To the Editor:
In 1994 we reported on a novel regimen consisting of cyclophosphamide (CY) combined with antithymocyte globulin (ATG) to condition 39 patients with aplastic anemia for marrow transplants from HLA-identical family members.1 The regimen was patterned after a successful second transplant regimen2 that, in turn, was developed on the basis of animal studies showing synergistic immunosuppression when an alkylating agent was alternated with ATG.3 The regimen's purpose was to reduce the risk of marrow graft rejection in aplastic anemia patients, most of whom had been previously transfused. Two of the 39 patients rejected their grafts, and both were successfully retransplanted. Three patients died. One, who had a 4-year history of aplastic anemia, died on day 5 with massive candidiasis that preceded the transplant. The second, who had a 13-year history of dyskeratosis congenita and a 2-year history of pneumonia, died on day 72 with pneumonia. The third, who had chronic graft-versus-host disease (GVHD), died on day 180 with a mixed Pseudomonas aeruginosa and cytomegalovirus pneumonia. Actuarial survival at 3 years was 92%, which compared favorably with the 72% survival rate in 39 historical patients conditioned with CY only who were matched for age and risk factors for rejection of GVHD. The current update with follow-up ranging from 3.2 to 8.2 years among surviving patients shows that the early survival advantage has persisted.
Thirty-nine consecutive patients were entered onto study from July 13, 1988 to April 17, 1993, and the results were analyzed as of January 1997. Patient ages ranged from 2 to 52 years (median, 24.5 years), 87% of the patients had been previously transfused, the median time from diagnosis to transplant was 2.4 months, and 41% of transplanted patients had previous unsuccessful treatment of their aplastic anemia by immunosuppressive therapy. Patients were conditioned for transplant by CY, 50 mg/kg intravenously on each of 4 successive days and, after the first, second, and third dose of CY, patients received ATG at 30 mg/kg intravenously per dose. Thirty-six hours after the last dose of CY, marrow was infused. Immunosuppression for prevention of GVHD consisted of a short course of methotrexate and at least 180 days of cyclosporine, as previously described.4 For each CY/ATG-treated patient, 1 historical patient receiving CY alone was chosen to be comparable in terms of age, laminar airflow room isolation, postgrafting immunosuppression, transfusion status, and the combination of patient and donor sex and donor parity. A major difference between the two groups of patients was that 62% of the historical patients received buffy coat cell infusions in addition to the marrow in an attempt at reducing the previously observed high graft rejection rate in multiply transfused individuals.5 Although this approach was successful with regard to rejection, patients receiving buffy coat cell infusions experienced a higher than usual incidence of chronic GVHD.6
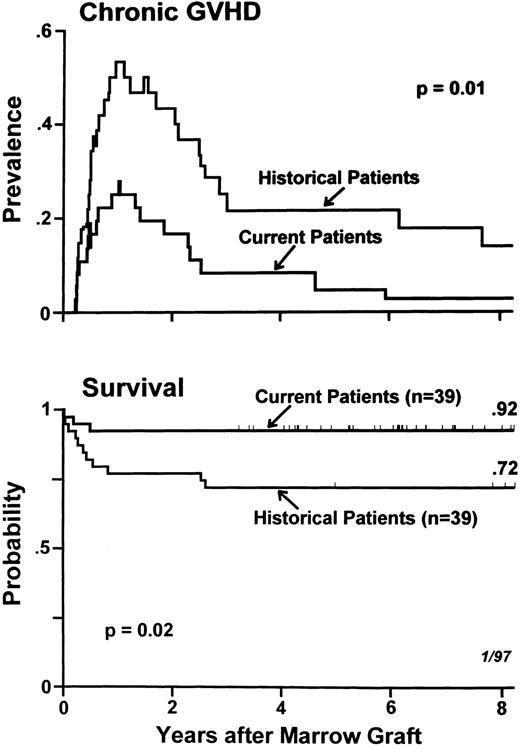
Graft rejection was seen in 5% of current and 8% of historical patients (P = .96), and acute GVHD was seen in 15% of current and 20% of historical patients (P = .64). Figure 1 updates the data on chronic GVHD and on survival. The prevalence curves for chronic GVHD describe both the times of onset of this complication and of its disappearance, along with discontinuation of its therapy. Chronic GVHD was not only less frequent but also appeared to be more responsive to therapy among current compared with historical patients. This explains the statistically significant difference (P = .01) between the prevalence curves for the two groups of patients, which began diverging at approximately 6 months after transplant. At 4 years after transplantation, 8% of current patients compared with 21% of historical patients still required treatment for chronic GVHD. The Kaplan-Meier estimate of survival at 8 years posttransplant was 92% in current patients compared with 72% among historical patients (P = .02).
Prevalences of chronic GVHD (top) and Kaplan-Meier estimates of survival (bottom) in 78 patients with aplastic anemia receiving HLA-identical marrow transplants and GVHD prophylaxis with methotrexate/cyclosporine, 39 of whom were conditioned with CY/ATG (current patients) and 39 received CY alone (historical patients). Tick marks indicate surviving patients.
Prevalences of chronic GVHD (top) and Kaplan-Meier estimates of survival (bottom) in 78 patients with aplastic anemia receiving HLA-identical marrow transplants and GVHD prophylaxis with methotrexate/cyclosporine, 39 of whom were conditioned with CY/ATG (current patients) and 39 received CY alone (historical patients). Tick marks indicate surviving patients.
Since the original report, 16 additional patients have been transplanted with this protocol. None of the 16 rejected the graft. Three patients died. One of them died on day 83 from respiratory syncytial virus pneumonia during a community based epidemic. The second died on day 210 from bilateral idiopathic pneumonia. The third died on day 223 from septicemia secondary to GVHD of gut and liver. Overall survival among the 55 patients was 89% at 8 years.
In conclusion, the CY/ATG regimen, when combined with postgrafting immunosuppression by methotrexate/cyclosporine, was well tolerated and was accompanied by low incidences of graft rejection and acute and chronic GVHD. The survival rate was 92%, a result that was significantly better than the 72% survival of a cohort of historical patients who were conditioned with CY alone and, in many cases, received viable donor buffy coat cell transfusions in addition to the marrow graft to reduce the risk of graft rejection. With a follow-up of up to 8.2 years (median, 5.2 years), no unusual long-term sequelae from the CY/ATG regimen have been seen among surviving patients.
ACKNOWLEDGMENT
Supported in part by Grants No. HL36444, CA18221, and CA15704 awarded by the National Institutes of Health, Department of Health and Human Services (Bethesda, MD).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal