fetal thymus.7 In mice, a certain population of thymocytes has been shown to have a potential to develop into both NK and T cells, suggesting that NK/T common precursors also exist in murine thymus.8 9 However, it still remains to be clarified how NK or NK/T common progenitors develop from multipotential hematopoietic progenitor cells and how and when NK/T common progenitors diverge to form separate NK and T-cell lineages.
The cytokine regulation of the growth and survival of early NK precursor cells also needs to be elucidated. Recently, mice lacking the common γ-chain (the common receptor component of interleukin-2 [IL-2], IL-4, IL-7, IL-9, and IL-15 receptors)10-16 were established and found to lack NK cells.17 18 This observation strongly suggested that cytokines which use the common γ-chain as a receptor component play important roles in regulating the growth and differentiation of NK cells. However, it still remains to be determined which cytokines are critical for NK cell development and how these cytokines interact to regulate the growth of NK cell progenitors. The roles of cytokines that do not use the common γ-chain also remain to be clarified.
To examine the developmental pathway and cytokine requirement of NK cells in more detail, it is necessary to establish a clonal culture system that supports the proliferation of NK cell progenitors without stromal cells. Here we report that murine fetal thymocytes plated in methylcellulose culture form immature NK cell colonies in the presence of IL-7, IL-15, and steel factor (SF, c-kit ligand). Both IL-7 and SF were found to be indispensable, and IL-2 and IL-15 increased the frequency of the immature NK cell colonies. IL-4, which also uses the common γ-chain as a receptor component, strongly inhibited the formation of immature NK colonies. We believe that this quantitative and clonal cell culture system is useful for analyzing the developmental pathway and cytokine regulation of NK cell progenitors.
MATERIALS AND METHODS
Mice and cell preparation.Female C57BL/6-Ly5.2 and male DBA/2 mice were purchased from Charles River (Raleigh, NC). Male C57BL/6-Ly5.1 congenic mice were purchased from Jackson Laboratories (Bar Harbor, ME). To obtain fetal thymocytes, female C57BL/6-Ly5.2 mice were mated with male DBA/2 or C57BL/6-Ly5.1 congenic mice for 18 hours. The day of vaginal plugging was designated as day 0 of gestation. Cell suspension of fetal thymocytes was prepared by gently pressing the thymus lobes between two slide glasses and by repeated pipetting.
Cytokines.Purified human recombinant IL-2, murine recombinant IL-13, and murine recombinant IL-3 were purchased from R&D system (Minneapolis, MN). Murine IL-4 and human IL-7 were provided by Sanofi Winthorp, Inc (Malven, PA). Purified simian recombinant IL-15 and recombinant murine SF were gifts from Elaine K. Thomas of Immunex (Seattle, WA). Purified recombinant human IL-11 was provided by P. Schendel of Genetic Institute (Cambridge, MA). Purified human erythropoietin (Epo) was a gift from Genetic Institute (Cambridge, MA). Unless otherwise specified, concentrations of cytokines used were as follows: IL-2, 100 ng/mL; IL-3, 10 ng/mL; IL-4, 10 ng/mL; IL-7, 200 U/mL; IL-13, 10 ng/mL or 50 ng/mL; IL-11, 100 ng/mL; IL-15, 100 ng/mL; SF, 100 ng/mL; Epo; 2 U/mL.
Monoclonal antibodies (MoAbs).The following MoAbs were used for flow cytometric analysis and cell sorting: biotin-conjugated-anti-CD3 (clone; 145-2C11) (Pharmingen, San Diego, CA),19 fluorescein isothiocyanate (FITC)-conjugated-anti-CD4 (YTS 191.1) (Caltag Laboratories, South San Francisco, CA), phycoerythrin (PE)-conjugated-anti-CD8 (YTS 169.4) (Caltag), PE-conjugated-anti-CD25 (PC61.5.3) (Caltag),20 FITC-conjugated-anti-Thy1.2 (30-H12) (Pharmingen),21 biotin-conjugated-anti-NK1.1 (PK136) (Pharmingen),22 PE-conjugated-anti-B220 (RA3-6B2) (Pharmingen),23 biotin-conjugated-anti-c-kit (ACK4),24 biotin-conjugated-anti-Mac-1 (M1/70),25 biotin-conjugated-anti-Gr-1 (RB6-8C5) (Pharmingen),26 biotin-conjugated-anti-TER (TER119) (Pharmingen),27 FITC-conjugated-anti-Ly5.1 (A20-1.7; kindly provided by Dr H. Fleming of Emory University, Atlanta, GA), FITC-conjugated-anti-Ly5 (30F11.1).28
Flow cytometric analysis and cell sorting.For direct staining of cells with FITC or PE-conjugated antibodies, 2 × 105 cells were incubated with appropriate dilutions of MoAbs for 20 minutes on ice. For indirect staining of cells with biotin-conjugated antibodies, 2 × 105 cells were first incubated with biotin-conjugated antibodies on ice for 20 minutes, then followed by staining with FITC-conjugated streptavidin (Caltag) for 15 minutes on ice. In all experiments, cells stained with appropriate isotype-matched control Igs were also prepared as negative controls. Cells were washed once with phosphate-buffered saline (PBS) supplemented with 1% deionized fraction-V bovine serum albumin (BSA) (Sigma, St Louis, MO), and analyzed and/or sorted using FACS Vantage (Becton Dickinson, Mountain View, CA).
Fetal thymus organ culture.Fetal thymic organ culture was performed as described by Jenkinson et al29 and Kingston et al30 with slight modifications. Thymic lobes from day 14 BDF1 fetuses were individually plated in the wells of a Terasaki plate (Nunc, Kamstrup, Denmark). Thirty microliters of medium containing 1 × 105 test cells was added to each well. The plate was then inverted to allow a lobe and cells to contact at the bottom of a hanging drop. After 20 hours of incubation at 37°C, the lobes were transferred individually onto filter membranes (Costar, Cambridge, MA; pore size, 8 μm) floating on the surface of medium using a pasteur pipette and cultured for 8 days. Cells were recovered from the lobes, stained with antibodies, and analyzed by flow cytometry. In some experiments, NK1.1+ Ly5.1+ cells were prepared from fetal thymus organ culture by sorting using FACS Vantage. Medium used for fetal thymic organ culture was RPMI 1640 (Sigma) supplemented with L-glutamine (2 mmol/L), nonessential amino acids (1 mmol/L; GIBCO Laboratories, Grand Island, NY), 2-mercaptoethanol (2-ME, 0.1 mmol/L; Sigma), and 10% fetal calf serum (FCS; Intergen, Purchase, NY).
Clonal culture for immature NK cell colonies.Methylcellulose culture was performed using 35-mm Falcon suspension culture dishes (Becton Dickinson Labware, Lincoln Park, NJ). Whole or fractionated fetal thymocytes were cultured in the medium consisting of α-medium (Flow Laboratories, Rockville, MD), 1.2% 1,500-cp methylcellulose (Shinetsu Chemical, Tokyo, Japan), 5% FCS, 1% deionized fraction-V BSA, and 0.1 mmol/L 2-ME in the presence of designated cytokines. Dishes were incubated at 37°C in a humidified atmosphere flushed with 5% CO2 for 14 days. In some experiments, c-kit+CD25− cells were individually plated in culture by micromanipulation as described previously.31
Clonal culture for myeloid and pre-B cells.Basic methods of culture for myeloid and pre-B cells have been described previously.31 32 Briefly, cells were cultured in 35-mm Falcon suspension culture dishes containing α-medium, 1.2% 1,500-cp methylcellulose, 30% FCS, 1% deionized fraction-V BSA, and 0.1 mmol/L 2-ME. For culture of myeloid lineage cells, IL-3, IL-11, and Epo were added to the medium. For pre-B cell culture, IL-7, IL-11, and SF were added to the medium. Dishes were incubated at 37°C in a humidified atmosphere flushed with 5% CO2 for 14 days.
Cytolytic assay.Ly5.1+NK1.1+ cells were prepared by sorting with FACS Vantage from the fetal thymus organ culture and cultured in the presence of IL-2 (500 ng/mL; 500 U/mL) for 7 days. Cells were washed twice and used as effector cells. As control effector cells, immature NK cell colonies were individually picked, pooled, and washed twice. NK-sensitive YAC-1 cells were obtained from American Type Culture Collection (Rockville, MD). YAC-1 cells, 1 × 106, were labeled with 100 μCi of sodium chromate (51Cr) for 2 hours at 37°C. Cells were washed three times and plated in the wells of round-bottomed 96-well plates at the concentration of 2,000 cells/100 μL. Varying numbers of effector cells in a volume of 100 μL were added to the target cells and the plates were incubated at 37°C for 4 hours. The plates were then centrifuged once and the radioactivity of 100 mL of determined using a gamma counter. Percent specific lysis was calculated as follows: (ER-SR)/(MR-SR), where ER is the experimental 51Cr release in the presence of effector cells, SR is a spontaneous release of 51Cr from culturing target cells alone, and MR the maximum 51Cr release in the presence of 0.5% Triton-X.
RESULTS
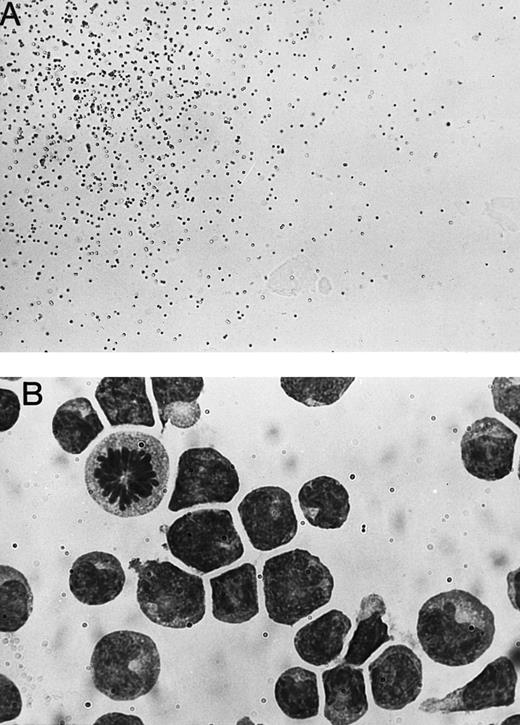
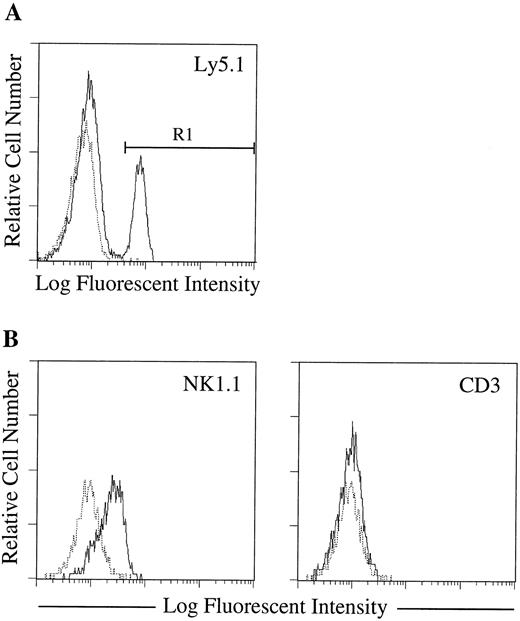
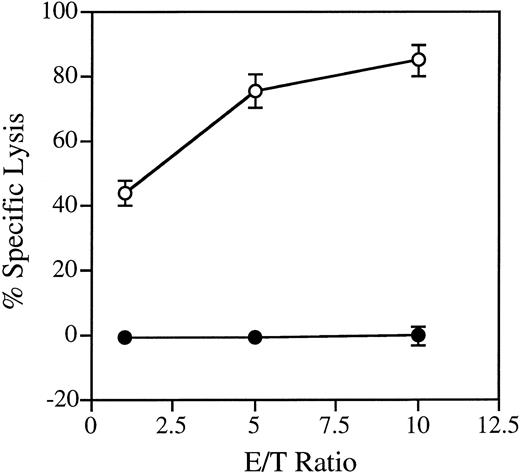
Establishment of a clonal culture system that supports the proliferation of immature NK cells.We chose day 14 murine fetal thymocytes as the material for growing candidate NK cell colonies because a relatively high percentage of the cells had been shown to have the potential of developing into NK cells.8 After trying several cytokine combinations, we found that fetal thymocytes cultured in methylcellulose with IL-7, IL-15, and SF form very diffuse colonies that could not be classified to known colony types (Fig 1A). Most of the cells in the colonies were small- to medium-sized round cells. May-Grünwald Giemsa staining of the colonies demonstrated that all cells in the colonies are blastic, showing no signs of cytoplasmic differentiation (Fig 1B). The nuclei often showed prominent nucleoli and mitotic cells were also common. The colony-forming efficiencies of the fetal thymocytes were about 1%. To further characterize the nature of cells in the colonies, we analyzed the expression of surface molecules by flow cytometry. As shown in Fig 2, cells in the colonies expressed Ly5, Thy-1, and CD25, but not myeloid markers (Mac-1, Gr-1), erythloid markers (TER), B-cell markers (B220), or mature NK- and T-cell markers (NK1.1, CD3, CD4, and CD8). These results suggested that the cells in the colonies are immature hematopoietic cells. Because the cells expressed Thy-1 and CD25, it was likely that these cells are immature NK or T cells. Therefore, we next examined T-cell and NK cell potentials using fetal thymus organ culture. This technique had been shown to support both NK and T-cell development.33 As shown in Fig 3, the cells converted to NK1.1+ but remained CD3−, indicating that the cells in the colonies are committed to NK cell lineage. We also examined the functional status of the NK1.1+ cells present in the fetal thymus organ culture. We used the standard 51Cr release assay and NK sensitive YAC-1 cells as targets. As shown in Fig 4, the NK1.1+ cells expressed cyotoxic activity toward YAC-1 cells. Figure 4 also shows that the cells in the immature NK cell colonies are functionally incompetent. These results confirmed that the colonies derived from the fetal thymocytes are immature NK cell colonies.
Photomicrographs of a immature NK cell colony. (A) One quarter of a representative immature NK cell colony seen on an inverted microscope. Original magnification (OM) × 40. (B) A portion of a May-Grünwald Giemsa–stained smear of the colony. OM × 100.
Photomicrographs of a immature NK cell colony. (A) One quarter of a representative immature NK cell colony seen on an inverted microscope. Original magnification (OM) × 40. (B) A portion of a May-Grünwald Giemsa–stained smear of the colony. OM × 100.
Surface phenotype of cells constituting the immature NK cell colonies. Thymocytes obtained from day 14 fetus were plated in methylcellulose culture with SF, IL-7, and IL-15. After 14 days of culture, immature NK cell colonies were individually picked, pooled, and stained with MoAbs specific for molecules indicated in each FACS profiles. Stained cells were analyzed by FACS Vantage using CellQuest software. Dotted and solid lines in each profiles represent cell stained with isotype-matched Ig and specific MoAbs, respectively.
Surface phenotype of cells constituting the immature NK cell colonies. Thymocytes obtained from day 14 fetus were plated in methylcellulose culture with SF, IL-7, and IL-15. After 14 days of culture, immature NK cell colonies were individually picked, pooled, and stained with MoAbs specific for molecules indicated in each FACS profiles. Stained cells were analyzed by FACS Vantage using CellQuest software. Dotted and solid lines in each profiles represent cell stained with isotype-matched Ig and specific MoAbs, respectively.
Surface phenotype of cells developing in the fetal thymus lobe culture. Fetal thymocytes from C57BL/6-Ly5.1/C57BL/6-Ly5.2 F1 mice (Ly5.1/Ly5.2) were plated in methylcellulose culture with SF, IL-7, and IL-15. After 14 days of culture, immature NK cell colonies were individually picked, pooled, and incubated in hanging drops for 20 hours with fetal thymus lobes from BDF1 (Ly5.2) mice. The lobes were cultured on filter membranes for an additional 8 days. Cells recovered from the lobes were stained with MoAbs specific for molecules indicated in each FACS profiles. (A) Expression of Ly5.1 (donor marker) by the cells from cultured lobes. (B) Expression of NK1.1 and CD3 by donor-derived cells. Only the gated cell population (R1) is shown in each FACS profile. Dotted and solid lines represent cells stained with isotype-matched Ig and specific MoAbs, respectively.
Surface phenotype of cells developing in the fetal thymus lobe culture. Fetal thymocytes from C57BL/6-Ly5.1/C57BL/6-Ly5.2 F1 mice (Ly5.1/Ly5.2) were plated in methylcellulose culture with SF, IL-7, and IL-15. After 14 days of culture, immature NK cell colonies were individually picked, pooled, and incubated in hanging drops for 20 hours with fetal thymus lobes from BDF1 (Ly5.2) mice. The lobes were cultured on filter membranes for an additional 8 days. Cells recovered from the lobes were stained with MoAbs specific for molecules indicated in each FACS profiles. (A) Expression of Ly5.1 (donor marker) by the cells from cultured lobes. (B) Expression of NK1.1 and CD3 by donor-derived cells. Only the gated cell population (R1) is shown in each FACS profile. Dotted and solid lines represent cells stained with isotype-matched Ig and specific MoAbs, respectively.
Cytolytic activity of NK1.1+ cells derived from immature NK cell colonies. Fetal thymocytes from C57Bl/6-Ly5.1/C57Bl/6-Ly-5.2 F1 mice were cultured under the conditions described in the legend for Fig 3. After 8 days of fetal thymus organ culture, Ly5.1+NK1.1+ cells were prepared by FACS sorting from the cells in the fetal thymus lobe culture, cultured for an additional 7 days with IL-2 (500 ng/mL), and assayed for cytolytic activity as described in Materials and Methods. As a control, the NK1.1− cells from the immature NK cell colonies were assayed for cytolytic activity. (○), NK1.1+ cells from the fetal thymus culture; (•), cells from the immature NK cell colonies.
Cytolytic activity of NK1.1+ cells derived from immature NK cell colonies. Fetal thymocytes from C57Bl/6-Ly5.1/C57Bl/6-Ly-5.2 F1 mice were cultured under the conditions described in the legend for Fig 3. After 8 days of fetal thymus organ culture, Ly5.1+NK1.1+ cells were prepared by FACS sorting from the cells in the fetal thymus lobe culture, cultured for an additional 7 days with IL-2 (500 ng/mL), and assayed for cytolytic activity as described in Materials and Methods. As a control, the NK1.1− cells from the immature NK cell colonies were assayed for cytolytic activity. (○), NK1.1+ cells from the fetal thymus culture; (•), cells from the immature NK cell colonies.
Myeloid and B-cell potentials of the cells from immature NK cell colonies.We next tested the cells in the immature NK cell colonies for possible possession of myeloid and B-cell potentials. Thymocytes obtained from day 14 fetuses were cultured in methylcellulose in the presence of IL-7, IL-15, and SF. Fourteen days later, immature NK cell colonies were individually picked, pooled, and recultured in the presence of IL-3, IL-11, and Epo for analysis of myeloid potential, or in the presence of IL-7, IL-11, and SF for B-cell potential. The cells in the colonies were unable to form myeloid or pre-B cell colonies whereas bone marrow cells formed myeloid and pre-B cell colonies under the same culture conditions (data not shown). These results confirmed that the colonies we identified contained NK committed progenitors.
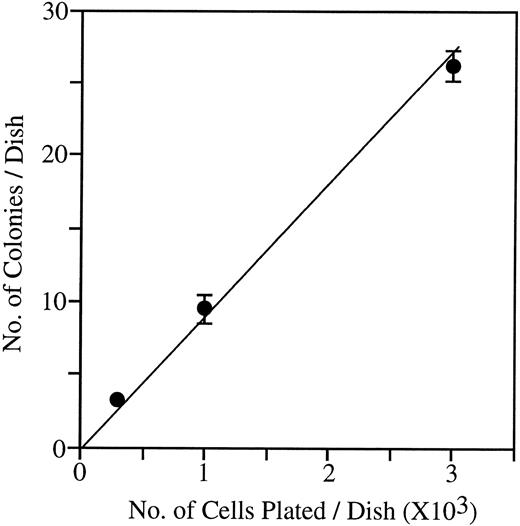
Linearity studies of the immature NK cell colony formation.To test if the observed colony formation could be used as an assay for quantifying NK progenitors, we plated 0.3 × 103, 1 × 103, and 3 × 103 fetal thymocytes in methylcellulose with IL-7, IL-15, and SF. The number of immature NK cell colonies were scored after 14 days of culture. As shown in Fig 5, a linear relationship was observed between the number of cells plated and the number of immature NK cell colonies. This result indicated that the methylcellulose culture may be used as an assay for NK progenitors.
A linearity study of immature NK cell colony formation from fetal thymocytes. 0.3 × 103, 1 × 103, and 3 × 103 of day 14 fetal thymocytes were cultured with IL-7, IL-15, and SF in methylcellulose and colonies were scored on day 14 of culture. Data represent mean ± SD of quadruplicate cultures.
A linearity study of immature NK cell colony formation from fetal thymocytes. 0.3 × 103, 1 × 103, and 3 × 103 of day 14 fetal thymocytes were cultured with IL-7, IL-15, and SF in methylcellulose and colonies were scored on day 14 of culture. Data represent mean ± SD of quadruplicate cultures.
Clonality of immature NK cell colonies.To confirm the single-cell origin of the immature NK cell colonies, we next plated individual cells into methylcellulose by use of micromanipulation. As described in detail later, progenitors for the immature NK cell are enriched most in the c-kit+CD25− fraction of fetal thymocytes. Therefore, we used c-kit+CD25− cells for confirmation of clonality by micromanipulation. We plated a total of 154 cells individually by using a micromanipulater into cultures containing IL-7, IL-15, and SF. Eight immature NK cell colonies developed. Flow cytometric analyses of the pooled colonies showed that the cells in the colonies express Thy-1 and CD25 but not B220, Gr-1, Mac-1, NK1.1, or CD3. These results established the clonal origin of the immature NK cell colonies.
Maturational stages of the progenitors for the immature NK cell colonies.Fetal thymocytes contain precursor cells at various maturational stages of T-cell and NK cell lineages.33-35 Although the majority of the precursor cells are committed to T-cell lineage, it has been suggested that earlier precursors have a potential to develop cells in other lineages cells including NK cells.8,9 Therefore, it was important to determine the maturational stages of the fetal thymocytes that form the immature NK cell colonies. CD25 and c-kit have been shown to be useful markers for separating different stages of intrathymic precursor cells.33 35 Therefore, we examined colony formation from thymocytes separated on the basis of CD25/c-kit expression. As shown in Table 1, most of the cells forming the immature NK cell colonies are c-kit+. Both CD25− and CD25+c-kit+ cells formed the immature NK cell colonies, although the frequency of colony forming cells in CD25− populations was slightly higher than that of CD25+ population.
Colony Formation From Subpopulation of Fetal Thymocytes Separated on the Basis of CD25/c-kit Expression
| Cells Plated . | No. of Colonies . | |||
|---|---|---|---|---|
| Type . | No./Dish . | GM . | Mast . | NK . |
| CD25−c-kit+ | 300 | 1 ± 1 | 1 ± 1 | 20 ± 2 (79)* |
| CD25+c-kit+ | 500 | 0 ± 1 | 1 ± 1 | 19 ± 1 (75) |
| CD25−c-kit− | 1,500 | 0 | 1 ± 1 | 2 ± 1 (8) |
| CD25+c-kit− | 4,000 | 0 | 0 ± 1 | 3 ± 1 (11) |
| Cells Plated . | No. of Colonies . | |||
|---|---|---|---|---|
| Type . | No./Dish . | GM . | Mast . | NK . |
| CD25−c-kit+ | 300 | 1 ± 1 | 1 ± 1 | 20 ± 2 (79)* |
| CD25+c-kit+ | 500 | 0 ± 1 | 1 ± 1 | 19 ± 1 (75) |
| CD25−c-kit− | 1,500 | 0 | 1 ± 1 | 2 ± 1 (8) |
| CD25+c-kit− | 4,000 | 0 | 0 ± 1 | 3 ± 1 (11) |
Designated populations of cells were prepared by FACS sorting from day 14 fetal thymocytes of BDF1 mice and cultured with IL-7, IL-15, and SF in methylcellulose. Colonies were counted on day 14 of culture. Data represent mean ± SD of quadruplicate cultures.
Total number of immature NK cell colonies in four plates is shown in parentheses.
Minimal cytokine requirements for formation of the immature NK cell colonies.The immature NK cell colonies were initially identified in culture containing IL-7, IL-15, and SF. We next examined the minimal cytokine requirement for the development of the immature NK cell colonies. As shown in Table 2, neither IL-7, IL-15, nor SF alone could support the formation of the colonies. A combination of IL-7 and SF supported formation of the immature NK cell colonies from both CD25− and CD25+c-kit+ cells. IL-15 was not essential for the colony formation, but increased the frequency of colonies. These results indicate that IL-7 and SF act synergisticaly, and are indispensable for supporting immature NK cell colonies.
Minimal Cytokine Requirement of Immature NK Colonies
| Cells Plated . | Cytokines . | No. of Colonies . | |||
|---|---|---|---|---|---|
| Type . | No./Dish . | . | GM . | Mast . | NK . |
| CD25−c-kit+ | 300 | IL-7 | 0 | 0 | 0 (0)* |
| IL-15 | 0 | 0 | 0 (0) | ||
| SF | 0 | 0 | 0 (0) | ||
| IL-7, IL-15 | 0 | 0 | 0 (0) | ||
| IL-7, SF | 0 ± 1 | 1 ± 1 | 11 ± 2 (45) | ||
| IL-15, SF | 0 | 1 ± 1 | 0 (0) | ||
| IL-7, IL-15, SF | 1 ± 1 | 0 ± 1 | 20 ± 2 (79) | ||
| CD25+c-kit+ | 500 | IL-7 | 0 | 0 | 0 (0) |
| IL-15 | 0 | 0 | 0 (0) | ||
| SF | 0 | 0 | 0 (0) | ||
| IL-7, IL-15 | 0 | 0 | 0 (0) | ||
| IL-7, SF | 0 ± 1 | 0 ± 1 | 12 ± 3 (46) | ||
| IL-15, SF | 0 | 0 | 0 (0) | ||
| IL-7, IL-15, SF | 1 ± 1 | 1 ± 1 | 16 ± 1 (64) | ||
| Cells Plated . | Cytokines . | No. of Colonies . | |||
|---|---|---|---|---|---|
| Type . | No./Dish . | . | GM . | Mast . | NK . |
| CD25−c-kit+ | 300 | IL-7 | 0 | 0 | 0 (0)* |
| IL-15 | 0 | 0 | 0 (0) | ||
| SF | 0 | 0 | 0 (0) | ||
| IL-7, IL-15 | 0 | 0 | 0 (0) | ||
| IL-7, SF | 0 ± 1 | 1 ± 1 | 11 ± 2 (45) | ||
| IL-15, SF | 0 | 1 ± 1 | 0 (0) | ||
| IL-7, IL-15, SF | 1 ± 1 | 0 ± 1 | 20 ± 2 (79) | ||
| CD25+c-kit+ | 500 | IL-7 | 0 | 0 | 0 (0) |
| IL-15 | 0 | 0 | 0 (0) | ||
| SF | 0 | 0 | 0 (0) | ||
| IL-7, IL-15 | 0 | 0 | 0 (0) | ||
| IL-7, SF | 0 ± 1 | 0 ± 1 | 12 ± 3 (46) | ||
| IL-15, SF | 0 | 0 | 0 (0) | ||
| IL-7, IL-15, SF | 1 ± 1 | 1 ± 1 | 16 ± 1 (64) | ||
CD25−c-kit+ or CD25+c-kit+ cells were prepared by FACS sorting from day 14 fetal thymocytes. Culture was performed in the presence of designated cytokines. Colonies were scored on day 14 of culture. Data represent mean ± SD of quadruplicate culture.
Total number of immature NK cell colonies in 4 dishes is shown in parentheses.
The effect of IL-2.The results shown in Table 2 indicated that IL-15 is a growth factor for immature NK cells. We next examined the effects of IL-2 because it has been shown that IL-2 and IL-15 share many biologic activities, including the ability to promote the growth of mature NK cells.6,36 As shown in Table 3, both IL-2 and IL-15 increased the frequencies of immature NK cell colonies in the presence of IL-7 and SF. Table 3 also shows that there are neither synergistic nor additive effects between IL-2 and IL-15. These results indicate that IL-2 and IL-15, previously shown to be growth factors for mature NK cells,6 36 are also growth factors for the NK cells at earlier stages of development.
Effects of IL-2 and IL-15 on the Formation of Immature NK Cell Colonies
| Cells Plated . | Cytokines . | No. of Colonies . | |||
|---|---|---|---|---|---|
| Type . | No./Dish . | . | GM . | Mast . | NK . |
| CD25−c-kit+ | 300 | IL-7, SF | 0 | 1 ± 1 | 11 ± 1 (42)3-150 |
| IL-2, IL-7, SF | 0 ± 1 | 1 ± 1 | 19 ± 1 (76) | ||
| IL-15, IL-7, SF | 0 | 0 | 18 ± 2 (71) | ||
| IL-2, IL-15, IL-7, SF | 0 | 1 ± 1 | 19 ± 2 (75) | ||
| CD25+c-kit+ | 500 | IL-7, SF | 0 | 1 ± 1 | 12 ± 1 (49) |
| IL-2, IL-7, SF | 0 ± 1 | 1 ± 1 | 24 ± 1 (94) | ||
| IL-15, IL-7, SF | 0 | 1 ± 1 | 19 ± 2 (76) | ||
| IL-2, IL-15, IL-7, SF | 0 | 1 ± 1 | 24 ± 1 (95) | ||
| Cells Plated . | Cytokines . | No. of Colonies . | |||
|---|---|---|---|---|---|
| Type . | No./Dish . | . | GM . | Mast . | NK . |
| CD25−c-kit+ | 300 | IL-7, SF | 0 | 1 ± 1 | 11 ± 1 (42)3-150 |
| IL-2, IL-7, SF | 0 ± 1 | 1 ± 1 | 19 ± 1 (76) | ||
| IL-15, IL-7, SF | 0 | 0 | 18 ± 2 (71) | ||
| IL-2, IL-15, IL-7, SF | 0 | 1 ± 1 | 19 ± 2 (75) | ||
| CD25+c-kit+ | 500 | IL-7, SF | 0 | 1 ± 1 | 12 ± 1 (49) |
| IL-2, IL-7, SF | 0 ± 1 | 1 ± 1 | 24 ± 1 (94) | ||
| IL-15, IL-7, SF | 0 | 1 ± 1 | 19 ± 2 (76) | ||
| IL-2, IL-15, IL-7, SF | 0 | 1 ± 1 | 24 ± 1 (95) | ||
CD25−c-kit+ or CD25+c-kit+ cells from day 14 fetal thymocytes were cultured in the presence of designated cytokines. Colonies were scored on day 14 of culture. Data represent mean ± SD of quadruplicate cultures.
Total number of immature NK cell colonies in 4 plates is shown in parentheses.
Effects of IL-4 and IL-13.IL-7, IL-2, and IL-15 use common γ-chain as a receptor component.10,13,14,16 We next examined the effects of IL-4 on the formation of immature NK cell colonies, because IL-4 also uses the common γ-chain as a receptor component11,12 and shares several biologic activities with IL-2.37,38 We also examined the effects of IL-13 along with IL-4, because many biologic activities of IL-4 have been shown to be substituted by IL-13.39 The results are presented in Table 4. Surprisingly, addition of 10 ng/mL IL-4 to the culture completely abolished the formation of immature NK cell colonies. Although 50 ng/mL of IL-13 slightly reduced the frequencies of immature NK cell colonies, 10 ng/mL IL-13 did not abrogate the formation of immature NK cell colonies.
Effects of IL-4 and IL-13 on Immature NK Cell Colonies
| Cells Plated . | Cytokines . | No. of Colonies . | |||
|---|---|---|---|---|---|
| Type . | No./Dish . | . | GM . | Mast . | NK . |
| CD25−c-kit+ | 300 | IL-7, IL-15, SF | 1 ± 1 | 1 ± 1 | 24 ± 2 (97)4-150 |
| IL-4 (10 ng/mL), IL-7, IL-15, SF | 1 ± 1 | 2 ± 1 | 0 (0) | ||
| IL-13 (10 ng/mL), IL-7, IL-15, SF | 1 ± 1 | 2 ± 1 | 22 ± 1 (86) | ||
| IL-13 (50 ng/mL), IL-7, IL-15, SF | 0 ± 1 | 1 ± 1 | 16 ± 2 (65) | ||
| CD25+c-kit+ | 500 | IL-7, IL-15, SF | 0 | 0 | 27 ± 1 (107) |
| IL-4 (10 ng/mL), IL-7, IL-15, SF | 0 ± 1 | 1 ± 1 | 0 (0) | ||
| IL-13 (10 ng/mL), IL-7, IL-15, SF | 0 | 1 ± 1 | 26 ± 1 (104) | ||
| IL-13 (50 ng/mL), IL-7, IL-15, SF | 0 | 0 | 18 ± 2 (80) | ||
| Cells Plated . | Cytokines . | No. of Colonies . | |||
|---|---|---|---|---|---|
| Type . | No./Dish . | . | GM . | Mast . | NK . |
| CD25−c-kit+ | 300 | IL-7, IL-15, SF | 1 ± 1 | 1 ± 1 | 24 ± 2 (97)4-150 |
| IL-4 (10 ng/mL), IL-7, IL-15, SF | 1 ± 1 | 2 ± 1 | 0 (0) | ||
| IL-13 (10 ng/mL), IL-7, IL-15, SF | 1 ± 1 | 2 ± 1 | 22 ± 1 (86) | ||
| IL-13 (50 ng/mL), IL-7, IL-15, SF | 0 ± 1 | 1 ± 1 | 16 ± 2 (65) | ||
| CD25+c-kit+ | 500 | IL-7, IL-15, SF | 0 | 0 | 27 ± 1 (107) |
| IL-4 (10 ng/mL), IL-7, IL-15, SF | 0 ± 1 | 1 ± 1 | 0 (0) | ||
| IL-13 (10 ng/mL), IL-7, IL-15, SF | 0 | 1 ± 1 | 26 ± 1 (104) | ||
| IL-13 (50 ng/mL), IL-7, IL-15, SF | 0 | 0 | 18 ± 2 (80) | ||
CD25−c-kit+ and CD25+c-kit+ cells sorted from day 14 fetal thymocytes were cultured in the presence of cytokines. Colonies were scored on day 14 of culture. Data represent mean ± SD of quadruplicate cultures.
Total number of colonies in 4 plates is shown in parentheses.
DISCUSSION
To examine the regulation of NK cell development, we established a clonal culture system that supports the proliferation of immature NK cells. c-kit+ fetal thymocytes plated in methylcellulose media containing IL-7, IL-15, and SF formed colonies consisting of committed NK progenitor cells. IL-7 and SF were found to be indispensable for supporting the formation of immature NK cell colonies and IL-15 and IL-2 increased the frequency of the colonies. IL-2, IL-7, and IL-15 use the common γ-chain and JAK-3 kinase as signal transduction molecules.40-42 Therefore, our results are consistent with the recent observations that mice lacking common γ-chain and JAK-3 kinase are devoid of mature NK cells.17,18,43 In our culture system, IL-7 appeared to be an important cytokine because no colonies formed without IL-7. However, it has been reported that mice lacking IL-7 receptor α-chain have normal numbers of functional NK cells.44,45 This indicates that other cytokines can compensate for the absence of IL-7 in NK cell development. Alternatively, there may be receptors for IL-7 that do not use IL-7 receptor α-chain as a component. SF and its receptor, c-kit, have been shown to be important for the growth of human immature and mature NK cells.46 47 Here we showed that SF also functions as a growth factor for murine immature NK cells. It appears that, in addition to common γ-chain–JAK-3 kinase signal transduction pathway, signaling through a transmembrane tyrosine kinase receptor is important for the growth of immature NK cells.
Surprisingly, IL-4 revealed strong inhibitory effects on the formation of the immature NK cell colonies. IL-4, like IL-2, IL-7, and IL-15, uses a common γ-chain10,13,14,16 and JAK-3 kinase as signal transduction molecules.40-42 IL-4 and IL-2 also share JAK-1 kinase as a signal transduction molecule.48,49 It is unlikely that IL-4 competes with IL-2, IL-7, or IL-15 for binding to common γ-chain because very low concentrations (10 ng/mL) of IL-4 are sufficient to abolish immature NK cell colony formation and IL-2, IL-7, and IL-15 at concentrations as high as 1 mg/mL did not affect the inhibitory effect of IL-4 (data not shown). Therefore, the inhibitory effects of IL-4 may be due to activation of IL-4 specific signaling pathways. It has been reported that IL-4 evokes specific responses, including tyrosine phosphorylation of 4PS/IRS-2 and activation of Stat6.48,50 51 Whether these molecules are involved in the IL-4–mediated growth inhibition of immature NK cells needs to be clarified.
Other investigators have reported that fetal thymus organ culture supplemented by cytokines supports the development of cytolytic cells. Widmer et al52 have shown that IL-7 and IL-2 increase the cytolytic activities of cultured fetal thymocytes while IL-4 inhibits development of cytolytic cells. More recently, Leclercq et al53 have reported that IL-15 also can induce the development of cytolytic cells in fetal thymus organ culture. In these reports, however, it was not clear what developmental stages of the cytolytic cells are regulated by these cytokines. In addition, the nature of cytolytic cells was not defined clearly. Our results presented in this report pertain to cytokine regulation of NK cells at the progenitor level.
Accumulating data suggest that NK and T cells share their developmental pathways. CD16+ or c-kit+ cells in murine thymus have been reported to have T and NK lineage potentials.8,9 More recently, Zúñiga-Pflücker et al35 have reported that c-kit+CD25− cells in murine fetal thymus have NK/T potentials whereas c-kit+CD25+ cells contain only T-cell committed progenitors. However, we found that c-kit+CD25+ cells are able to form immature NK cell colonies. The apparent discrepancy may be due to differences in the sensitivities of their in vivo and our cell culture assays. Regardless, our results suggest that the expression of CD25 is not an absolute marker of commitment to T-cell lineage. We are not certain whether the NK cell colonies we identified are derived solely from NK committed progenitors or both committed and NK/T common progenitors because our culture conditions may not be permissive to proliferation of T-cell progenitors. To clarify this point, a culture system that supports clonal proliferation of T-cell progenitors needs to be established.
Although this appears to be the first report of a culture system which supports proliferation of murine immature NK cell growth without stromal cell elements, cytokine-based culture systems for human NK cells and progenitors have already been reported. Sánchez et al7 have shown that certain populations of human fetal thymocytes cultured with IL-2, IL-7, and SF give rise to mature NK cells. Shibuya et al47 and Mrozek et al54 have shown generation of mature NK cells from human CD34+lin− cells in culture with IL-2 and SF or IL-15 and SF, respectively. A major difference exists between the human and our murine culture system in that our murine assay is directed to early stages of NK cell development only (Figs 2 and 3).
At the present time we are not certain why NK progenitors are present in the thymus. It has been shown that athymic nude mice have a normal number of mature NK cells.55 It is possible that there are several pathways of NK cell development, including the one occurring in thymus. It is unlikely that the colony formation described in this report applies only to the intrathymic NK progenitors, because we recently observed the immature NK cell colony formation from fetal liver cells similar to that from fetal thymocytes (unpublished observation). The clonal assay for immature NK cell progenitors described in this report will be useful in the studies of the origins of NK cells and characterization of the cytokine regulation of the early stages of NK cell development.
ACKNOWLEDGMENT
We thank Dr Haiqun Zeng for assistance in cell sorting and Dr Pamela N. Pharr, Dr Stewart D. Lyman, and Anne G. Leary for assistance in preparation of this manuscript.
Supported by National Institutes of Health Grants No. DK32294 and DK/HL48714, Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
Address reprinte requests to Makio Ogawa, MD, PhD, Ralph H. Johnson Medical Center, 109 Bee St, Charleston SC 29401-5799.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal