Abstract
This report describes the prognostic significance of the intensity of surface membrane antigen expression in a series of 1,231 children older than 1 year with newly diagnosed B-precursor acute lymphoblastic leukemia (ALL) treated on Pediatric Oncology Group (POG) treatment protocols. All patients had dual-color flow cytometric immunophenotyping performed at a central reference laboratory with a standard panel of monoclonal antibodies. The flow cytometers used in the study were calibrated with a standard fluorescence microparticle that permitted conversion of relative fluorescence channels to standard units of mean equivalents of soluble fluorochrome (MESF). In univariate analysis, fluorescence intensity of CD45 and CD20 was significantly associated with event-free survival (EFS), whereas other markers showed no significant correlation with outcome. Patients whose blasts were greater than the 75th percentile of intensity for CD45 (corresponding to 18,000 MESF units with CD45-FITC, or about 8% of the intensity of normal lymphocytes) fared significantly worse than those with lower-density CD45, and those whose blasts were greater than the 25th percentile of intensity for CD20 (corresponding to 17,900 MESF units with CD20-PE) had a poorer EFS. The intensity of both CD45 and CD20 was independently correlated with outcome. There was no significant correlation between intensity of expression of either antigen and traditional clinical risk factors, ploidy, or t(9; 22) or t(1; 19). All patients with t(4; 11) had CD45 intensity greater than the 75th percentile, but CD45 intensity retained its prognostic significance after adjusting for t(4; 11). In multivariate analysis, both CD45 intensity greater than the 75th percentile and CD20 intensity greater than the 25th percentile were significantly correlated with poor outcome independently of previously reported poor prognostic factors including National Cancer Institute (NCI) risk group, ploidy, trisomies of 4 and 10, and adverse translocations including t(1; 19), t(9; 22), and t(4; 11). We conclude that in childhood B-precursor ALL, the intensity of expression of CD20 and CD45 provides prognostic information not available from simple consideration of antigen expression as positive or negative, and adds to that obtained from traditional clinical and biologic risk factors.
IMMUNOPHENOTYPIC characterization of acute lymphoblastic leukemia (ALL) in childhood is important for directing therapy and predicting outcome. With the large number of monoclonal antibodies directed against hematopoietic cell surface antigens, it is now possible to demonstrate considerable phenotypic heterogeneity among cases of ALL. Although classification into major immunologic categories is well accepted,1-5 the importance of detailed subclassification is more controversial. Markers such as CD10, CD24, or myeloid cell surface antigens have been associated with outcome, although the independent prognostic importance of these is less well established.6-14
Although flow cytometry is widely used for performing immunophenotyping studies in acute leukemia, little attention has been given to standardizing the criteria for concluding that antigens are present on leukemic cells. In most studies, rigid criteria based on “percent positive” cells are used, even though procedures for standardizing the denominator used to calculate these percentages are rarely specified. Moreover, antigen distributions in leukemia are often such that it is not appropriate to analyze them as percent positives; specifically, distributions may be unimodal but of low intensity so that they overlap with control distributions. Arbitrarily enumerating events to the right of a negative control may result in the calculation of an inappropriate numeric value.
Fluorescence intensity may provide a better descriptor of antigen distribution on leukemic cells than percent positives. Unfortunately, commercial flow cytometers measure only relative fluorescence in units called channels. Changes in instrument set-up, service, or replacement, as well as changes in monoclonal antibodies, all change the relative channel value of the same fluorescent signal. This limits the ability to use relative fluorescence to compare studies performed over time.
In the past few years, methods for standardizing fluorescence using fluorescent microparticles have been developed.15-18 These methods take advantage of the fact that measurable amounts of fluorochromes can be attached to beads and assayed in the flow cytometer. Relative channel values are generated and plotted against the known values for the fluorochrome, expressed in units known as mean equivalents of soluble fluorescein (MESF), to produce a standard curve. The fluorescent channel of an unknown sample can then be read from this standard curve. In this way, it is possible to measure fluorescence in absolute rather than relative terms.
In this study, we used a set of fluorescent microparticles to standardize fluorescence intensity measurement of monoclonal antibody–defined cell surface antigens in pediatric B-precursor ALL. The fluorescence intensity of two markers, CD45 and CD20, is highly prognostic of outcome independently of traditional clinical and other biologic risk factors.
SUBJECTS AND METHODS
Patient population.From January 1991 to January 1994, 1,314 patients aged 1.0 to 21.9 years were entered onto Pediatric Oncology Group (POG) treatment studies for patients with newly diagnosed B-precursor ALL. Although the study did not terminate until November 1994, patients entered after January 1994 were excluded from the study after we became aware of a change in calibration procedures for the microparticles (Schwartz A., personal communication, December 1995). Specimens from all patients were sent by overnight courier to the immunophenotyping reference laboratory at Duke University (through late 1993) or Johns Hopkins University (after 1993), to the cytogenetics reference laboratory at the University of Alabama at Birmingham, and to the ploidy reference laboratory at St. Jude Children's Research Hospital. Cytogenetic and ploidy studies were performed as previously described.19 Informed consent for reference laboratory studies and for treatment was obtained using forms approved by local institutional review boards.
Among 1,314 patients, 1,231 had sufficient material to perform a complete analysis of antigen density for all markers; 1,258 had satisfactory data for determination of antigen density of CD20 and CD45. Although the material was suitable for confirmation of B-precursor phenotype in the remaining patients, these had either excessive contamination with uninvolved peripheral blood or insufficient cells for complete phenotypic characterization and therefore were not included in this analysis. Eight hundred twenty-two of 1,231 patients had informative cytogenetic studies (ie, at least two abnormal metaphases).
Immunophenotyping.Ficoll-Hypaque–enriched blasts at a concentration of 5 × 106/mL were stained in a final volume of 100 μL by two-color immunofluorescence with a panel of monoclonal antibodies directly conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE). Stained cells were analyzed by flow cytometry on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA), which was calibrated daily with a set of standardized beads (Caribbean Microparticles Inc, San Juan, Puerto Rico). These consisted of a set of beads each with a different known amount of either FITC or PE expressed in units of MESF. A standard curve was constructed each day by plotting MESF values for the beads against the median channel in which the peak was displayed.18 Fluorescence intensity data were collected using a logarithmic amplifier, but channel values were expressed on a linearized 256- or 1,024-channel scale.
Antibodies CD10, CD34, and CD45 were tested with FITC conjugates, and CD19, CD20, CD22, and HLA-DR were tested with PE conjugates. CD9 and CD24 were tested by indirect immunofluorescence using an FITC-conjugated Fab′(2) goat antimouse secondary antibody. Appropriate isotype controls were also tested. Although the duration of the study was such that it was not possible to use a single lot of antibody, the same vendor was used for the entire study and there was relatively little between-lot variability in fluorescence intensity. All antibodies were from Becton Dickinson, except for CD19-PE and CD9 (Immunotech, Westbrook, ME) and CD24 (Boehringer, Mannheim, Germany). A minimum of 5,000 events were collected for each pair of antibodies, and list mode data were analyzed using Lysys or, in later experiments, Lysys II software (Becton Dickinson). For analysis, an initial display of forward-angle light scatter versus right-angle light scatter was used to construct a mononuclear cell gate, and the reactivity of cells in the gate was determined for each marker. Antibodies were considered positive when there was a significant shift (usually about 20 channels with 256-channel full scale) in the curve of an antibody-stained sample compared with an isotype control, provided it could be determined that this shift was not due to reactivity of normal cells contaminating the gate used for analysis. This latter point was determined from inspection of all the antibody combinations run, based on knowledge of the expected position of normal cells on multiparameter displays. In addition to this method of visually comparing displays to determine positivity, the percent positive events was also recorded using traditional methods based on setting the gate on mononuclear cells and comparing control and test histograms. With this method, analysis was made using the arbitrary value of 20% as the criterion for positivity.
In addition to recording whether each marker was positive or negative, we also recorded the intensity of antigen expression based on determining the median channel value of the positive population. Although channel values are expressed in arbitrary units, these were converted to standard MESF units by reading from the standard curve obtained by plotting the known MESF values of the standard beads against their channel values. In some experiments, MESF values for blasts were compared directly with MESF values for residual normal lymphocytes in the population. In these experiments, blast populations were distinguished from the residual lymphocytes on dual-parameter displays of CD45 versus right-angle light scatter, separate gates were established on each population, and the median channel of each gated population was used to compute the corresponding MESF value from the same curve.
Treatment.Patients were assigned to standard-risk (POG 9005) or poor-risk (POG 9006) protocols based on age, white blood cell count, and blast ploidy. Briefly, children were assigned to POG 9005 using previously published age and white blood cell count criteria or if DNA ploidy was greater than 1.16 irrespective of age and white blood cell count.20 Other patients were considered poor risk. In addition, patients with central nervous system disease, t(9; 22), or t(1; 19) were considered poor risk independently of other factors. Sixty-five percent of the patients were assigned to 9005 and 35% to 9006, although the two studies had equivalent numbers of failures. Infants less than 1 year old were not included in this study.
Standard-risk patients were treated with vincristine, L-asparaginase, and prednisone for induction and randomized for intensification to either 12 courses of intravenous (IV) methotrexate (MTX) (1 g/m2 over 24h/IV 6-mercaptopurine (6-MP) (1 g/m2 over 6h) or 12 courses of oral MTX (30 mg/m2 per dose every 6 h for six doses)/IV 6-MP. The poor-risk protocol consisted of four-drug induction (vincristine, L-asparaginase, prednisone, and daunorubicin) followed by randomization for intensification to either 12 courses of the IV MTX/IV 6-MP regimen or an alternating drug regimen of IV MTX/6-MP, followed by VM-26 and cytosine arabinoside, followed by another MTX/6-MP course, and finally followed by daunorubicin, cytosine arabinoside, vincristine, prednisone, and PEG asparaginase. All patients received standard-dose MTX/6-MP as continuation therapy for 2 years and central nervous system prophylaxis with intrathecal MTX or triple intrathecal medications.
Statistical methods.Univariate analyses of prognostic markers were conducted using the log-rank test21 stratified for study (standard-risk v high-risk) using event-free survival (EFS) as the dependent variable. EFS is defined as the time from registration to relapse, progression, death, second cancer, or last contact, whichever occurs first. EFS curves were constructed by the Kaplan-Meier method22 with the standard errors of Peto et al.21 The overall results should take priority over all subset results (ie, standard- v poor-risk), as subsets have much lower statistical power to observe true differences. In addition, subset results were not an a priori objective of the study. To assess cutoff points and the independent importance of the CD variables found to be significant, multivariate analysis was constructed by the forward stepwise Cox method for proportional hazards, with EFS as the dependent variable.23 Independent variables included four binary variables (negative, and cutoffs at the first three quartiles) for each significant CD variable, along with National Cancer Institute (NCI) consensus risk group, study (standard- v poor-risk, the latter defined by age ≥ 10 years or white blood cell count >50,00024); trisomy of both chromosomes 4 and 10; or bad translocation [t(4; 11), t(9; 22), or t(1; 19)]. The risk ratio is the estimated ratio of instantaneous likelihood of failure, ie, adverse to favorable value of the factor. Clinical correlations were made by the Kruskal-Wallis nonparametric test for quantitative factors and the Pearson χ2 test for qualitative factors.
The prognostic significance of each factor was assessed using a traditional 20% positive cutoff and as five groups using intensity of expression, including negative plus each of the four quartiles for cases in which blasts were considered positive (ie, cutoff points were defined at the 25th, 50th, and 75th percentiles of the fluorescence intensity variable MESF for each marker). This division into multiple groups allowed for investigation of nonmonotonic trends (for example, a trend other than one in which the higher the value of a variable, the more favorable the outcome). The decision to use quartiles was arbitrary, but follows the common practice among clinical investigators to place patients into prognostic groups while allowing for flexible choices of cutoff points.
RESULTS
Prognostic significance of surface antigen expression.Each marker in the panel was evaluated in univariate analysis to determine its prognostic significance for EFS. Expression of CD10, CD19, HLA-DR, CD34, CD9, or CD24 was not significantly correlated with outcome whether results were expressed as positive using the arbitrary 20% positive cutoff or based on intensity (Table 1). CD22 was significant only when the percent positive cutoff was used; examination of this latter phenomenon in more detail revealed that this was due to a high failure rate (12 patients) among a small number of patients (n = 23) in whom CD22 was less than 20%. However, CD20 expression was an adverse prognostic marker using either the arbitrary percentage cutoff for positivity or based on intensity, whereas CD45 expression was only significant when intensity was considered.
Prognostic Significance of Antigen Expression in Childhood B-Precursor ALL by Univariate Analysis (P values)
| Antigen . | All . | Intensity . | ||
|---|---|---|---|---|
| . | Patients . | All . | Standard-Risk . | Poor-Risk . |
| . | > 20% . | Patients . | Patients . | Patients . |
| CD10 | .08 | .14 | .28 | .28 |
| CD19 | * | .65 | .11 | .69 |
| CD20 | .01 | .005 | .34 | .01 |
| CD22 | <.001 | .33 | .38 | .54 |
| HLA-DR | * | .63 | .84 | .76 |
| CD34 | .92 | .60 | .73 | .48 |
| CD9 | .91 | .18 | .81 | .18 |
| CD24 | .28 | .68 | .53 | .04 |
| CD45 | .81 | <.0001 | .08 | .0001 |
| Antigen . | All . | Intensity . | ||
|---|---|---|---|---|
| . | Patients . | All . | Standard-Risk . | Poor-Risk . |
| . | > 20% . | Patients . | Patients . | Patients . |
| CD10 | .08 | .14 | .28 | .28 |
| CD19 | * | .65 | .11 | .69 |
| CD20 | .01 | .005 | .34 | .01 |
| CD22 | <.001 | .33 | .38 | .54 |
| HLA-DR | * | .63 | .84 | .76 |
| CD34 | .92 | .60 | .73 | .48 |
| CD9 | .91 | .18 | .81 | .18 |
| CD24 | .28 | .68 | .53 | .04 |
| CD45 | .81 | <.0001 | .08 | .0001 |
Analysis of failure rates of patients in different groups was conducted with the log-rank test. In the column marked “v 20%,” patients were considered positive for each marker if 20% of blasts were positive; in columns marked “intensity,” patient populations were determined based on intensity distributions. Standard-risk and poor-risk patients were treated on study protocols 9005 and 9006, respectively.
Only 3 patients were CD19− and 2 patients HLA-DR−.
Intensity distributions for CD45 and CD20 were examined in more detail. MESF values for CD45-FITC that corresponded to the 25th, 50th, and 75th percentiles were represented by 6,250, 10,900, and 18,000 MESF units, respectively; for CD20-PE, the corresponding values were 17,900, 31,900, and 62,900. To put this in perspective, 24 cases with MESF values for the blasts closest to the 75th percentile cutoff (mean MESF, 17,600 ± 800) were compared with normal lymphocytes in the same specimen; the corresponding value for CD45 on these residual normal lymphocytes was 236,000 ± 70,000. Expressed as a relative value, the average ratio of the MESF value of CD45 on the blasts to that on the lymphocytes was .081 ± .024. There were insufficient normal residual CD20+ B cells to calculate a similar relative value for CD20.
Results were also investigated separately for standard- and poor-risk patients treated on studies 9005 and 9006, respectively. In five-way analysis, no marker was significant among standard-risk patients, although a trend was seen with CD45 intensity (P = .08). However, among poor-risk patients, both CD45 and CD20 intensities were significantly associated with outcome. CD24 intensity was marginally significant in higher-risk patients.
Prognostic significance of CD45 and CD20 intensity.Table 2 presents treatment failure rates as a function of fluorescence intensity of CD45. Patients who are CD45− or CD45+ up to the 75th percentile of intensity show little difference in outcome, but patients who are CD45-brightest (4th quartile) are at much higher risk of treatment failure. Kaplan-Meier plots of EFS for these patients are illustrated in Fig 1. Table 3 illustrates similar data for CD20 fluorescence intensity. The increased risk of treatment failure is seen at a lower intensity level (at the 2nd quartile and above). For both CD45 and CD20, fluorescence intensity shows a better correlation with treatment failure than does classification as positive or negative.
Prognostic Significance of Fluorescence Intensity of CD45 Expression in Childhood B-Precursor ALL by Univariate Analysis
| CD45 Intensity . | No. . | No. of Failures . | Expected . |
|---|---|---|---|
| Negative | 195 | 25 | 28.5 |
| 1st quartile | 278 | 35 | 49.9 |
| 2nd quartile | 285 | 41 | 49.8 |
| 3rd quartile | 288 | 47 | 49.9 |
| 4th quartile | 244 | 72 | 41.9 |
| CD45 Intensity . | No. . | No. of Failures . | Expected . |
|---|---|---|---|
| Negative | 195 | 25 | 28.5 |
| 1st quartile | 278 | 35 | 49.9 |
| 2nd quartile | 285 | 41 | 49.8 |
| 3rd quartile | 288 | 47 | 49.9 |
| 4th quartile | 244 | 72 | 41.9 |
P < .0001.
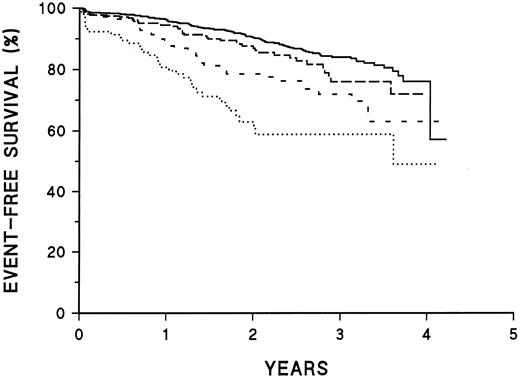
Prognostic significance of CD45 intensity. Kaplan-Meier survival plots of patients classified as shown in Table 2. ——, CD45− or intensity in dimmest quartile; –––, CD45 intensity in second quartile; ---, CD45 intensity in third quartile; ⋅⋅⋅, CD45 intensity in brightest quartile. The curves are significantly different (P < .001).
Prognostic significance of CD45 intensity. Kaplan-Meier survival plots of patients classified as shown in Table 2. ——, CD45− or intensity in dimmest quartile; –––, CD45 intensity in second quartile; ---, CD45 intensity in third quartile; ⋅⋅⋅, CD45 intensity in brightest quartile. The curves are significantly different (P < .001).
Prognostic Significance of Fluorescence Intensity of CD20 Expression in Childhood B-Precursor ALL by Univariate Analysis
| CD20 Intensity . | No. . | No. of Failures . | Expected . |
|---|---|---|---|
| Negative | 466 | 65 | 84.5 |
| 1st quartile | 204 | 35 | 41.0 |
| 2nd quartile | 211 | 40 | 35.1 |
| 3rd quartile | 226 | 39 | 32.8 |
| 4th quartile | 189 | 42 | 27.4 |
| CD20 Intensity . | No. . | No. of Failures . | Expected . |
|---|---|---|---|
| Negative | 466 | 65 | 84.5 |
| 1st quartile | 204 | 35 | 41.0 |
| 2nd quartile | 211 | 40 | 35.1 |
| 3rd quartile | 226 | 39 | 32.8 |
| 4th quartile | 189 | 42 | 27.4 |
P < .005.
In multivariate analysis, CD45 and CD20 intensity are independent prognostic factors for poor outcome. In this analysis, the strongest prognostic relationship of CD20 is above and below the median rather than at the 25th percentile. Figure 2 shows a Kaplan-Meier plot of outcome of all patients using risk groups defined by the combination of CD45 and CD20 intensity. The poorest EFS was seen among patients who were both CD45-bright and CD20-bright. Such patients accounted for about 8% of total patients; these represented about 14% of patients registered to the high-risk study and 5% of patients on the standard-risk study. The 3-year EFS of this poor-risk group is 59% ± 13%, compared with 84% ± 2% among patients who are neither CD45- nor CD20-bright. Other groups (CD45-bright, CD20-less bright; or CD20-bright, CD45-less bright) show intermediate survival.
Prognostic significance of the combination of CD45 and CD20 intensity. Kaplan-Meier survival plots of all patients classified by risk group based on intensity of CD45 and CD20 expression. CD45 poor risk implies < 75th percentile of intensity; CD20 poor risk implies < 50th percentile of intensity. ——, CD45 and CD20 good risk (57% of patients); –––, CD45 good risk, CD20 poor risk (24% of patients); ---, CD45 poor risk, CD20 good risk (11% of patients); ⋅⋅⋅, CD45 and CD20 poor risk (8% of patients). The curves are significantly different (P < .001).
Prognostic significance of the combination of CD45 and CD20 intensity. Kaplan-Meier survival plots of all patients classified by risk group based on intensity of CD45 and CD20 expression. CD45 poor risk implies < 75th percentile of intensity; CD20 poor risk implies < 50th percentile of intensity. ——, CD45 and CD20 good risk (57% of patients); –––, CD45 good risk, CD20 poor risk (24% of patients); ---, CD45 poor risk, CD20 good risk (11% of patients); ⋅⋅⋅, CD45 and CD20 poor risk (8% of patients). The curves are significantly different (P < .001).
Correlation of CD45 and CD20 intensity with other prognostic parameters.Patients with bright CD20 or CD45 were not significantly different from other patients with respect to other known important prognostic factors including age, white blood cell count, incidence of hyperdiploidy, trisomies of 4 and 10, or presence of the Philadelphia chromosome (data not shown). However, all 12 patients with t(4; 11) were in the CD45-bright (>75th percentile) group; CD45 intensity remained prognostic even after adjusting for t(4; 11) patients.
Multivariate analysis.Each marker shown to be of prognostic significance in univariate analysis was put into a multivariate Cox linear regression model along with other known prognostic factors including NCI risk group, ploidy involving trisomies of chromosome 4 and 10, adverse translocations [t(1; 19), t(9; 22), or t(4; 11)], and treatment regimen. A second analysis was made substituting flow hyperdiploidy (DNA index [DI] > 1.16) for the cytogenetic analysis, because there were significantly more patients for whom there were informative data with flow ploidy compared with cytogenetics.
In these analyses, the intensity of expression of CD45 (>75th percentile) and CD20 (>25th percentile) was again independently prognostic. The cutoff point for CD45 was again at the 75th percentile and that for CD20 was at the 25th percentile, as it had been in univariate analysis. Additional variables found to be significant included NCI risk group, trisomies of 4 and 10, and adverse translocations. In patients with only satisfactory flow ploidy, NCI risk group and flow hyperdiploidy (DI > 1.16) were significant. The final models are illustrated in Tables 4 and 5.
Multivariate Analysis of Risk of Treatment Failure in Childhood B-Precursor ALL Patients With Informative Cytogenetic and Immunophenotyping Studies (N = 822)
| Adverse Variable . | P . | Estimated Risk Ratio . |
|---|---|---|
| . | . | (95% confidence limits) . |
| NCI poor-risk group | <.001 | 2.1 (1.5-2.9) |
| Not both +4 and +10 | .002 | 2.3 (1.4-4.0) |
| Brighter than 1st-quartile CD20 | .006 | 1.6 (1.1-2.2) |
| Brightest-quartile CD45 | .008 | 1.6 (1.1-2.2) |
| t(1; 19), t(4; 11), or t(9; 22) | .04 | 1.5 (1.0-2.3) |
| Adverse Variable . | P . | Estimated Risk Ratio . |
|---|---|---|
| . | . | (95% confidence limits) . |
| NCI poor-risk group | <.001 | 2.1 (1.5-2.9) |
| Not both +4 and +10 | .002 | 2.3 (1.4-4.0) |
| Brighter than 1st-quartile CD20 | .006 | 1.6 (1.1-2.2) |
| Brightest-quartile CD45 | .008 | 1.6 (1.1-2.2) |
| t(1; 19), t(4; 11), or t(9; 22) | .04 | 1.5 (1.0-2.3) |
Multivariate Analysis of Risk of Treatment Failure in Childhood B-Precursor ALL Patients With Satisfactory Ploidy and Immunophenotypic Studies (N = 1,211)
| Adverse Variable . | P . | Estimated Risk Ratio . |
|---|---|---|
| . | . | (95% confidence limits) . |
| NCI poor-risk group | <.001 | 2.1 (1.5-2.9) |
| DNA < 1.16 | .009 | 1.7 (1.1-2.4) |
| Brightest-quartile CD45 | <.001 | 1.9 (1.4-2.6) |
| Brighter than 1st-quartile CD20 | .004 | 1.5 (1.1-1.9) |
| Adverse Variable . | P . | Estimated Risk Ratio . |
|---|---|---|
| . | . | (95% confidence limits) . |
| NCI poor-risk group | <.001 | 2.1 (1.5-2.9) |
| DNA < 1.16 | .009 | 1.7 (1.1-2.4) |
| Brightest-quartile CD45 | <.001 | 1.9 (1.4-2.6) |
| Brighter than 1st-quartile CD20 | .004 | 1.5 (1.1-1.9) |
DISCUSSION
The independent prognostic significance of expression of particular surface membrane antigens within an immunologically defined subgroup of ALL is controversial. In part, this reflects the close association between expression of particular surface markers and other biologic measurements of disease expression, particularly cytogenetics.25-28 However, varying analytic approaches used for measuring these antigens and for defining positivity may also play a role.
In this study, we calibrated the flow cytometers with a well-characterized fluorescent microparticle to quantify the fluorescence intensity of antigen expression. We found that the description of antigen expression using intensity was more powerful than either qualitative or quantitative dichotomous discrimination of “positives” and “negatives” for predicting early EFS in pediatric B-precursor ALL. Specifically, patients with the brightest expression of CD45 (>75th percentile) or relatively bright (>25th percentile) CD20 expression had an increased risk of treatment failure independently of each other and of other traditional risk factors including age, white blood cell count, DNA ploidy, or adverse chromosomal translocations. The risk ratios of treatment failure in final multivariate models for CD45 or CD20 ranged from 1.5 to 1.9, compared with relative risks of approximately 2.0 for other traditional adverse risk factors. Although these analyses were based on the entire cohort of patients, the clinical relevance of these results appeared most pronounced in the high-risk stratum.
We found no significant association between CD20 intensity and any other clinical or laboratory features known to be associated with adverse prognosis. However, there was a strong association between bright CD45 expression and t(4; 11): all patients with t(4; 11) expressed CD45 at intensity levels that put them in the upper quartile. In fact, preliminary studies suggest that bright CD45 expression is a more sensitive method for predicting t(4; 11) than our previously published algorithm based on expression of CD10, CD24, and CD15.22 Despite this strong correlation, the adverse prognostic association of bright CD45 expression was not completely explained by the poor outcome of t(4; 11) patients, as only 12 patients in our series had t(4; 11). It also appears unlikely that the adverse outcome of CD45-bright patients can be explained by other 11q23 translocations involving the MLL gene, as non-t(4; 11) MLL translocations are relatively rare in this patient population (Behm, Carroll, and Shuster, unpublished observations, May 1996).
In normal B-cell development, CD45 is expressed more intensely as B cells mature.29 However, we do not believe that the poor outcome of this patient population reflects the fact that their leukemic blasts represent a later stage of maturation. In the first place, as already noted, CD45 is particularly intense on the subset of “immature” leukemias associated with t(4; 11). In addition, there was no correlation between intensity of CD45 expression with other differentiation-related markers such as CD34. Whether CD45 expression per se is somehow important in determining response to therapy or whether it is a surrogate for an as yet undefined biologic marker is uncertain. CD45 is a tyrosine phosphatase, and as such may play a role in the regulation of growth via tyrosine kinase phosphorylation pathways.30 Whether this plays any role in leukemia remains to be determined.
Although CD45 is ubiquitous on hematologic cells, about 15% to 20% of B-precursor ALL is negative for CD45,6 although the sensitivity of the method used for detection may affect this number. Behm et al6 showed that patients with CD45− blasts fared better than CD45+ cases; however, this was due to the high correlation of CD45 negativity and hyperdiploidy. They did not investigate the bright CD45+ patients as a separate subgroup. However, Caldwell et al7 also showed that patients with bright CD45 fared worse than those with lower-density expression. However, the series was relatively small and multivariate analysis was not performed. Moreover, the patient population was heterogeneous with respect to immunophenotype, and unlike our series, it included some patients with T-ALL. However, in light of our results, it is likely that Caldwell et al were measuring a similar correlation between outcome and CD45 intensity.
CD20 has rarely been considered an important prognostic factor in childhood B-precursor ALL.9 Although we found a modest relationship between CD20 expression and outcome when expressed as positive or negative using an arbitrary percent positive cutoff, it was not nearly as strong as the consideration of intensity of expression; in multivariate analysis, intensity of expression was an independent significant prognostic factor. Thus, the failure of other investigators to note the importance of CD20 expression may be due to analytic problems in standardizing the most important discriminating values. Differences in therapy may also contribute to variability within the literature.
Relatively few investigators have examined the intensity of expression of surface markers other than CD45 in ALL.31-34 Look et al,31 and more recently Lavabre-Bertrand,33 studied the relationship of CD10 intensity to a number of patient characteristics. The latter study showed that CD10 intensity correlated with cytogenetic abnormalities, and also demonstrated that leukemic blasts often expressed levels of CD10 different from those of normal B precursors.
Ideally, for our results to be compared across studies and ultimately to be used to stratify patients, it would be useful to have an absolute reproducible value for a poor prognostic marker. The MESF value of 18,000, representing our 75th percentile value for CD45, has some limitations as such a marker. First, the change in calibration value of the bead since the inception of this study would likely slightly change the absolute value for this cutoff in subsequent studies. Second, even if this problem had not occurred, differences in antibody formulation will produce variations in this absolute value. A possible mechanism to overcome these limitations is suggested by the recent study by Bikoue et al.35 These investigators quantified the number of antigenic sites for CD45 on normal lymphocytes, monocytes, and granulocytes and found them to be stable across individuals. This suggests that normal cells might serve as internal standards for quantitative measurement. Although we did not have sufficient granulocytes or monocytes to evaluate, most cases had residual normal lymphocytes that could be readily separated from the blast population. When we compared the blast intensity of our cases at the 75th percentile cutoff with that of residual lymphocytes, we found that their fluorescence intensity corresponded to a relative intensity value of about 8% that of normal lymphocytes. An attempt to standardize CD20 cutoff values in a similar fashion was unsuccessful; this may, in part, be due to the small number of normal B cells that were present in the specimens. It would have been more difficult to determine a target value for the CD20 cutoff in any case because, depending on the analysis, the cutoff point for CD20 intensity as a significant variable was sometimes at the 25th percentile and sometimes at the 50th percentile. In future studies, we will investigate whether the use of an internal standard can provide a more reproducible method for measuring fluorescence intensity than the set of beads used in this study. In addition, the bead-based technologies used by Bikoue et al and other groups make possible direct readings of antibody binding sites independently of instrument, antibody, and fluorochrome.15,18 33 Such an approach to a more reproducible method of antigen density determination will also be investigated in future studies.
Although it might be tempting to conclude that patients whose blasts express CD45 at a level greater than 8% of that seen in normal lymphocytes should be treated more aggressively, we would urge caution before adopting such a strategy. In the first place, our own approach in the POG has been to confirm a new prognostic finding in a second prospective study before adopting it in our treatment design. Second, we do not yet know the interlaboratory or even the intralaboratory reproducibility of quantitation of intensity by this method. Finally, we should emphasize that the 75th percentile value was an arbitrary cutoff based on dividing our patient population into quartiles, and there is no inherent biologic significance to these cutoffs. However, arbitrary cutoffs are commonly used for all prognostic factors that are continuous variables, including age, white blood cell count, and ploidy.
However, despite the immediate practical limitations, our investigation has shown that measurement of antigen density of CD45 and CD20 provides important prognostic information in childhood B-precursor ALL. These results could lead to investigations on possible biologic attributes of leukemic cells with these phenotypic properties. In addition, future studies directed at confirming these results and further improving interlaboratory standardization could lead to more accurate methods for selecting therapy for children with ALL.
Presented in part at the 37th Annual Meeting of the American Society of Hematology, Seattle, WA, December 2-5, 1995.
Address reprint requests to Michael J. Borowitz, PhD, POG 9000, c/o Pediatric Oncology Group, Operations Office, 645 N Michigan Ave, Suite 910, Chicago, IL 60611.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal